Abstract
Rhamnolipids are among the best-known biosurfactants. Severe foaming occurs in aerobic rhamnolipid fermentation and negatively affects operation and economics of the biosurfactant production. In this study the foaming properties were examined with samples taken along a Pseudomonas aeruginosa fermentation that produced 55 g l−1 rhamnolipids with a maximum volumetric productivity of 0.080 g l−1 h−1 and a maximum specific productivity of 0.013 g g−1 h−1. For a better understanding of the process, the broth samples were also centrifuged to prepare cell-free supernatants and cell suspensions in water, and all samples were evaluated under fixed foaming conditions. In addition to the time profiles of foam rise, the initial foaming rates and maximum foam volumes were determined. Contrary to the general assumption, the cells, not rhamnolipids, were the main foaming agents in the fermentation. Soluble components including rhamnolipids had secondary roles. Supernatant foaming was higher after the culture entered the rhamnolipid-producing stationary phase; however, the foaming appeared to decrease with increasing rhamnolipid concentrations at high concentrations (>15 g l−1). The pH effects on foaming of broths, supernatants, and cell suspensions were also studied. Broth foaming was 55 and 80 % less at pH 5.5 and 5.0, respectively, compared to that at pH 6.5. Cell growth and rhamnolipid production at lower pH should be included in future studies. In addition, strain selection or genetic engineering and medium modification to reduce cell hydrophobicity are suggested as useful strategies to address the foaming issue of rhamnolipid fermentation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During recent decades there have been increasing concerns over global warming, depleting petroleum sources, and environmental pollution. These concerns have prompted active research in developing or modifying technologies for improved sustainability. Replacing petroleum-based products by ones derived from renewable resources is a significant part of this effort. Biosurfactants produced by microorganisms are potential alternatives to synthetic surfactants [1]. With similar surface-active properties, biosurfactants can offer advantages over their synthetic counterparts in having higher biodegradability, biocompatibility, and lower toxicity to mammals [2, 3].
Rhamnolipids are among the most effective biosurfactants [3]. Most commonly produced by Pseudomonas aeruginosa [4], rhamnolipids have a wide range of potential applications as bioremediation enhancers [5, 6], detergents [7], antibacterial, antifungal, and anti-biofouling agents [8–10], and pharmaceuticals [11, 12]. The production cost of rhamnolipids was estimated at $5–20 per kg, varying with the fermentation scale from 20,000 to 100,000 l, whereas the production cost of synthetic surfactants was $1–3 per kg [13]. Reducing the production cost is very important for improving the market competitiveness of rhamnolipids. The main operational challenges in rhamnolipid production include the extreme foaming property of broth in aerobic fermentation and the relatively low productivity and yield [14].
Rhamnolipids have typically been produced by aerobic fermentation systems with high agitation and submerged sparging of fine oxygen or air bubbles [15–18]. Extensive and stable foaming occurs in such systems and causes serious operational problems such as wall growth of cells, overflow of foams, clogging of gas outlet filters, and higher chance of contamination [16, 19]. Adding large amounts of antifoaming agents increases the cost and can complicate the downstream separation and purification [15]. Müller et al. [17] used a mechanical foam breaker in their rhamnolipid production, but they still had to sacrifice 43 % of the bioreactor volume (15 l out of 35 l) as the headspace containing the heavy foams. The whole bioreactor (35 l) was eventually filled with the culture broth expanded by the fine bubbles trapped/emulsified as stable, high gas holdup. To avoid the foaming problem associated with aeration, Kronemberger et al. [19] used an extensive membrane structure in their rhamnolipid fermentation, relying only on the oxygen molecules permeating through the membrane wall for oxygen supply.
Severe foaming routinely occurred in the studies of aerobic rhamnolipid fermentation performed in this laboratory. Despite many reports on the occurrence of foaming, the contributions of broth components in rhamnolipid fermentation were not systematically investigated or reported. In this work, the foaming properties of broth samples taken along rhamnolipid fermentation were examined. These samples had different rhamnolipid concentrations, cell concentrations, and vegetable oil contents. Contributions of cells themselves and the cell-free supernatants were separately determined. The effect of pH was also studied. It should be noted that the study was performed with a P. aeruginosa strain previously selected for its high rhamnolipid productivity [20]. During the selection study some low-productivity stains were observed to produce more non-rhamnolipid metabolites. The use of those strains, and other cultivation conditions and substrates, can affect the foaming behaviors of the broths. The goal of this study was to identify the major factor(s) that caused the severe broth foaming in the fermentation using the selected strain with high rhamnolipid productivity. The findings would lead to potential strategies for reduction and/or better control of foaming, for improving productivity of aerobic rhamnolipid fermentation.
Materials and Methods
Bacterial Culture Preparation
A P. aeruginosa strain E03-40 isolated from soil samples taken near a biodiesel plant was used for the fermentation in this study. The strain was selected because of its high rhamnolipid productivity [20]. The cell culture was maintained frozen in 2-ml centrifuge tubes containing 85 % of 30 g l−1 tryptic soy broth (TSB) (Sigma-Aldrich, St. Louis, MO) and 15 % glycerol (Sigma-Aldrich). For inoculum preparation, the content of a tube was added to 10 ml TSB medium and incubated for 8 h at 34 °C in a shaker (Thermo Scientific, MAXQ 5,000) operating at 280 rpm. The 10-ml culture was subsequently added to 90 ml TSB in a 500-ml Erlenmeyer flask and cultured for 20 h under the same conditions. The culture thus prepared was used as the fermentation inoculum.
Fermentation
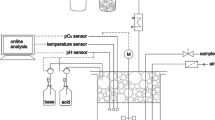
A 2-l New Brunswick Scientific, BIOFLO 110 fermentor with a 1-l working volume was used for the fermentation. The broth temperature was maintained at 32 °C. The pH was controlled at 6.7 ± 0.2 by addition of 1 N H2SO4 or NaOH. Agitation, with two six-blade turbines, was fixed at the speed of 600 rpm. Pure oxygen was used for control of dissolved oxygen concentration (DO) in the fermentation broth. With a set point of 10 % (air saturation), DO typically fluctuated between 5 and 30 % responding to the oxygen flow rate automatically adjusted by the primary control unit software of the fermentor.
The medium, excluding the carbon source, contained 3 g l−1 KH2PO4, 2.86 g l−1 NH4Cl, 2.5 g l−1 yeast extract, 2.5 g l−1 peptone, 0.75 g l−1 NaCl, 0.45 g l−1 MgSO4·7H2O, 0.05 g l−1 FeSO4·7H2O, 0.015 g l−1 CaCl2·2H2O, 0.015 g l−1 MnCl2·4H2O, and 1 ml l−1 of a trace element solution. The trace element solution had 0.75 g l−1 MnSO4·H2O, 0.75 g l−1 ZnSO4·7H2O, 0.15 g l−1 H3BO3, 0.08 g l−1 FeCl3·6H2O, 0.08 g l−1 CoCl2·6H2O, 0.075 g l−1 CuSO4·5H2O, and 0.05 g l−1 Na2MoO4. The medium was designed to have the nitrogen source as the limiting substrate.
Soybean oil was used as the carbon source. The initial medium had 37 g l−1 soybean oil. Along with the fermentation, soybean oil and/or other fresh medium components were supplemented four times by different methods, to create broth samples of different cell concentrations, rhamnolipid concentrations, and soybean oil contents for the foaming study. As described in the “Results” and “Discussion”, the different methods of oil and medium supplementation also provided important insights into the possible strategy of long-term rhamnolipid production at improved productivity.
The four different supplementation methods used are described in the following: (1) Batch addition of soybean oil only—At 24 h, a batch of 37 g soybean oil, with no other medium components, was added into the fermentor. (2) Batch replacement of part of the broth with fresh medium and soybean oil—At 321 h, all carbon substrates, including soybean oil and the glycerol and fatty acids released from oil hydrolysis, were consumed. About 200 ml broth was removed from the fermentor and 200 ml fresh medium plus 37 g soybean oil were added in a single step. (3) Continuous slow feeding of soybean oil only—at 583 h, all added carbon substrates were again depleted. Soybean oil, without other medium components, was this time continuously added with a peristaltic pump at a slow rate of 0.13 g h−1. (4) Batch replacement of part of the broth with fresh medium and continuous slow feeding of soybean oil—At 922 h, 250 ml broth was again removed and replaced by the same amount of fresh medium without soybean oil, followed by the same slow feeding of soybean oil at 0.10 g h−1 till the end of the study (at almost 1,300 h).
Foaming Study
The foaming study was done with 30-ml liquid samples in a 100-ml graduated cylinder (PYREX no. 3,046). Air was introduced by a metered pump (BioChem Technology, Malvern, PA) at 100 ml h−1 through silicone tubing connected to an air diffuser stone. The air flow rate was selected to give a volume of gas per volume of liquid per minute (VVM) similar to that in the fermentor. The air diffuser stone was placed at the bottom of the graduated cylinder. After the air flow was started, the foam volume was recorded as a function of time. Multiple samples were taken at different fermentation times for the foaming study, as shown in Table 1. The cell and rhamnolipid concentrations and soybean oil amounts present in these samples are also given in the table. To evaluate the foaming properties of cells and broth supernatant separately, some fermentation broth samples were centrifuged (Thermo Scientific, Sorvall Legend X1R centrifuge) at 7,000 rpm (5,700 g) for 15 min to separate the supernatant from the cells. The cells were resuspended in deionized water with the same volume as the original broth sample volume. Then the cell suspension and the broth supernatant were separately evaluated for the foaming properties. In some cases the same sample was evaluated at multiple pH values, adjusted with 1 N HCl, to study the pH effect.
Analysis
Intracellular Protein Concentration and Cell Dry-Weight Concentration
Broth samples taken along the fermentation were centrifuged (Eppendorf, Centrifuge 5415D) at 8,000 rpm (5,900 g) for 10 min. Supernatant was collected and frozen for further analysis. The cells were resuspended in deionized water and centrifuged again. The cell pellet was then resuspended in 4 ml 0.2 N NaOH and cooked for 20 min at 100 °C for cell lysis. Then the intracellular protein concentration was analyzed by using the standard Bradford method. Absorbance was measured at 595 nm by a UV/Vis spectrophotometer (model 1,601, Shimadzu). The intracellular protein concentration was divided by 0.32 for conversion to an approximate cell dry-weight concentration. The conversion ratio 0.32 had been obtained from a separate study performed to establish the correlation between cell dry-weight measurement and intracellular protein concentration analysis (details not reported).
Glycerol Concentration
The concentration of glycerol was determined by using high-performance liquid chromatography (HPLC; Shimadzu, LC-10A with a refractive index detector, RID-10A). The mobile phase was 0.1 % phosphoric acid at the flow rate of 0.14 ml min−1. The column used was a Supelcogel H column (25 cm × 4.6 mm, Supelco).
Rhamnolipid Concentration
The broth supernatant collected by centrifugation was diluted to the range of 3–90 mg l−1 rhamnolipids, suitable for the anthrone analysis. The supernatant pH was then adjusted to 2–3 by addition of 1 N HCl. The acidified supernatant was extracted by fourfold volume of ethyl acetate. The ethyl acetate extract (5 ml) was collected and vaporized to remove the solvent. A 1.7-ml aliquot of a 0.05 M NaHCO3 solution was then added to redissolve the extracted material including rhamnolipids. An anthrone reagent (3.3 ml), composed of 2 g l−1 of anthrone in concentrated H2SO4, was added into the solution and reacted for 16 min at 95 °C. The solution was then cooled to room temperature and the absorbance was measured at 625 nm. Pure rhamnose solutions of known concentrations were similarly analyzed to establish the calibration equation for converting the absorbance values to rhamnose concentrations. Finally, the rhamnolipid concentrations were estimated by multiplying the measured rhamnose concentrations by 2.73. The value 2.73 was determined as follows: The sample was analyzed by a mass spectrometer (MS) (HP Bruker Esquire LC) following the procedures used in previous work by this group for MS characterization of rhamnolipids [20, 21]. The rhamnolipid structures detected are summarized in Table 2, together with their molecular weights (MW) and the percentages of total ion intensity (TIC) peak areas (out of the summed area of all peaks). The sample was found to contain predominantly (about 81.6 %) monorhamnolipids. By assuming that the molar percentages were the same as the TIC percentages, the average MW could be estimated as 531.7 for the rhamnolipid mixtures. Similarly, it could be estimated that 1.18 (=0.816 × 1 + 0.184 × 2) moles (194.4 g) of rhamnose would be released from each mole of the rhamnolipid mixture during the anthrone analysis. Accordingly, for this rhamnolipid mixture the measured rhamnose concentration (grams per liter) could be multiplied by the ratio of 531.7/194.4 = 2.73 for conversion to the rhamnolipid concentration (grams per liter).
Ammonium Concentration
Ammonium concentrations in fermentation broths were measured as ammonia–nitrogen (NH3–N) by using HACH water quality test strips (no. 2755325) after dilution to measurable concentrations of no more than 6 mg l−1.
Results
Fermentation
As described in the “Materials and Methods” section, to provide broth samples of different properties, this fermentation experiment was done with four different strategies of supplementation of soybean oil and fresh medium components. The observed profiles of cell, glycerol, and rhamnolipid concentrations are shown in Fig. 1.
Cell Concentration
After the first day, all the nitrogen sources provided in the fresh medium were depleted and the cell concentration (based on intracellular protein measurements) peaked at approximately 10 g l−1. The intracellular protein concentration decreased in the stationary phase to give an apparent cell concentration of about 6 g l−1 during 100–320 h. At 321 and 922 h, portions of the broth (200 and 250 ml, respectively) were replaced with fresh medium. The cell concentration dropped immediately owing to dilution and then quickly grew back (to about 7.5–8.0 g l−1) with the nitrogen sources (and other components) introduced in the fresh medium. Once the supplemented nitrogen sources were depleted, the cell concentration would decrease again.
Glycerol Concentration
P. aeruginosa cells are known to produce lipase for hydrolyzing triglycerides (soybean oil in this case) to fatty acids and glycerol for assimilation and metabolism [22]. In this study the glycerol concentration was followed as an indication of carbon source availability and soybean oil depletion. It was observed that when a batch of soybean oil was added to the broth, the glycerol concentration would increase as a result of faster generation (by soybean oil hydrolysis) than consumption (by cells). The increase would then slow down as the glycerol concentration approached and remained at about 4 g l−1, suggesting the existence of feedback inhibition of soybean oil hydrolysis [23]. When soybean oil was (nearly) depleted, the glycerol concentration would decrease (as consumption became faster than generation). This profile allowed a rather easy way of detecting the depletion of soybean oil, without the need to follow the concentrations of many glycerides and fatty acids associated with soybean oil metabolism.
Rhamnolipid Concentration
Rhamnolipid concentration increased along the entire fermentation, except on the two occasions when the broth was diluted with the fresh medium replacement. The maximum rhamnolipid concentration reached 48.9 g l−1 (without adjustment for dilution). During the first two stages of fermentation, the rhamnolipid volumetric productivity was similar at 0.056 and 0.054 g l−1 h−1. During the third stage, when soybean oil was made available to cells by slow feeding at 0.13 g h−1, the rhamnolipid production reduced significantly to 0.010 g l−1 h−1. The volumetric productivity, however, recovered to 0.072 g l−1 h−1 during the final stage, when the soybean oil was still fed slowly at 0.1 g h−1 but a portion of the broth was first replaced with fresh medium (at 922 h). The lower productivity observed during the third stage was therefore unlikely to be caused by the slow feeding of soybean oil. Instead, it appears that the rhamnolipid production would be negatively affected by long-term maintenance under nitrogen source-limited stationary phase and/or by depletion of certain other nutrient(s). Which of the two potential causes were actually responsible is yet to be determined. Nonetheless, both could be remediated by fresh medium replacement as shown by the results of this study. This finding can be very important. Rhamnolipids are typically overproduced by non-growing cells. If the productivity can be maintained in a prolonged stationary phase, the downtime and operations between short batches are eliminated; the time and substrates consumed for growing the cells in every batch are also avoided. The rhamnolipid productivity and yield and the overall process economics may be significantly improved. Future studies to identify the limiting factor(s) and demonstrate the improved fermentation design are warranted.
Foaming of Fermentation Broth
Figure 2 shows the profiles of increasing foam volume (=total volume − original liquid volume) with time in the foaming study for the broth samples taken along the fermentation. For presentation clarity not all the samples studied are included in the figure. Similar profiles were seen for the samples of supernatants and cell suspensions prepared as described in the “Materials and Methods” section. The fermentation broths were complex. Effects of different broth components on foaming are described in the following sections.
Effects of Cells
Figure 3 shows the foaming profiles observed for the broth samples taken from the fermentation at 23 and 47 h. Profiles for the (cell-free) supernatant of the 23-h sample and the cell suspension of the 47-h sample were included for comparison. It is clear that in this early stage of rhamnolipid fermentation, the supernatant was not very foamy while the hydrophobic P. aeruginosa cells [24] were the primary cause of broth foaming. Note that rhamnolipid concentrations were low (about 4 g l−1 at 23 h and 7 g l−1 at 47 h) and soybean oil contents were high in these samples. Later the supernatant became more foamy, as shown in Fig. 4 where the foaming profiles are compared for broth, supernatant, and cell suspension of the sample taken from the fermentation at 174 h. At that point soybean oil had been consumed to a much lower content (glycerol concentration started to decrease at about 220 h, Fig. 1) and rhamnolipid concentration was higher at about 15 g l−1. Nonetheless, the cells remained the stronger foaming factor than the supernatant throughout the fermentation.
From the time profiles of foaming (as those shown in Figs. 2, 3, 4), the maximum foam volumes attainable and the initial foaming rates could be derived; the latter from the initial periods showing approximately linear increase of foam volume. These two foaming properties are summarized in Fig. 5 for all the samples of broths, supernatants, and cell suspensions evaluated (except those with pH being adjusted for studying the pH effect on foaming). As a group the cell suspensions clearly contributed more than the cell-free supernatants to the foaming of whole broth samples, in both initial foaming rates and maximum foam volumes.
Effects of Fresh Medium Addition
Some observations made in the foaming study gave insights into the effects of non-cell medium components on foaming. First we describe the effect of medium nutrients (except soybean oil) on foaming. This effect could be best seen from the comparison of foaming properties of the respective samples taken at 922 and 923 h of fermentation. At 922 h the cells had been without fresh medium nutrients for 600 h; the last fresh medium addition was done at 321 h (Fig. 1). The 922-h sample was taken right before a medium replacement: 250 ml broth was removed and replaced with 250 ml fresh medium without soybean oil. The 923-h sample was taken about 30 min after the medium replacement was completed. Owing to the dilution effect, both cell and rhamnolipid concentrations were lower at 923 h (Table 1).
The foaming profiles of these samples were followed and compared for broths, supernatants, and cell suspensions. Instead of showing these six profiles, the corresponding initial foaming rates and maximum foam volumes are summarized in Fig. 6. The two broth samples showed similar foaming rates but the 923-h sample reached a much larger foam volume (32 ml as compared with 18 ml for the broth at 922 h). Given that the foaming difference was caused by a medium replacement, one might expect the difference to come more from different foaming properties of the two supernatants than from those of the two cell suspensions. Rather surprisingly, the results showed the opposite. The foaming profiles, and thus the foaming rates and maximum foam volumes, of the two supernatants were almost the same. On the other hand, the cell suspension of the 923-h sample showed significantly faster foaming and higher foam volume than that of the 922-h sample. Apparently, the cells responded rapidly to the medium replacement and were responsible for the higher foaming observed with the broth of 923 h. Because the change occurred in a very short time (<1 h), it is hypothesized that the higher foaming was due to changed cell surface properties (e.g., increased hydrophobicity) as a result of rapid adsorption or other interactions of some fresh medium components on/with the cell surface. Future study is needed to evaluate this hypothesis.
It should be mentioned that the immediate increase in broth foaming following fresh medium addition or replacement was reproducibly observed in many rhamnolipid fermentations performed in this laboratory. The results of this study provided a better understanding of the phenomenon.
Effects of Soybean Oil Content
Soybean oil content is expected to affect foaming. It is common to use oils to decrease the foam formation in fermentation processes [25, 26]. According to Rols and Goma, the oils would increase the gas bubble size and make the foam less stable [27]. In this study the soybean oil effect was seen most clearly when the foaming properties were compared for the samples taken at 321 and 341 h of fermentation. After the 321-h sample was taken, 200 ml broth was removed and replaced with 200 ml fresh medium plus 37 g soybean oil. The two broth samples showed similar initial foaming rates (0.9–1 ml min−1) but very different maximum foam volumes: 47 ml for the broth at 321 h but only 24 ml for the broth at 341 h (foaming profiles not shown). As described in the previous section, the addition of only fresh medium (without oil) at 922 h also did not change the initial foaming rate much but increased the maximum foam volume. The results of this study indicated that the primary effect of soybean oil on foaming was to reduce the maximum foam volume, presumably by destabilizing the foams.
Effects of Rhamnolipid Concentration
The effects of non-cell broth components such as rhamnolipids could be more clearly seen from the foaming of cell-free supernatants collected by centrifugation. The maximum foam volumes and initial foaming rates of supernatants are plotted in Fig. 7 against the rhamnolipid concentrations. Rhamnolipids are well-known foaming agents [18, 28]. It is rather surprising to find that, except for the supernatant with a very low rhamnolipid concentration (from the sample taken at 23 h when the culture just entered the stationary phase), the supernatant foaming mostly decreased with increasing rhamnolipid concentrations. This observation did not mean rhamnolipids were non-foaming or antifoaming. The responsible mechanisms remain to be elucidated. Presence of different concentrations of soybean oil in the cell-free supernatants could be a reason. The higher viscosity in supernatants with higher rhamnolipid concentrations might be a factor. The other possibility is that rhamnolipids at higher concentrations formed larger and different forms of micelles or physical aggregates [29] that had lower foaming effects.
Effects of pH on Foaming
The study of the pH effect was perfomed with the broth sample taken at 1,179 h of fermentation. The broth sample was centrifuged to create the supernatant and cell suspension samples. The three groups of samples were adjusted to several pH values and then subjected to the foaming study. The maximum foam volumes obtained with these samples are summarized in Fig. 8 to show the effect of pH on foaming. It is clearly shown that the foaming decreased significantly as the pH was lowered from 6.5 to 5.0 for all three groups of samples (broth, supernatant, and cell suspension). At pH 5.0 the maximum foam volume of the broth sample was only about 20 % of that of the original sample at pH 6.5. The foam sizes were larger and less uniform at pH 5.0 (up to 4 mm in diameter) when compared with the foams at higher pH (all about 2 mm in diameter).
The pH dependency could be attributed to the changes of electrostatic repulsion due to the charged foaming agents (cells, rhamnolipids, and others) adsorbed on bubble surfaces; stronger electrostatic repulsion would deter coalescence and cause faster and more stable foaming. Bacterial cells are charged owing to the presence of biomolecules such as peptidoglycan, lipopolysaccharides, and lipoproteins on their cell wall [30]. As the pH was lowered from neutrality, the net negative charge of the cells decreased [30, 31], exerting lower repulsion between the bubbles. The foaming of pure rhamnolipid solutions has also been reported to decrease with decreasing pH [28]. The pK a of a monorhamnolipid mixture in water was reported to be about 4.8 or 5.5 (measured by two different methods) at concentrations above the critical micelle concentration (about 40 mg l−1) [32]. Smaller fractions of rhamnolipids would be present as negatively charged ions as the pH was lowered from neutrality.
It may be worth noting that the foaming of supernatants was higher at pH 5.9 than at pH 6.5, which was inconsistent with the foaming properties reported for pure rhamnolipid solutions at lower concentrations (0.1 g l−1) [28]. The discrepancy could be related to the observation described in the previous section, i.e., certain foam-affecting mechanism(s) might be associated with the high rhamnolipid concentrations involved in this study.
Discussion
Rhamnolipids or precursors to rhamnolipids had been thought to be responsible for the highly foaming nature of rhamnolipid fermentation broths. The results of this study showed that, at least for the productive strain of P. aeruginosa used, the cells themselves were the most important foaming factor. The contribution of cells to foaming was particularly overwhelming during the phase of active cell growth. This finding is important to process design and productivity. The actively growing cells have much higher specific oxygen consumption rates than the cells in the stationary phase [19]. The maximum concentration of actively growing cells whose respiration needs could be met by aeration was the maximum cell concentration employable in the fermentation design. And in the highly foaming rhamnolipid fermentation, the maximum aeration rate usable was limited by the controllable foaming of the broth.
It had been reported that hydrophobic cells could concentrate at the gas/liquid interface and stabilize the foams [25]. In our previous study, the relative hydrophobicity of various bacterial cells was measured by the partitioning of cells to the water–hexadecane interface [24]. Briefly, the pregrown cultures were washed twice with pH 7 phosphate buffer and diluted to the same concentration, with an optical density (OD) of 0.40 ± 0.01 at 400 nm. The cell suspension (3.2 ml) was then vortexed with hexadecane (6 ml) for 2 min. The mixture was allowed to stand for 30 min for phase separation. The OD400 of the aqueous phase was measured again. The percentage of OD reduction, due to partitioning of the cells to the interface, was an indicator of cell hydrophobicity. The % reduction was 36.7 ± 4.5 for P. aeruginosa but only 5.1 ± 4.7 and 10.8 ± 2.8 for two other common Gram-negative bacteria Escherichia coli and Pseudomonas putida. P. aeruginosa cells were clearly more hydrophobic and could have promoted the foaming of fermentation broths.
In the current study the foaming of cell suspensions was not done with washed cells. Therefore, the possibility exists that the strong foaming was associated with the hydrophobicity imparted by rhamnolipids or other metabolites bound to the cell surface. Nonetheless, from the process point of view, the bound materials are part of the cells in the fermentation. This possibility does not affect the conclusion that the cells are the dominant foaming agents in the fermentation.
The study results clearly indicated the value of reducing the cell hydrophobicity and/or other unidentified properties causing severe cell-associated foaming. This reduction may be achieved by several approaches. One approach is to select or genetically engineer productive species or strains with lower surface hydrophobicity. Adjustment of growth medium may also be helpful, particularly because the addition of fresh medium to the broth sample taken at 922 h was shown to significantly increase the foaming of cell suspension. It is worthwhile to identify the responsible medium component(s) and then evaluate the effects on foaming, cell growth, and rhamnolipid production when the component(s) was reduced, substituted, or provided in batches of low concentrations. Yet another approach suggested by the results of this study is to control the fermentation at lower pH values such as 5.5 or 5. Future studies are warranted to evaluate the cell growth rate and rhamnolipid yield and productivity at lower pH. Successfully addressing the foaming issue will have a significant impact on the process economics of rhamnolipid production.
The most important conclusion obtained in the study is that the P. aeruginosa cells themselves were the dominant foaming agents in the rhamnolipid fermentation investigated in this study. This finding is contrary to the assumption of rhamnolipids being responsible in the previous literature reports. Other important factors for controlling foaming were the presence of sufficient contents of oil and the use of low enough pH (5.5 or 5). Soluble components in broth supernatants including rhamnolipids played secondary roles in foaming, such as increasing foam stability. The study results clearly showed the value of understanding and reducing the directly cell-associated foaming. Future studies are suggested: (1) to select or genetically engineer the productive species or strains with less hydrophobic cell surface, (2) to identify the medium component(s) that promoted cell-associated foaming and modify the supply strategy of the nutrient(s), and (3) to find the lower fermentation pH for reduced foaming without negative effects on cell growth or rhamnolipid production.
References
Desai JD, Banat IM (1997) Microbial production of surfactants and their commercial potential. Microbiol Mol Biol Rev 61:47–64
Sen R (2010) Biosurfactants. Springer, Berlin
Nitschke M, Costa SG, Contiero J (2005) Rhamnolipid surfactants: an update on the general aspects of these remarkable biomolecules. Biotechnol Prog 21:1593–1600
Abdel-Mawgoud AM, Lépine F, Déziel E (2010) Rhamnolipids: diversity of structures, microbial origins and roles. Appl Microbiol Biotechnol 86:1323–1336
Lai CC, Huang YC, Wei YH, Chang JS (2009) Biosurfactant-enhanced removal of total petroleum hydrocarbons from contaminated soil. J Hazard Mater 167:609–614
Mulligan CN (2005) Environmental applications for biosurfactants. Environ Pollut 133:183–198
Makkar R, Cameotra S (2002) An update on the use of unconventional substrates for biosurfactant production and their new applications. Appl Microbiol Biotechnol 58:428–434
Das P, Mukherjee S, Sen R (2008) Improved bioavailability and biodegradation of a model polyaromatic hydrocarbon by a biosurfactant producing bacterium of marine origin. Chemosphere 72:1229–1234
Raya A, Sodagari M, Pinzon NM, He X, Zhang Newby B, Ju LK (2010) Effects of rhamnolipids and shear on initial attachment of Pseudomonas aeruginosa PAO1 in glass flow chambers. Environ Sci Pollut Res 17:1529–1538
Mukherjee S, Das P, Sen R (2006) Towards commercial production of microbial surfactants. Trends Biotechnol 24:509–515
Stipcevic T, Piljac T, Isseroff RR (2005) Di-rhamnolipid from Pseudomonas aeruginosa displays differential effects on human keratinocyte and fibroblast cultures. J Dermatol Sci 40:141–143
Stipcevic T, Piljac A, Piljac G (2006) Enhanced healing of full-thickness burn wounds using di-rhamnolipid. Burns 32:24–34
Lang S, Wullbrandt D (1999) Rhamnose lipids—biosynthesis, microbial production and application potential. Appl Microbiol Biotechnol 51:22–32
Timmis KN (2010) Handbook of hydrocarbon and lipid microbiology. Springer, Berlin
Ochsner U, Hembach T, Fiechter A (1996) Production of rhamnolipid biosurfactants. Downstream processing biosurfactants carotenoids. Springer, Berlin
Guerra-Santos L, Käppeli O, Fiechter A (1984) Pseudomonas aeruginosa biosurfactant production in continuous culture with glucose as carbon source. Appl Environ Microbiol 48:301–305
Müller MM, Hörmann B, Syldatk C, Hausmann R (2010) Pseudomonas aeruginosa PAO1 as a model for rhamnolipid production in bioreactor systems. Appl Microbiol Biotechnol 87:167–174
Sha R, Meng Q, Jiang L (2012) The addition of ethanol as defoamer in fermentation of rhamnolipids. J Chem Technol Biotechnol 87:368–373
Kronemberger FA, Anna LMMS, Fernandes ACLB, Menezes RR, Borges CP, Freire DMG (2008) Oxygen-controlled biosurfactant production in a bench scale bioreactor. Appl Biochem Biotechnol 147:33–45
Pinzon NM, Ju LK (2009) Analysis of rhamnolipid biosurfactants by methylene blue complexation. Appl Microbiol Biotechnol 82:975–981
Chayabutra C, Ju LK (2001) Polyhydroxyalkanoic acids and rhamnolipids are synthesized sequentially in hexadecane fermentation by Pseudomonas aeruginosa ATCC 10145. Biotechnol Prog 17:419–423
Gupta R, Gupta N, Rathi P (2004) Bacterial lipases: an overview of production, purification and biochemical properties. Appl Microbiol Biotechnol 64:763–781
Goto M, Goto M, Nakashio F, Yoshizuka K, Inoue K (1992) Hydrolysis of triolein by lipase in a hollow fiber reactor. J Membr Sci 74:207–214
Wang H, Sodagari M, Chen Y, He X, Newby BZ, Ju LK (2011) Initial bacterial attachment in slow flowing systems: effects of cell and substrate surface properties. Colloid Surf B 87:415–422
Junker B (2007) Foam and its mitigation in fermentation systems. Biotechnol Prog 23:767–784
Jones A, Porter M (1998) Vegetable oils in fermentation: beneficial effects of low-level supplementation. J Ind Microbiol Biotechnol 21:203–207
Rols J, Goma G (1991) Enhanced oxygen transfer rates in fermentation using soybean oil-in-water dispersions. Biotechnol Lett 13:7–12
Zhang Q, Ju LK (2011) Rhamnolipids as affinity foaming agent for selective collection of β-glucosidase from cellulase enzyme mixture. Enzyme Microb Technol 48:175–180
Champion JT, Gilkey JC, Lamparski H, Retterer J, Miller RM (1995) Electron microscopy of rhamnolipid (biosurfactant) morphology: effects of pH, cadmium, and octadecane. J Colloid Interface Sci 170:569–574
Farahat M, Hirajima T, Sasaki K, Doi K (2009) Adhesion of Escherichia coli onto quartz, hematite and corundum: extended DLVO theory and flotation behavior. Colloids Surf, B 74:140–149
Sharma P, Hanumantha Rao K (2003) Adhesion of Paenibacillus polymyxa on chalcopyrite and pyrite: surface thermodynamics and extended DLVO theory. Colloids Surf B 29:21–38
Lebrón-Paler A, Pemberton JE, Becker BA, Otto WH, Larive CK, Maier RM (2006) Determination of the acid dissociation constant of the biosurfactant monorhamnolipid in aqueous solution by potentiometric and spectroscopic methods. Anal Chem 78:7649–7658
Acknowledgments
The study was supported by the US Department of Transportation, Office of the Secretary, grant no. DTOS59-07-G-00050. The authors would like to thank Dr. Shida Miao, a postdoctoral research associate in the group, for performing the LC–MS analysis of the rhamnolipid samples.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Sodagari, M., Ju, LK. Cells Were a More Important Foaming Factor than Free Rhamnolipids in Fermentation of Pseudomonas aeruginosa E03-40 for High Rhamnolipid Production. J Surfact Deterg 17, 573–582 (2014). https://doi.org/10.1007/s11743-013-1535-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-013-1535-4