Abstract
In order to determine the structure-performance relationship of nonionic-zwitterionic hybrid surfactants, N,N-dimethyl-N-dodecyl polyoxyethylene (n) amine oxides (C12EO n AO) with different polyoxyethylene lengths (EO n , n = 1–4) were synthesized. For homologous C12EO n AO, it was observed that the critical micelle concentration (CMC), the maximum surface excess (Γm), CMC/C 20, and the critical micelle aggregation number (N m,c) decreased on going from 1 to 4 in EO n . However, there were concomitant increases in surface tension at the CMC (γ CMC), minimum molecular cross-sectional area (A min), adsorption efficiency (pC 20), and the polarity ([I 1/I 3]m) based on the locus of solubilization for pyrene. The values of log CMC and N m,c decreased linearly with EO n lengthening from 1 to 4, although the impact of each EO unit on the CMC of C12EO n AO (n = 1–4) was much smaller than that typically seen for methylene units in the hydrophobic main chains of traditional surfactants. Compared to the structurally related conventional surfactant N,N-dimethyl-N-dodecyl amine oxide (C12AO), C12EO n AO (n = 1–4) have smaller CMC, A min, and CMC/C 20, but larger pC 20, Γm, and N m,c with a higher [I 1/I 3]m. This may be attributed to the moderately amphiphilic EO n (n = 1–4) between the hydrophobic C12 tail and the hydrophilic AO head group.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nonionic-ionic hybrid surfactants, especially those having a polyoxyethylene chain segment between the hydrophilic head group and hydrophobic tail, always display dual properties of both nonionic and ionic surfactants [1, 2]. For example, they show better electrolyte properties and water hardness tolerance. The traditional representatives are alcohol ether sulfates (AES) [3], alcohol ether carboxylates (AEC) [4], and sodium alkyl-benzyl polyoxyethylenated propanesulfonates (ABEPS) [2]. However, the synthesis and surface activity of nonionic-zwitterionic hybrid surfactants have seldom been reported to date.
One US patent [5] reveals the synthesis of N,N-dimethyl-N-alkyl polyoxyethylene amine and the corresponding amine oxides from commercially available AES. One Japanese patent [6] reports the synthesis of N,N-dimethyl-N-alkyl polyoxyethylene amine-based betaines from commercially available fatty alcohols. Due to the length distribution of the alkyl tails and polyoxyethylene segments in commercially available AES and polyethoxylated fatty alcohols (AEO n ), the corresponding amine oxides and betaines are mixtures of different molecules. Thus, it is hard to determine the structure-performance relationships of these hybrid surfactants when they are synthesized using commercially available AES or AEO n as starting materials.
Here, N,N-dimethyl-N-dodecyl polyoxyethylene (n) amine oxides (C12EO n AO) with different polyoxyethylene lengths (EO n , n = 1–4) were synthesized (see Fig. 1). Many important physicochemical parameters of these hybrid surfactants, such as the critical micelle concentration (CMC), the surface tension at the CMC (γ CMC), the adsorption efficiency (pC 20), the maximum surface excess (Γ m), the minimum molecular cross-sectional area (A min), the value of CMC/C 20, and the mean micelle aggregation number (N m), were determined using surface tension and fluorescence probe methods. The dependences of CMC, A min, and the critical aggregation number N m,c on polyoxyethylene length (EO n ) were deduced.
Experimental Section
Materials
1-Bromododecane (Br–C12), polyethylene glycol (EO n , n = 1–4), sodium hydride (NaH), dimethylamine (DMA), and H2O2 (30 wt%) were used as received without further purification. C12EO n AO (n = 1–4) were synthesized according to the previously reported method [7], and their molecular structures were confirmed by means of electrospray ionization mass spectrometry (ESI–MS) and 1H nuclear magnetic resonance (1H NMR). The results of ESI–MS and 1H NMR, and the corresponding interpretation using C12EO3AO as a representative example, can be seen in the Electronic Supplementary Material (ESM) Fig. 1S and Fig. 2S as complementary data. Deionized water was obtained from a Millipore Milli-Q water purification system (Millipore, USA).
Analytical Methods
Surface tension (γ, mN m−1) measurements were conducted on a drop volume tensiometer at 25 ± 0.1 °C. The outer radius of the glass capillary was 0.58 mm. In the procedure for γ measurements, a sufficient aging time is necessary for the pendant drop surface to reach an equilibrium state. The aging time of the drop surface was determined from a plot of γ versus the drop detachment time t (min) (ESM Fig. 3S). Finally, the drop volume was corrected by the Harkins–Brown method [8]. The surface activity parameters, specifically CMC, γ CMC, pC 20, Γ m, and A min, were obtained or calculated [9] from γ-log c (the concentration of surfactant) curves (Fig. 2). The calculation of Γ m and the related regression coefficients are listed in ESM Table 1S as supplementary data. The CMC values were confirmed by the pyrene I 1/I 3 method [10].
The micellar aggregation (N m) was measured by the time-resolved fluorescence method [11–13]. Time-resolved fluorescence measurements were performed using an FLS920 lifetime measurement spectrometer. An excitation pulse width of 1.5 ns was provided by a nanosecond flash lamp containing hydrogen gas as the medium at a pressure of 0.40 ± 0.02 bar. The pulse repetition rate was 40 kHz. The fluorescence was excited at 335 nm and detected at 393 nm with a fast photomultiplier tube. The fluorescence photon counts were accumulated using the technique of time-correlated single-photon counting (ESM Fig. 4S). The slit width and the excitation level were maintained at values such that the count rate did not exceed 2,000 cps in order to prevent pulse pile-up.
Results and Discussion
Surface Activity Parameters of C12EO n AO (n = 1–4)
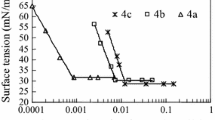
Plots of γ versus log c for C12EO n AO (n = 1–4) used in this work and for the corresponding structurally related traditional counterpart C12AO are shown in Fig. 2. It can be seen that γ gradually decreases to a plateau region with increasing surfactant concentration. This decrease in surface tension indicates that the surfactant molecules are adsorbed at the air/solution interface, and the discontinuity seen in the γ-log c curves suggests the formation of micelles in aqueous solution. Here, it is worth mentioning that no evidence of any surface tension minima was found in the γ-log c curves [14]. The surface activity parameters, such as CMC, γ CMC, pC 20, Γ m, and A min, were obtained and/or calculated [9] from the γ-log c curves and are listed in Table 1.
As can be seen in Table 1, the CMC value for C12AO determined in this work is in good agreement with the value of 1.18 × 10−3 mol L−1 (ring method) reported in the literature [15], despite the difference in γ determination methods. All of the CMC values of the hybrid surfactants studied in this work are of the order of 10−4 mol L−1 and are smaller than that of C12AO. A similar relationship holds for sodium dodecylpolyoxyethylene sulfates (C12EO n S, n = 2–4) and the corresponding sodium dodecyl sulfate (C12S) [2]. This may be attributed to the hydrophilicity of the hybrid surfactants in this work incrementally increasing from hydrophobic (C12) to moderately amphiphilic EO n (n = 1–4) to a hydrophilic AO group. As regards the change in hydrophilicity at the water/air interface, one can argue that it changes from hydrophobic (air), through a moderately amphiphilic interfacial zone (EO n , n = 1–4), to hydrophilic (water) [1], whereas the hydrophilicity of the structure-related counterpart, C12AO, changes discontinuously from hydrophobic (C12) to hydrophilic (AO) without an intermediate moderately amphiphilic transition zone (EO n , n = 1–4). This accounts for the higher surface activity of the hybrid surfactants used in this work.
Furthermore, the CMC values of C12EO n AO decreased with the length of the EO n segment (n) increasing from 1 to 4. A linear correlation between log CMC and n was observed, as shown in Fig. 3. Generally, for a homologous series of traditional surfactants, the CMC follows the empirical Stauff–Klevens rule, which indicates a logarithmic relationship between CMC and the number of carbon atoms in the alkyl main chain, as shown in the following Eq. (1):
where A and B are constants for a particular homologous series and temperature, and n C is the number of carbon atoms in the hydrocarbon main chain. The constant A varies with the nature and number of hydrophilic groups, while B is a constant that reflects the effect of each additional methylene group on the CMC. Based on the Stauff–Klevens rule, the relationship between EO n length (n) and log CMC (in 10−4 mol L−1) for C12EO n AO (n = 1–4) was determined as Eq. (2):
For traditional zwitterionic and nonionic surfactants, the constant B is approximately 0.5, which means that the CMC decreases by a factor of about 10 for each two methylene groups added to the hydrophobic main chain [16]. Here, the constant B for C12EO n AO (n = 1–4) is obviously smaller than that for traditional zwitterionic and/or nonionic surfactants. This indicates that the impact of each EO unit on the CMC of hybrid surfactants C12EO n AO (n = 1–4) is much smaller than that of a methylene group in the hydrophobic main chain, and is more akin to that of a methylene group in a side chain [17]. This could provide further evidence in support of the above hypothesis that the chain segment of EO n serves as a moderately amphiphilic zone between the hydrophilic head group and the hydrophobic tail [1].
The value of pC 20 reflects the efficiency of surfactant adsorption at the air/water interface. The larger the value of pC 20 is, the higher is the adsorption efficiency of the surfactant. As shown in Table 1, the value of pC 20 increased significantly from 3.55 to 4.20 when n was increased from 0 to 1, indicating that the efficiency of C12EO1AO adsorption at the air/water interface was larger than that of C12AO; a similar tendency has been observed for C12S and C12EO n S (n = 2–4) [2]. However, pC 20 for C12EO n AO showed a slight increase with increasing length of EO n from 1 to 4, which could be attributed to the moderately amphiphilic EO n segment between the hydrophilic head group (AO) and the hydrophobic tail (C12).
The CMC/C 20 ratios of the hybrid surfactants in this work are smaller than that of C12AO. The CMC/C 20 ratio is correlated with structural factors in the micellization and adsorption processes. A surfactant with a larger CMC/C 20 ratio has a greater tendency to adsorb at interfaces than to form micelles. Thus, the CMC/C 20 data in Table 1 indicate that C12EO n AO surfactants have a lower tendency to adsorb at interfaces than to form micelles. Additionally, the CMC/C 20 ratio of C12EO n AO shows a slight decrease with increasing length of EO n from 1 to 4, which is opposite to the trend for traditional polyoxyethylenated (POE) nonionic surfactants [17]. For POE nonionic surfactants, the CMC/C 20 ratio increases with increasing the number of EO units at a constant hydrophobic chain length. Thus, the EO n chain in C12EO n AO hybrid surfactants cannot be considered as a purely hydrophilic group as in POE nonionics.
The minimum molecular cross-sectional areas (A min) of C12EO n AO (n = 1–4) surfactants are smaller than that of C12AO, and hence their maximum surface excesses (Γ m) are accordingly larger. However, the values of A min for C12EO n AO increase with increasing length of EO n from 1 to 4, which may be attributed to coiling of the longer EO n chain [2, 19]. On the other hand, the EO n chain in the hybrid surfactant of C12EO n AO is probably suitably predisposed to form intermolecular hydrogen bonds with H2O at the air/water interface, as a result of which the γ CMC increases slightly with increasing EO n chain length from 1 to 4. Similar trends have been observed for the surfactants C12EO n S (n = 2–4) [2] and POE nonionics [18]. The difference in γ CMC between C12AO and C12EO1AO may be ascribed to their different molecular structures.
Polarity of the Micellar Microenvironment
The emission spectrum of pyrene features five vibrational bands, of which the first (I 1, around 373 nm) is enhanced in a polar microenvironment, whereas the third (I 3, around 384 nm) is not sensitive to the surrounding environment. It is known that pyrene preferentially dissolves in hydrophobic regions. Thus, the ratio I 1/I 3 can be exploited to probe the formation of micelles and the micropolarity of surfactant aggregates [20]. It can be seen in Fig. 4 that when the surfactant concentration reaches the CMC, the value of I 1/I 3 decreases rapidly. Thus, the correlation of I 1/I 3 and c may be employed to determine the CMC of surfactants (ESM Fig. 5S) [10]. The CMC values for C12AO and C12EO n AO in this work are listed in Table 1; the corresponding log CMC-n relationship was obtained according to Eq. (3) and is presented graphically in Fig. 3.
As can be seen from Table 1, Eqs. (2) and (4), the results of CMC (and the correlation of log CMC vs. n) for C12AO and C12EO n AO determined by the surface tension method are in good agreement with those determined by the pyrene I 1/I 3 ratio method.
For aqueous micelle solutions of C12AO and C12EO n AO, the values of I 1/I 3 (defined as [I 1/I 3]m) are 0.93 (C12AO), 0.94 (C12EO1AO), 0.95 (C12EO2AO), 1.01 (C12EO3AO), and 1.02 (C12EO4AO), respectively. Pyrene is a strongly hydrophobic probe and its solubility in water is very low (2–3 μM). In the presence of micelles, it is preferentially solubilized in the interior hydrophobic regions of these aggregates. The inner cores of typical micelles can be considered as hydrocarbon-like. However, considering that pyrene I 1/I 3 in water is around 1.56 and in pure hydrocarbon is around 0.61 [21], it seems that the locus of solubilization of pyrene can be neither the inner hydrocarbon-like cores nor the surfaces of micelles in the cases of C12AO and C12EO n AO. Furthermore, considering the rigid planar structure of pyrene, it seems that its locus of solubilization is probably the palisade layer, i.e. the hydrophobic-hydrophilic transition zone in these micelles [22, 23]. In the case of C12AO micelles, the palisade layer may consist of methylene groups near the end of the hydrophilic AO group, while for C12EO n AO (n = 1–4) this layer is probably made up of part of the EO units due to its moderately amphiphilic character. Hence, the polarity of the palisade layer in the C12AO micelles is likely to be somewhat lower than that in C12EO n AO (n = 1–4) and accordingly the [I 1/I 3]m values of C12EO n AO (n = 1–4) are slightly larger than that of C12AO. Moreover, the [I 1/I 3]m of C12EO n AO shows a slight increase with increasing n from 1 to 4 (see Fig. 4).
Micellar Aggregation Number N m
Plots of N m versus c for the hybrid surfactants C12EO n AO (n = 1–4) used in this work and the corresponding traditional surfactant C12AO are shown in Fig. 5. It can be seen that N m increases with increasing surfactant concentration. A quite good empirical linear correlation between N m and c was observed within a certain surfactant concentration range from 3 × CMC to 10 × CMC. Similar correlations have been observed for imidazolium-based cationic and zwitterionic surfactants [9, 17]. The corresponding mathematical equations for N m and c are listed in Table 2 (the coefficient constants are given in parentheses). Therefore, the critical micellar aggregation number (N m,c) can be obtained by extrapolating the linear equations for N m and c back to the CMC values, and are listed in Table 2. Here, the parameter N m,c describes the number of surfactant monomers for the first micelle corresponding to the defined CMC at a certain temperature, which is difficult to obtain by other routine methods.
As shown in Table 2, the N m,c of C12AO and C12EO4AO are approximately equal, which indicates that both surfactants should have almost the same degree of compactness in the micelles. It has been thought that water can enter the micelles and extend up to four carbons from the head group [21]. In micelles with compact head groups, the I 1/I 3 ratios are lower, indicating lower water penetration compared to that in micelles with larger head groups [21]. However, the data in Table 1 demonstrate that the A m values of C12EO n AO (n = 1–3) are smaller than that of C12AO, while the N m,c of C12EO n AO (n = 1–3) are larger than that of C12AO (see Table 2). All of these results indicate that the C12EO n AO (n = 1–3) micelles are more compact, allowing less water penetration, compared to the C12AO micelles. On this basis, the [I 1/I 3]m of C12EO n AO (n = 1–3) might be expected to be smaller than that of C12AO, but the data in Fig. 4 demonstrate the opposite trend. As discussed in the above section on the polarity of the micellar microenvironment, the locus of solubilization for pyrene is likely to be the palisade layer of C12EO n AO (n = 1–4) and C12AO micelles, and the polarity of this layer of C12EO n AO (n = 1–4) micelles is higher than that of C12AO micelles due to the higher polarity of the EO n chain compared to methylene groups. Therefore, as shown in Fig. 4, the [I 1/I 3]m values of C12EO n AO (n = 1–4) are larger than that of C12AO, despite almost equal N m,c values for C12AO and C12EO4AO systems.
For the homologous hybrid surfactants C12EO n AO (n = 1–4), with increasing n from 1 to 4, A m increases (see Table 1) while N m,c gradually decreases (Fig. 6). An approximately linear empirical correlation of N m,c and n can be described by Eq. (4):
Conclusions
Nonionic-zwitterionic hybrid surfactants C12EO n AO with different polyoxyethylene chain lengths (n = 1–4) have been synthesized. For homologous C12EO n AO, the values of CMC, Γ m, CMC/C 20, and N m,c decrease with increasing n in EO n from 1 to 4. Concomitantly, the parameters of γ CMC, A min, pC 20, and [I 1/I 3]m of the locus of solubilization for pyrene increase. The log CMC and N m,c decrease linearly with EO n lengthening from 1 to 4, although the impact of each EO unit on the CMC of C12EO n AO (n = 1–4) is much smaller than that of a methylene unit in the hydrophobic main chains of traditional surfactants. Compared to the structurally related conventional surfactant C12AO, C12EO n AO (n = 1–4) have smaller CMC, A min, and CMC/C 20, but larger pC 20, Γ m, and N m,c, with a higher [I 1/I 3]m. This may be attributed to the moderately amphiphilic EO n (n = 1–4) between the hydrophobic C12 tail and the hydrophilic AO head group.
References
Catanoiu G, Blunk D, Stubenrauch C (2012) Novel ethoxylated inositol derivatives-hybrid carbohydrate/oligoethylene oxide surfactants. J Colloid Interface Sci 371:82–88
Wang X, Yan F, Li Z, Zhang L, Zhao S, An J, Yu J (2007) Synthesis and surface properties of several nonionic-anionic surfactants with straight chain alkyl-benzyl hydrophobic group. Colloids Surf A 302:532–539
Alargova RG, Ivanova VP, Kralchevsky PA, Mehreteab A, Broze G (1998) Growth of rod-like micelles in anionic surfactant solutions in the presence of Ca2+ counterions. Colloids Surf A 142:201–218
Gong R, Han L, Gao C, Shu M, Che S (2009) Molecular design of AEC tri-block anionic surfactant towards rational synthesis of targeted thick-walled mesoporous silica. J Mater Chem 19:3404–3411
Koebner A, Potts HA (1965) N-dialkyl-aklyl- and alkaryl-oxyalkyl-amine oxides. US Patent 3, p 206,512
Yoshinobu N (1985) Ampholytic surface-active betaine compound and production thereof. JP Patent 60(089):458
Hou L, Zhang H, Chen H, Xia Q, Huang D, Meng L, Liu X (2014) Synthesis and surface properties of N, N-dimethyl-N-dodecyl polyoxyethylene amine-based surfactants: amine oxide, betaine and sulfobetaine. J Surfactants Deterg 17:403–408
Harkins WD, Brown F (1919) The determination of surface tension (free surface energy), and the weight of falling drops: the surface tension of water and benzene by the capillary height method. J Am Chem Soc 41:499–524
Liu X, Dong L, Fang Y (2011) A novel zwitterionic imidazolium-based ionic liquid surfactant: 1-carboxymethyl-3-dodecylimidazolium inner salt. J Surfactants Deterg 14:497–504
Aguiar J, Carpena P, Molina-Bolívar JA, Ruiz CC (2003) On the determination of the critical micelle concentration by the pyrene 1:3 ratio method. J Colloid Interface Sci 258:116–122
Atik SS, Nam M, Singer LA (1979) Transient studies on intramicellar excimer formation: a useful probe of the host micelle. Chem Phys Lett 67:75–80
Infelta PP, Grätzel M (1979) Statistics of solubilizate distribution and its application to pyrene fluorescence in micellar systems: a concise kinetic model. J Chem Phys 70:179–186
Jover A, Meijide F, Núñez ER, Tato JV (1997) Aggregation number for sodium deoxycholate from steady-state and time-resolved fluorescence. Langmuir 13:161–164
Mohamed A, Trickett K, Chin SY, Cummings S, Sagisaka M, Hudson L, Nave S, Dyer R, Rogers SE, Heenan RK, Eastoe J (2010) Universal surfactant for water, oils, and CO2. Langmuir 26:13861–13866
Goracci L, Germani R, Rathman JF, Savelli G (2007) Anomalous behavior of amine oxide surfactants at the air/water interface. Langmuir 23:10525–10532
Rosen MJ (2004) Surfactants and interfacial phenomena, 3rd edn. Wiley, New Jersey, pp 144–145
Liu X, Hu J, Huang Y, Fang Y (2013) Aggregation behavior of surface active dialkylimidazolium ionic liquids [C12C n im]Br (n = 1–4) in aqueous solutions. J Surfactants Deterg 16:539–546
Rosen MJ, Cohen AW, Dahanayake M, Hua X (1982) Relationship of structure to properties in surfactants 10. Surface and thermodynamic properties of 2-dodecyloxypoly(ethenoxyethanol)s, C12H25(OC2H4) x OH, in aqueous solution. J Phys Chem 86:541–545
Rosch M, Schick MJ (1996) Nonionic surfactants, surfactant science series 1. Marcel Dekker, New York, pp 22–23
Tariq M, Podgorsěk A, Ferguson JL, Lopes A, Gomes MFC, Pádua AAH, Rebelo LPN, Lopes JNC (2011) Characteristics of aggregation in aqueous solutions of dialkylpyrrolidinium bromides. J Colloid Interface Sci 360:606–616
Kalyansundaram K, Thomas JK (1977) Environmental effects on vibronic band intensities in pyrene monomer fluorescence and their application in studies of micellar systems. J Am Chem Soc 99:2039–2044
Kumbhakar M, Goel T, Mukherjee T, Pal H (2005) Nature of the water molecules in the palisade layer of a Triton X–100 micelle in the presence of added salts: a solvation dynamics study. J Phys Chem B 109:14168–14174
Turro NJ, Baretz BH, Kuo PL (1984) Photoluminescence probes for the investigation of interactions between sodium dodecyl sulfate and water-soluble polymers. Macromolecules 17:1321–1324
Acknowledgments
This work was jointly supported by the National Natural Science Foundation of China under Grant No. 21071065, the Scientific Research Foundation for the Returned Overseas Chinese Scholars (2011), State Education Ministry, Qinlan Project of Jiangsu Province, and Zhejiang Zanyu Technology Co. Ltd. of Zhejiang Province.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Cheng, H., Zhang, H., Liu, X. et al. Effect of Polyoxyethylene Chain Length on the Physicochemical Properties of N,N-Dimethyl-N-dodecyl Polyoxyethylene Amine Oxide Hybrid Surfactants (C12EO n AO, with n = 1–4). J Surfact Deterg 18, 487–493 (2015). https://doi.org/10.1007/s11743-014-1650-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-014-1650-x