Abstract
Rhamnolipids are interesting microbial surfactants having great industrial importance. However, the main obstacles towards an economic production of rhamnolipids are low productivity and high raw-material costs. Therefore, this study aimed at optimization of the culture media as well as culture conditions using response surface methodology for maximum rhamnolipid production by Pseudomonas aeruginosa isolate P6, a promising rhamnolipid-producing isolate. The optimum medium for maximum rhamnolipid production was found to be a mineral salts medium with glycerol 2 % v/v as the carbon source. The optimum cultivation conditions using response surface methodology were found to be an incubation temperature of 30 °C, an agitation rate of 250 rpm, an inoculum size of 5 % v/v and unlike most studies, an initial pH of 7.5. The resulting model predicted data points that corresponded well to the experimental values. Optimization resulted in a threefold increase in rhamnolipid production reaching 7.54 g/L. The data are potentially useful for further industrial exploitation of rhamnolipid production by the studied isolate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biosurfactants are a group of surface active molecules produced by a wide variety of microorganisms [1]. They have several advantages over chemical surfactants, such as lower toxicity; higher biodegradability and better environmental compatibility [2]. Rhamnolipids (RL), mainly produced by Pseudomonas aeruginosa (P. aeruginosa), constitute one of the most important classes of biosurfactants because of their advantageous characteristics [3]. With respect to their production, they show high concentrations as compared to other biosurfactants, and several renewable materials, like used oils or wastes from the food industry, can be used as carbon sources [4]. Unique properties of RL, including detergency, foaming, emulsifying, sequestering, solubilizing, and wetting make them suitable to be used in a wide range of industrial applications such as cosmetics, food, pharmaceutical formulations and bioremediation of pollutants [5].
However, problems in their production, such as low productivity, expensive raw materials and high costs for downstream processing, prevented them from being applied in bulk applications [6]. Reducing the production cost is very important for improving the market competitiveness of rhamnolipids [7]. Thus, to make this process cost-competitive, several strategies have been accepted worldwide, including optimizing the fermentation medium and optimizing the fermentation conditions [8].
Optimizing the fermentation medium involves the selection of cheap carbon sources together with overall media optimization in order to obtain maximum production with minimum costs. Optimizing the fermentation conditions involves selection of the optimal culture conditions that will induce the maximum productivity. A number of factors have been reported to affect RL production including the nature of the carbon source, temperature, agitation rate and pH [9]. Although a considerable number of studies on the optimization of RL production have been reported recently [10–12], more studies are still required to find and optimize new promising isolates producing this powerful biosurfactant. A statistical optimization strategy based on response surface methodology (RSM) is usually used to make the optimization process easier. This strategy helps the industry to design the best media containing cheaper substrates and to use the most favorable environmental conditions for improved biosurfactant production.
Therefore, the aim of the present study was directed towards physiological optimization of RL production by the P. aeruginosa soil isolate P6 by optimizing the media used and the use of RSM to optimize the environmental conditions for maximum RL production.
Materials and methods
Microorganism
The P. aeruginosa isolate P6 is a promising RL producer obtained through a screening program in our previous study (unpublished data). This isolate was stored in Luria–Bertani broth (LB broth) (Lab M, Topley House, England) containing 20 % glycerol at −20 °C.
Culture Media
The basal mineral salts medium, MSM [13] and the soybean oil MSM, SMSM [14] were prepared and used in this study for optimization of RL production. Medium SMSM was MSM with C source and multivalent cations replaced with the optimum ones and its composition was as follows [14] (conc/ml): Soy bean oil (20 ml), NaNO3 (2.50 g), MgSO4·7H2O (2.85 g), NaCl (1.00 g), KCl (1.00 g), ZnSO4 (0.16 g), FeCl3·6H2O (0.013 g), MnCl2·4H2O (0.2 g), Na2MoO4·2H2O (0.012 g), H3BO3 (0.062 g), CoSO4·7H2O (0.028 g), H3PO4 85 % (10.0 ml) and distilled H2O to 1000 ml. The pH of this media was adjusted to 7 using KOH pellets before sterilization.
Fermentative Production of RL
The seed culture was prepared by inoculating a loopful from a fresh culture into a 250-ml Erlenmeyer flask containing 25 ml Trypticase soy broth. The flask was incubated at 250 rpm and 30 °C for 15–16 h. After that the culture obtained was centrifuged at 10,000 rpm for 10 min and the resulting cells were washed once and resuspended in the fermentation medium to a count of 5 × 109 cfu/ml.
The production process was carried out using 50-ml aliquots of the fermentation medium under test in 250-ml Erlenmeyer flasks. The flasks were inoculated with 2 % v/v of the seed culture prepared above and incubated in a shaking incubator at 250 rpm and 30 °C. At specified time intervals, the fermentation broth was sampled for determination of biomass and RL concentration.
Analytical Methods
Biomass Determination
Cellular growth (biomass g/L) was expressed in terms of dry cell weight which was calculated from optical density (OD600) measurements using the equation of a calibration curve constructed between OD600 and dry cell weight of the tested P. aeruginosa isolate P6 as described in previous studies [15]. To measure the OD600, one ml of the culture broth was centrifuged at 10,000 rpm for 10 min and the resulting cells were washed once, resuspended in saline and appropriately diluted. In the case of experiments containing soybean oil as carbon source, the culture broth was initially mixed vigorously with equal volumes of hexane and centrifuged to remove residual oil [16, 17]. The resulting pellet was then washed once, resuspended in saline and appropriately diluted to measure its OD600.
RL Concentration
RL concentration was determined using the modified colorimetric orcinol assay [18, 19]. The RL in the supernatant was first extracted as described by Wu and Ju [20]. Briefly, the supernatant was acidified to pH 2 and left overnight at 4 °C. It was then extracted twice by mixing and vigorously shaking with an equal volume of ethyl acetate. Finally, the separated organic phases were pooled and evaporated at 80 °C. The obtained residue was dissolved in distilled H2O adjusted to pH 7 using 2.5 N NaHCO3 to be used for the orcinol assay. A control (uninoculated medium) was prepared for each experiment to act as blank during spectrophotometric measurement in the orcinol assay.
The orcinol assay was performed according to Chandrasekaran and Bemiller [18]. An aliquot of 100 μL of the diluted aqueous extract was added to 900 μL orcinol reagent (0.19 % orcinol in 53 % H2SO4) and heated in a water bath at 80 °C for 30 min. The mixture was allowed to cool to room temperature and the developed color was measured at 421 nm against the blank. The concentration of RL in the sample was calculated from an equation of a standard calibration curve prepared in a previous study using a standard RL solution (A 421nm = 0.0047 × RL conc) [14], considering the dilution factor (D.F.) of the diluted aqueous extract, as follows:
Studying Different Factors Affecting RL Production
Effect of Different Media Components on RL Production
Effect of Replacement of Glucose in MSM with Other Carbon Sources and Testing the Production in MSM and SMSM at Different Concentrations of Two Selected Carbon Sources
In our previous study, it was proven that isolate P6 produces maximum biomass of 1.62 g/L after 2 days of incubation and maximum RL concentration of 2.5 g/L after 6 days of incubation when grown in MSM (containing glucose as the carbon source) at 30 °C and 250 rpm (unpublished data). The carbon source (glucose) present in MSM was replaced with other carbon sources, namely glycerol and soy bean oil, both at 2 % v/v. The media with the tested carbon source was then inoculated and incubated as described before. The carbon source which proved to yield maximum RL production was selected and tested at various concentrations (1, 2, 3, 4, 8 % v/v). In parallel, soybean oil originally present in SMSM was also tested at various concentrations (1, 2, 4, 8, 10 and 15 % v/v).
RL Production by Isolate P6 in MSM and SMSM with Dual Carbon Sources
In an attempt to enhance RL production, a dual carbon source system was investigated. For MSM with glycerol (which proved to be optimal for RL production in MSM), the additional carbon source was soybean oil (the original carbon source present in SMSM) and for SMSM with the selected soybean oil concentration, the additional carbon source was glycerol. In both cases, the additional carbon source was tested at various concentrations (0, 0.5, 1 and 2 % v/v). RL production was compared in such media and the best media was chosen for further experiments.
Effect of Different Environmental Fermentation Conditions
Response Surface Methodology (RSM) for the Optimization of RL Production
Factors such as temperature (represented by the coded variable A), agitation rate (represented by the coded variable B), inoculum size (represented by the coded variable C) and pH (represented by the coded variable D) were optimized by RSM since these were previously reported to be important factors affecting RL production [9]. A complete factorial design was used and in total, 13 runs of experiments were carried out (Tables 1, 2) using the best chosen media. Two response values, the RL concentration (R, g/L) and biomass (Y, g/L) were employed. After 6 days incubation, the response values were measured accordingly. The design of experiments was performed using Design Expert® v. 7.0 (DesignExpert ® Software, Stat-Ease Inc., Statistics Made Easy, Minneapolis, MN, USA).
Experimental Verification of RSM Results
A set of optimal culture conditions was generated using the numerical optimization function in the Design Expert software and a new shaking flask fermentation experiment was carried out using these optimal parameters. The RL production and biomass values were measured and compared with the results obtained using unoptimized conditions.
Statistical and Graphical Analysis
All experiments were carried out in triplicate and the values plotted are the means of triplicate results while error bars indicate the standard deviation of the data.
In the RSM tests, all the experiments were carried out in triplicate and the mean of three readings was recorded. All data analysis, response surfaces and model diagnostic plots were generated using Design Expert® v. 7.0 (DesignExpert ® Software, Stat-Ease Inc., Statistics Made Easy, Minneapolis, MN, USA).
Results and Discussion
Different Factors Affecting RL Production by Isolate P6
Effect of Different Media Components
RL Production in MSM with Different Carbon Sources and in SMSM
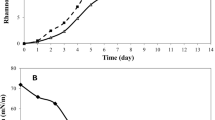
Cell growth and RL production are greatly affected by the medium components and the environmental fermentation conditions [9]. One of the factors having a marked effect on RL production is the carbon source used in bacterial culture [21]. In this study, the effect of replacing glucose with other carbon sources in MSM and the effect of using SMSM was tested. The tested carbon sources were glycerol (2 % v/v) and soybean oil (2 % v/v) as these were the most promising carbon sources reported in earlier studies [11, 14]. Results in Fig. 1 showed that maximum RL production was obtained using MSM containing glycerol (2 % v/v) as the carbon source and SMSM when compared to RL production in MSM containing glucose or soybean oil as the carbon source. Therefore, in the present study, high RL production by P. aeruginosa isolate P6 was achieved using either soluble carbon sources (glycerol) in MSM or insoluble carbon sources (soybean oil) in SMSM. This may indicate that such RL not only assist in the emulsification of water insoluble substrates but also may participate in another physiological role in the bacterial cell [19]. Therefore, these two media were selected for further studies. MSM containing glycerol was called GMSM.
Regarding biomass, maximum biomass was obtained with SMSM followed by MSM with soy bean oil 2 % indicating that soybean oil resulted in better growth than glycerol and glucose. These findings are in agreement with a previous study where an increase in biomass was observed with media containing soybean oil or safflower oil over that with glycerol [11]. Moreover, results showed that SMSM resulted in both a higher biomass and a higher RL production than MSM containing soybean oil 2 %. As mentioned previously, medium SMSM was MSM with C source and multivalent cations replaced with the optimum ones. These results proved that the multivalent cations, and not only the carbon and nitrogen sources, are critical factors in the media for both growth and RL production. Guerra-Santos et al. [22] demonstrated that by limiting the concentrations of salts of magnesium, calcium, potassium, sodium, and trace elements, a higher yield of rhamnolipid can be achieved. Moreover, the highest final RL concentrations (30 °C, pH 6.3, sunflower oil) were observed in calcium-free media as observed by Giani et al. [23].
The production profiles (Fig. 1) indicated that in the case of glycerol in MSM, although the RL appeared at the same time with the microbial growth, production still increased after biomass reached a plateau. For this reason, the product formation appeared to be partly growth-associated as described by other authors [24]. However, in the case of soybean oil in MSM and SMSM, RL production began to increase after biomass reached a plateau and therefore, the production of RL in this case is typical of a secondary metabolite. Therefore, it can be concluded that the growth or non growth association of RL production is culture medium dependant rather than strain dependant. The literature describes the occurrence of different kinetic profiles for RL. P. aeruginosa PA1 had been reported to produce RL which increased significantly in the stationary phase [25]. However, the growth-associated production of RL had also been noted by several researches [4, 26].
Effect of Variable Concentrations of the Selected Carbon Sources
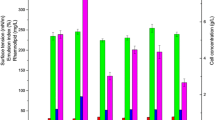
The carbon sources that showed maximum RL production in MSM (glycerol) and in SMSM (soybean oil) were tested at different concentrations. As illustrated in Fig. 2, maximum RL production (6.75 g/L) and bacterial growth (2.19 g/L) by isolate P6 was obtained using 2 % v/v glycerol in case of GMSM. In case of SMSM, maximum RL concentration (6.24 g/L) was obtained using 2 % v/v soybean oil, however, biomass increased as soybean oil concentration increased. Similar results were obtained in previous studies [14, 27] which reported that low concentrations of glycerol and soybean oil concentrations were favorable for maximum RL production.
RL Production by Isolate P6 in GMSM and SMSM with Dual Carbon Sources
Based on these results, we attempted to enhance RL production by using dual carbon source media combining the two selected carbon sources by different ratios. GMSM flasks containing different concentrations of soybean oil, and SMSM flasks containing different concentrations of glycerol were tested for RL production. GMSM containing 0 % soybean oil resulted in the maximum RL production of 6.65 g/L followed by SMSM containing 0 % glycerol, while maximum biomass was obtained using SMSM containing 0 % glycerol, reaching 2.8 g/L (Fig. 3). Results showed that the addition of soybean oil to GMSM led to a sharp decline in RL production, an observation which was not noted in case of the addition of glycerol in SMSM, where a gradual decline was demonstrated. This indicates that dual carbon sources were less favorable for RL production relative to the sole carbon sources. Results in this study are in agreement with a previous study [28], where addition of crude oil to a media containing glucose as the carbon source before bacterial inoculation led to a decrease in biosurfactant production. In contrast, concerning biomass, a gradual increase was observed as the concentration of soybean oil increased in GMSM. Similar results were obtained in previous studies were biomass in media containing soybean oil was higher than values obtained using media without the oil [11, 14, 29]. However, biomass gradually decreased as the concentration of glycerol increased in SMSM. Therefore, for maximum RL production, GMSM was selected for further studies. Glycerol is a very interesting option as a substrate due to its widespread availability, relatively low cost, and its predicted increased market excess as biodiesel production increased in several countries [30, 31]. These results are different from most previous studies which reported that vegetable oils were the best substrates for the induction of RL production with P. aeruginosa, and that final concentrations were generally higher than those for non-hydrophobic substrates [32, 33]. However, in 2002, Santa Anna et al. [25] showed that the maximum RL production was achieved when glycerol was used as carbon source in comparison to using hydrocarbons or vegetable oils. Moreover, in 2008, Wu et al. showed that glucose and glycerol were superior to olive oil and soybean oil as carbon sources in the RL production by P. aeruginosa EM1 [34]. This implies that the carbon source preference for the RL production is bacterial strain dependant [34].
Effect of Different Environmental Fermentation Conditions
RSM for the Optimization of RL Production
To optimize the environmental fermentation conditions, RSM was used. RSM is a collection of mathematical and statistical techniques that are useful for the modeling and analysis of problems in which a response of interest is influenced by several variables and the purpose is to optimize this response [35]. Several studies have reported the use of this technique for optimization of biosurfactant production [1, 36, 37]. In this study, we undertook a complete factorial design for optimization of RL production by isolate P6. The design of experiments, modeling, data analysis by ANOVA, generation of response surfaces and diagnostic plots were conducted using the Design Expert software.
Concerning the factors temperature and agitation, Table 3 showed the actual values of these two factors, the design, the experimental results, and the values predicted by the fitted equations obtained by the Design Expert software. These fitted equations for the two responses are given by Eqs. 1 and 2 for RL and biomass, respectively as follows:
ANOVA results for both responses are shown in Table 4. ANOVA verifies the adequacy of the models and explains the significance of the factors with effect on RL production and biomass. The P value was used as a tool to determine the significance of each of the coefficients. The Model F values of 3392.68 and 457.00 for RL production and biomass, respectively, implied that the models are significant. For RL production, there is only a 1.21 % chance that a “Model F value” this large could occur due to noise (P value = 0.012) whereas for biomass, there is only a 3.31 % chance that a “Model F value” this large could occur due to noise (0.0331). Moreover, A and B were found to be significant model terms for both responses (Table 4). Low values of the coefficient of variation (CV) of 0.48 and 0.21 % were obtained for responses R1 and Y1, respectively which indicates the good reliability of the experimental values. The CV reveals the level of precision with which the treatments are compared. Usually, as the CV value increases, the reliability of the experiment decreases [38]. For the first response, RL production (R1), the fit of the model was expressed with the coefficient of determination R 2 that was 0.9999, indicating that 99.99 % of variability in the response could be explained by the model. The Predicted R-squared (Pred R 2) of 0.9976 was obtained which was in reasonable agreement with the Adjusted R-squared (Adj R 2) which was found to be 0.9996. Adequate (Adeq) Precision measures the signal to noise ratio. A ratio greater than 4 is usually desirable [39]. In this model, an adequate precision ratio of 112.699 was obtained which indicated an adequate signal. This model can be used to navigate the design space. In case of the second response, biomass (Y1), there was no lack of fit and the fit of the model was indicated by the high R 2 that was 0.9989, suggesting that the model could explain 99.89 % of response variability. The Pred R 2 of 0.9825 was in reasonable agreement with the Adj R 2 of 0.9967. A ratio of 48.497 Adeq indicated an adequate signal. This model can be used to navigate the design space [39].
The three-dimensional (3D) plots between the input factors for both responses are shown in Fig. 4. These plots not only present details about the interaction between two parameters but also enable a simple prediction of the optimal experimental conditions [38]. From Fig. 4, and by using numerical optimization function in the Design Expert software, optimum conditions for maximum RL production were found to be a temperature of 30 °C and agitation rate of 250 rpm. On the other hand, maximum biomass of 2.45 g/L was obtained at a temperature of 37 °C, and agitation rate of 250 rpm. Often, in production processes, different conditions for cell growth and production may be observed [40].
Concerning the factors inoculum size and pH, the actual values of these two factors, the design, the experimental results obtained, and the values predicted by the fitted equations. are shown in Table 5. The fitted equations for the two responses are given by Eqs. 3 and 4 for RL and biomass, respectively as follows:
In case of RL production, ANOVA showed that C, D and D 2 were significant model terms whereas CD and C 2 were not significant (P value = 0.3096 and 0.9209, respectively). Moreover, Box-Cox plots for power transforms recommended a transformation to inverse square root. Therefore, model reduction and transformation was performed and a simplified equation was generated as follows:
ANOVA results of the quadratic model for RL production in Table 6 revealed that the Model F value was 538.18 which implied that the model is significant. There is only a 0.01 % chance that a “Model F value” this large could occur due to noise (P value <0.0001). A low value of CV of 0.53 % was obtained. A high R 2 of 0.9969 expressed the fit of the model, and indicated that this model could describe 99.69 % of variability in the response. The predicted R 2 of 0.9915 was in reasonable agreement with the adjusted R 2 of 0.9951. The Adequate Precision ratio of 63.768 indicates an adequate signal and that this model can be used to navigate the design space [39].
In case of biomass, the Model F value of 135.68 implied that the model is significant. There is only a 0.02 % chance that a “Model F value” this large could occur due to noise (P value = 0.0002). In this case, C, D, C 2 and D 2 were significant model terms (Table 6). A low value of CV of 0.72 % was obtained and the fit of the model was expressed with the R 2 that was 0.9927, implying that 99.27 % of the response variability could be explained by this model. The Pred R 2 of 0.9630 was in reasonable agreement with the Adj R 2 of 0.9854. Adeq precision ratio of 35.702 indicated an adequate signal and that this model can be used to navigate the design space [39].
The 3D plots between the input factors are shown in Fig. 5 and revealed that biomass reached the peak at 5 % inoculum size and a pH of 7. In case of RL production, maximum value was reached at 5 % inoculum size and a pH between 7 and 7.5. Using numerical optimization function in the Design Expert software, optimized conditions for maximum RL production were found to be inoculum size of 5 % and a pH of 7.5.
Model Diagnostics
To validate our models, graphical summaries for case statistics were constructed as follows.
-
(a)
Box Cox plots
The Box–Cox plots for power transformations for the two generated models in case of temperature and agitation showed that the models were sufficient with no need for further transformation, where the current lambda (λ = 1) is within the 95 % confidence range of the best lambda value (see supplementary material Fig. S1a and S1b).
Concerning inoculum size and pH, in case of RL production, a transformation to inverse square root was recommended (see supplementary material Fig. S2a). This improved the R 2 and modified results are shown in Fig. S2b (supplementary material). In case of biomass, the model was proven to be sufficient with no need for further transformation, where the current lambda (λ = 1) is within the 95 % confidence range of the best lambda value (see supplementary material Fig. S2c).
-
(b)
Predicted vs actual plots
The plots showed that the points were distributed near to the straight line which indicated that actual values were very close to the predicted ones (see supplementary material Fig. S3a, S3b, S4a and S4b).
-
(c)
Residuals vs run plots
The points were scattered around the mean for both responses which indicated that the models fit the data (see supplementary material Fig. S5a, S5b, S6a and S6b).
The ANOVA results also showed that all the tested factors (temperature, agitation, inoculum size and pH) had a significant effect on RL production and biomass. However, among these factors, inoculum size and pH had the most significant effect on RL production since these two factors showed the lowest P values. An increase in inoculum size generally improves the growth and growth related activities of the organism up to a certain limit after which there could be a reduction in microbial activity due to nutrient limitations. Lower inoculum size requires a longer time to grow to optimum number to consume the substrate and form the required product [41]. In this study, optimum inoculum size was found to be 5 % v/v.
The pH of the medium greatly influences many enzymatic and secondary metabolites production. It was previously reported that RL production in different Pseudomonas spp. is at its maximum at a pH range from 6 to 7, depending on the strain used [42–44]. Moreover, Mulligan et al. reported that optimum pH for RL production by P. aeruginosa was 6.2 and that it stops producing RL at a pH higher than 7.5 [45]. Another recent study also reported that RL concentration declined and reached its lowest point at a pH of 8 [46]. In contrast to these reports, maximum RL production in this study was obtained at an initial pH of 7.5, and could still be produced at a pH of 8, but in lower concentrations. A pH lower than 7 also decreased RL production, which suggests that the bacterial isolate was highly sensitive to pH for RL production.
Temperature is also one of the critical factors that affect the production of RL. Wei et al. measured RL production between 25 °C and 47 °C and found that the optimum temperature was between 30 °C and 37 °C [21]. Therefore, these two values were tested and result revealed that the production of RL reached a maximum at 30 °C, however biomass reached a maximum at 37 °C.
The speed of agitation was previously reported to be a significant factor affecting RL production rate [46]. Wei et al. reported that increasing the agitation rate from 50 to 200 rpm increased the RL production by P. aeruginosa J4 about 80 %. However, further increasing the agitation rate decreased the transfer efficiency of oxygen into the liquid medium, giving rise to inadequate conditions for the RL production [21]. The effect of agitation on the RL concentration and biomass in this study was tested at 200 and 250 rpm and the results obtained showed that the velocity of 250 rpm resulted in an increase in RL production over a velocity of 200 rpm. These results were in agreement with previous studies which showed that at 250 rpm, the RL biosynthesis reached its maximum value [47].
Experimental Verification Test
RL production using the recommended optimal levels of the four factors (30 °C, 250 rpm, 5 % v/v and pH 7.5) reached 7.54 g/L. This value is close to the value predicted by the model which was 7.30 g/L which demonstrates the validity of the model. As depicted in Fig. 6, the optimal levels used resulted in a 1.2-fold increase in RL production over that obtained using optimized media but unoptimized culture conditions (6.3 g/L), and a threefold increase in RL production over that obtained using unoptimized media and unoptimized culture conditions (2.5 g/L).
Conclusion
In conclusion, these results showed that experimental design and response surface methodology are successful tools for optimizing culture conditions leading to a higher RL concentration. Application of RSM enhanced the RL production threefold and a maximum RL concentration of 7.54 g/L was reached using MSM containing 2 % v/v glycerol as the carbon source. The optimum recommended conditions were found to be a temperature of 30 °C, agitation rate of 250 rpm, inoculum size of 5 % v/v and a pH of 7.5. The maximum RL concentration obtained in this study is greater than most of the comparable data cited in the literature, which used glycerol as a carbon source. Santa et al. [25] obtained 3.16 g/L RL (expressed as rhamnose), using P. aeruginosa PA1 and glycerol as the sole carbon source (3 % v/v). Da Rosa et al. reported a maximum RL concentration of 4.15 g/L [27]. In 2010, Silva et al. reported a RL concentration of 8 g/L using 3 % glycerol as the carbon source [4], which is close to results obtained in this study. Therefore, isolate P6 can be considered to be a promising bacterial isolate for further industrial exploitation.
Abbreviations
- MSM:
-
Mineral salts media
- RSM:
-
Response surface methodology
- RL:
-
Rhamnolipids
References
Luo Z, Yuan XZ, Zhong H, Zeng GM, Liu ZF, Ma XL, Zhu YY (2013) Optimizing rhamnolipid production by Pseudomonas aeruginosa ATCC 9027 grown on waste frying oil using response surface method and batch-fed fermentation. J Cent South Univ 20:1015–1021
Cameotra SS, Makkar RS, Kaur J, Mehta SK (2010) Synthesis of biosurfactants and their advantages to microorganisms and mankind. Adv Exp Med Biol 672:261–280
Rikalovic MG, Vrvic MM, Karadzic IM (2015) Rhamnolipid biosurfactant from Pseudomonas aeruginosa—from discovery to application in contemporary technology. J Serb Chem Soc 80:279–304
Silva SNRL, Farias CBB, Rufino RD, Luna JM, Sarubbo LA (2010) Glycerol as substrate for the production of biosurfactant by Pseudomonas aeruginosa UCP0992. Colloids Surf B 79:174–183
Sinumvayo JP, Ishimwe N (2015) Agriculture and Food Applications of Rhamnolipids and its Production by Pseudomonas aeruginosa. J Chem Eng Process Technol 6:223
Md Noh NA, Mohd Salleh S, Yahya ARM (2015) Enhanced rhamnolipid production by Pseudomonas aeruginosa USM-AR2 via fed-batch cultivation based on maximum substrate uptake rate. Lett Appl Microbiol 58:617–623
Sodagari M, Ju LK (2014) Cells were a more important foaming factor than free rhamnolipids in fermentation of Pseudomonas aeruginosa E03−40 for high rhamnolipid production. J Surfactants Deterg 17:573–582
Mukherjee S, Das P, Sen R (2006) Towards commercial production of microbial surfactants. Trends Biotechnol 24:509–515
Pornsunthorntawee O, Wongpanit P, Rujiravanit R (2010) Rhamnolipid biosurfactants: production and their potential in environmental biotechnology. In: Ramkrishna S (ed) Biosurfactants. Landes Bioscience and Springer Science and Business Media, New York, pp 211–221
AL-Araji LIY, Abd. Rahman RNZR, Basri M, Salleh AB (2007) Optimisation of rhamnolipids produced by Pseudomonas aeruginosa 181 using response surface modeling. Ann Microbiol 57:571–575
Rahman KS, Rahman TJ, McClean S, Marchant R, Banat IM (2002) Rhamnolipid biosurfactant production by strains of Pseudomonas aeruginosa using low-cost raw materials. Biotechnol Prog 18:1277–1281
Sahoo S, Datta S, Biswas D (2011) Optimization of culture conditions for biosurfactant production from Pseudomonas aeruginosa OCD1. J Adv Sci Res 2:32–36
Bodour AA, Drees KP, Maier RM (2003) Distribution of biosurfactant-producing bacteria in undisturbed and contaminated arid Southwestern soils. Appl Environ Microbiol 69:3280–3287
Abdel-Mawgoud AM (2008) Production and characterization of some microbial surfactants. Master degree dissertation. Ain Shams University, Cairo
Alfermann AW, Dombrowski K, Petersen M, Schmauder HP, Schweizer M, Völksch B, Günther T (1997) Basic scientific techniques for biotechnology-analytical methods—growth and cell viability. In: Schmauder HP, Schweizer M (eds) Methods in biotechnology. Taylor & Francis, London, pp 13–14
Kosaric N, Vardar-Sukan F (2014) Biosurfactants: production and utilization-processes, technologies, and economics. CRC Press, Boca Raton
Leitermann F, Syldatk C, Hausmann R (2008) Fast quantitative determination of microbial rhamnolipids from cultivation broths by ATR-FTIR Spectroscopy. J Biol Eng 2:13
Chandrasekaran EV, BeMiller JN (1980) Constituent analysis of glycosaminoglycans. In: Whistler RL, Wolfrom ML (eds) Methods in carbohydrate chemistry. Academic Press, New York, pp 89–96
Koch AK, Käppeli O, Fiechter A, Reiser J (1991) Hydrocarbon assimilation and biosurfactant production in Pseudomonas aeruginosa mutants. J Bacteriol 173:4212–4219
Wu J, Ju LK (1998) Extracellular particles of polymeric material formed in n-hexadecane fermentation by Pseudomonas aeruginosa. J Biotechnol 59:193–202
Wei YH, Chou CL, Chang JS (2005) Rhamnolipid production by indigenous Pseudomonas aeruginosa J4 originating from petrochemical wastewater. Biochem Eng J 27:146–154
Guerra-Santos L, Käppeli O, Fiechter A (1986) Dependence of Pseudomonas aeruginosa continuous culture biosurfactant production on nutritional and environmental factors. Appl Microbiol Biotechnol 24:443–448
Giani C, Wullbrandt D, Rothert R, Meiwes J (1996) Pseudomonas aeruginosa and its use in a process for the biotechnological preparation of L-rhamnose. US patent 5501966 A
Borges WS, Moura AAO, Filho UC, Cardoso VL et al (2015) Optimization of the operating conditions for rhamnolipid production using slaughterhouse-generated industrial float as substrate. Braz J Chem Eng 32:357–365
Santa Anna LM, Sebastian GV, Menezes EP, Alves TLM et al (2002) Production of biosurfactants from Pseudomonas aeruginosa PA1 isolated in oil environments. Braz J Chem Eng 19:159–166
Benincasa M, Contiero J, Manresa MA, Moraes IO (2002) Rhamnolipid production by Pseudomonas aeruginosa LBI growing on soapstock as the sole carbon source. J Food Eng 54:283–288
Da Rosa CFC, Michelon M, Burkert JFDM, Kalil SJ, Burkert CAV (2010) Production of a rhamnolipid-type biosurfactant by Pseudomonas aeruginosa LBM10 grown on glycerol. Afr J Biotechnol 9:9012–9017
Rocha C, San-Bias F, San-Bias G, Vierma L (1992) Biosurfactant production by two isolates of Pseudomonas aeruginosa. World J Microb Biot 8:125–128
Suryanti V, Hastuti S, Wahyuningsih TD, Mudasir M, Muliawati DI (2009) Biosurfactants production by pseudomonas aeruginosa using soybean oil as substrate. Indones J Chem 9:107–112
Pagliaro M, Ciriminna R, Kimura H, Rossi M, Della Pina C (2007) From glycerol to value-added products. Angew Chem Int Ed 46:4434–4440
Da Silva GP, Mack M, Contiero J (2009) Glycerol: a promising and abundant carbon source for industrial microbiology. Biotechnol Adv 27:30–39
Maier RM, Soberón-Chávez G (2000) Pseudomonas aeruginosa rhamnolipids: biosynthesis and potential applications. Appl Microbiol Biotechnol 54:625–633
Henkel M, Müller MM, Kügler JH, Lovaglio RB, Contiero J, Syldatk C, Hausmann R (2012) Rhamnolipids as biosurfactants from renewable resources: concepts for next-generation rhamnolipid production. Process Biochem 47:1207–1219
Wu JY, Yeh KL, Lu WB, Lin CL, Chang JS (2008) Rhamnolipid production with indigenous Pseudomonas aeruginosa EM1 isolated from oil-contaminated site. Bioresour Technol 99:1157–1164
Murthy MSRC, Swaminathan T, Rakshit SK, Kosugi Y (2000) Statistical optimization of lipase catalyzed hydrolysis of methyloleate by response surface methodology. Bioprocess Eng 22:35–39
Nalini S, Parthasarathi R (2014) Production and characterization of rhamnolipids produced by Serratia rubidaea SNAU02 under solid-state fermentation and its application as biocontrol agent. Bioresour Technol 173:231–238
Turbekar R, Malik N, Thakare D (2015) Optimization studies for enhancing rhamnolipid production by Pseudomonas aeruginosa RT using response surface methodology. Int J Res Stud Biosci 3:65–73
Ghribi D, Abdelkefi-Mesrati L, Mnif I, Kammoun R, Ayadi I, Saadaoui I, Maktouf S, Chaabouni-Ellouze S (2012) Investigation of antimicrobial activity and statistical optimization of Bacillus subtilis SPB1 biosurfactant production in solid-state fermentation. J Biomed Biotechnol 2012:373682. doi:10.1155/2012/373682
Abdel-Hafez SM, Hathout RM, Sammour OA (2014) Towards better modeling of chitosan nanoparticles production: screening different factors and comparing two experimental designs. Int J Biol Macromol 64:334–340
Abalos A, Maximo F, Manresa MA, Bastida J (2002) Utilization of response surface methodology to optimize the culture media for the production of rhamnolipids by Pseudomonas aeruginosa AT10. J Chem Technol Biotechnol 77:777–784
Kashyap P, Sabu A, Pandey A, Szakacs G, Soccol CR (2002) Extra-cellular l-glutaminase production by Zygosaccharomyces rouxii under solid-state fermentation. Process Biochem 38:307–312
Zhu L, Yang X, Xue C, Chen Y, Qu L, Lu W (2012) Enhanced rhamnolipids production by Pseudomonas aeruginosa based on a pH stage-controlled fed-batch fermentation process. Bioresour Technol 117:208–213
Chen SY, Wei YH, Chang JS (2007) Repeated pH-stat fed-batch fermentation for rhamnolipid production with indigenous Pseudomonas aeruginosa S2. Appl Microbiol Biotechnol 76:67–74
Chen SY, Lu WB, Wei YH, Chen WM, Chang JS (2007) Improved production of biosurfactant with newly isolated Pseudomonas aeruginosa S2. Biotechnol Prog 23:661–666
Mulligan CN, Sharma SK, Mudhoo A (eds) (2014) Biosurfactants: research trends and applications. CRC Press, Boca Raton
Moussa TAA, Mohamed MS, Samak N (2014) Production and characterization of di-rhamnolipid produced by Pseudomonas aeruginosa TMN. Braz J Chem Eng 31:867–880
Lotfabad TB, Shourian M, Roostaazad R, Najafabadi AR, Adelzadeh MR, Noghabi KA (2009) An efficient biosurfactant-producing bacterium Pseudomonas aeruginosa MR01, isolated from oil excavation areas in south of Iran. Colloids Surf B 69:183–193
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
El-Housseiny, G.S., Aboulwafa, M.M., Aboshanab, K.A. et al. Optimization of Rhamnolipid Production by P. aeruginosa Isolate P6. J Surfact Deterg 19, 943–955 (2016). https://doi.org/10.1007/s11743-016-1845-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-016-1845-4