Abstract
Although the biosurfactant rhamnolipid has been previously characterized as having low foam ability, its fermentation is largely impeded by severe foaming. Hence, the investigation of this paradox is critically important for improving the mass production of rhamnolipid. Unexpectedly, the hydrophobic cell, instead of rhamnolipid, has been claimed to explain such severe foaming in rhamnolipid fermentation. This study tried to systematically investigate the severe foaming in fermentation, aiming to propose an effective strategy for foam control. The overflowing foam sustained a super high stability in terms of half-time for over 30 min. The major product of rhamnolipid largely contributed to the severe foaming in the fermentation process whereas other products like cells elicited much more limited effects. Furthermore, the foam stability of the fermentation broth increased with rhamnolipid concentration and noticeably increased with agitation speed. In the classic Bikerman foam test system without stirring, rhamnolipid showed foam stability as low as Tween 20 which is well known for its poor foam stability. However, in a stirring Bikerman system, rhamnolipid exhibited a foam stability almost as high as sodium dodecyl sulfate (SDS) at 10 g/L and even surpassed SDS at a higher concentration of 20 g/L. Hence, the extraordinarily increased foam stability of rhamnolipid with both agitation and concentration could explain the severe foaming at its late-stage fermentation when rhamnolipid-rich solution is mechanically agitated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rhamnolipid (RHA), the best-known biosurfactant with excellent surface/interfacial activities and environmentally friendly properties, has a wide range of potential applications as detergents, antibacterial, antifungal, antibiofouling agents, and pharmaceuticals [1–4]. In addition, our group has recently extended its applications to the treatment of the oily sludge [5] and demulsification of waste crude oil [6].
Nevertheless, the current fermentation of rhamnolipid at a low yield and small scale causes a high production cost, which is about ten times that of conventional surfactants [7, 8], and thus has largely inhibited its industrial applications. Such limitations on the fermentation are largely due to the severe foaming during fermentation [9–11]. Severe foaming can inhibit cell growth and product accumulation by reducing the bioavailability of substrates as well as the mass transfer efficiency of oxygen [12]. More importantly, the excessively accumulated foam can overflow from the effluent gas outlet of the bioreactor and thereby have several detrimental impacts to the fermentation, including the loss of product, nutrients, and cells accompanied by a high risk of contamination [11]. This foaming could hinder fermentation and become a key factor for impeding scale-up [11].
Although some approaches including anaerobic [13] or solid fermentation [14, 15] have been developed to avoid foam formation, aerobic fermentation still seems to be the sole approach for the high-yield production of rhamnolipid [16]. Hence, effective foam control has become the most urgently required technology in rhamnolipid fermentation. In this respect, the identification of the factors for severe foaming in the rhamnolipid fermentation has been the subject of research [17]. Similar to other fermentations where the secreted metabolites with high surface activity (like biosurfactant, protein, etc.) cause severe foaming under aeration [18], the biosurfactant rhamnolipid has been accepted as the major component dominating the severe foaming during fermentation [19–22]. However, a recent study by Sodagari and Ju claimed that the hydrophobic cell could contribute more to the foaming than rhamnolipid itself in rhamnolipid fermentation [17].
In our opinion, rhamnolipid should be the dominant component causing the severe foaming. Nevertheless, rhamnolipid has been found to have low foaming ability in comparison with conventional surfactants such as sodium dodecyl sulfate (SDS) [23, 24]. Therefore, this paper will systematically study the major contributions of broth components (like rhamnolipid, cell, etc.) to the severe foaming behavior in rhamnolipid fermentation and the mechanism involved.
Materials and Methods
Rhamnolipid Fermentation
The strain of Pseudomonas aeruginosa ZJU211 (CCTCC M209237) was isolated from heavily oil-contaminated soil and applied to produce rhamnolipids [4]. The composition of culture medium is shown in Table 1 [25]. The pH of culture medium was 6.8. During the fermentation, 2 % of oil was fed at 48 and 60 h during the fermentation.
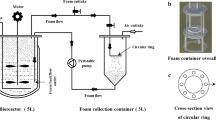
The rhamnolipid fermentation was conducted in a 10-L bioreactor (GUJS-10C, Eastbiotech, Zhenjiang, China) with a work volume of 5 L. The mixture of oxygen and nutrients was achieved by agitation under a speed of 300 rpm while a foam circulation system was set up. As shown in Fig. 1, the overflowing foam was introduced via the gas effluent pipe into a 100-L foam collector and then subjected to stirring with a mechanical impeller to break up the foam at 200 rpm. Then, the enriched culture broth, namely the collapsed foam, was pumped back to the bioreactor.
During the fermentation, the culture broth was sampled for determination of rhamnolipid and cell content. In each assay, the culture broth was centrifuged (H-1650R, Xiangyi, Hunan, China) for 15 min at 15,000 rpm (13,800×g) and 15 °C. The supernatant was taken for measuring rhamnolipid content using the anthrone–sulfuric assay [26] while the sediment was suspended in deionized water for detecting the cell content. The absorbance was then measured at 650 nm using water as a blank [27]. Optical density (OD650) was converted to the cell concentration by a standard curve.
Separation of Main Components From the Culture Broth
For the 60-h culture broth which was rich in low carbon substrates of oil, the components in the culture broth could be categorized into three parts: rhamnolipid, cell, and the residuals.
In cell separation, the 60-h culture broth was centrifuged at 15,000 rpm for 15 min and separated into two parts: (a) the supernatant, cell-free culture broth (labeled as “Cell-free”) and (b) sediment (cell components). The cell components after three washes with deionized water were then resuspended in deionized water at an equivalent volume as the initial culture broth and were then ready for use as a cell solution (labeled as component “Cell”).
The cell-free culture broth obtained above was further acidified to pH 2–3 and centrifuged at 15,000 rpm at 15 °C for 5 min before the precipitated rhamnolipids were removed. The supernatant free of either cell or rhamnolipids, after adjustment with 1 M NaOH to pH 7–8, is labeled as “Others” in Fig. 3. After three purification steps by dissolution–centrifugation–precipitation, the obtained rhamnolipid precipitate with a purity of over 85 % and a content of about 40 % was dissolved in deionized water at the same rhamnolipid concentration as the initial culture broth and was labeled as “RHA”. The other rhamnolipid solution at varying concentrations can be constituted by dissolving rhamnolipid precipitates into a different volume of deionized water.
Preparation of Various Culture Broths for Foaming
The cell-free culture broth was individually obtained after the centrifugation as indicated above. The rhamnolipid-free (“R-free”) culture broth was obtained by resuspending the cell parts into the cell/rhamnolipid-free supernatant culture broth. The culture broth in the absence of other components (“C-free”) was obtained by suspending the cell and rhamnolipid components together in deionized water at an equivalent concentration to the initial culture broth.
Foam Generation
The typical Bikerman column was used to generate foams. About 30 ml of surfactant solution or culture broth at pH 7.0 was used to fill a cylinder with an inner diameter of 4 cm and a height of 30 cm, and then humidified N2 was sparged at a flow rate of 150 ml/min for 2 min at 15 ± 2 °C.
To simulate the foam formation in the bioreactor, 5-L foaming solution in a 10-L bioreactor (model JUGS-2, Eastbiotech, Zhenjiang, China) installed with a stirring impeller was used. The foam was produced through air sparging at a flow of 0.5 L/min and stirring (0–300 rpm) at 15 ± 2 °C.
Evaluation of Foam Properties
The tested solution was evaluated in terms of foaming ability as well as foam stability.
In the Bikerman foam test, the foam height (cm) in the cylinder when gas flow immediately stopped was recorded as an index for the evaluation of the foaming ability. The foam stability was determined by the half-life time (min) which is defined as the time in which the foam height declined to half of its original value [28] at 15 ± 2 °C.
For culture broth in a 10-L bioreactor, the ratio of foam rate/air rate (or the “over-run ratio”) was taken as a measure of foaming ability. The half-time (min) of the over-running foam collected in the cylinder was selected as a criterion for foam stability similar to the Bikerman foam test.
Another foam property, the average water content (w), was measured by a gravimetric method. Foam was collected using a beaker and the foam mass was then measured. The average water content of foam was calculated as following (the mass of air was neglected while the density of liquid (bulk foaming solution) was set at 1 g/ml):
where V and m are the volume and mass of the collected foam, respectively.
Data Analysis
All values presented in this study were given as mean ± standard error (number of samples ≥3).
Results
Foam Stability of Fermentation Broth
Rhamnolipid fermentation was carried out as described in Fig. 1 and then examined for foaming behavior by analyzing the overflowing foam rate, accumulation volume, and water content. Meanwhile, the culture broth was sampled to evaluate its foam properties, cell growth, and productivity.
The foaming behavior of culture broth at varying time points in the fermentation is presented in Table 2. The overflowing foam showed an increasingly flow rate with fermentation time although the entering air flow rate had to be continuously reduced because of the increasing volume occupied by the foam in the collecting tank. At the late fermentation stage after 48 h, the ratio of the overflowing foam/air flow rate maintained a high level of 0.86 while the accumulated foam approached 50–70 L in volume, indicative of conditions difficult for foam collapse. Also, the average water content of the foam increased with the fermentation time, achieving a high steady-state value of about 4.5 % after 60 h of fermentation. It seems that the foaming problem was getting much severer with the fermentation time, in particularly at the late stage of fermentation.
The foam properties of the culture broth were assayed using the typical Bikerman approach. Between 24 and 72 h of fermentation, the foam height of the culture broth increased slightly from 13 to 20 cm while the foam stability sharply increased from 4 min to about 30 min (Fig. 2a); in contrast, before 24 h of fermentation, the broth presented much more limited foaming ability (data not shown). Correspondingly, both rhamnolipid productivity and cell mass accumulation greatly increased with the fermentation time, reaching about 30 g/L for rhamnolipid and 12 g/L for cell at 72 h (Fig. 2b).
Major Component for Foam Formation in Culture Broth
The fermentation broth was sampled at 60 h to identify the major component of the foam. Rhamnolipid and cells etc. were firstly isolated from the culture broth and then respectively examined for their foaming ability and foam stability in the typical Bikerman column.
The foaming ability of each component is presented in Fig. 3a. The separated rhamnolipids showed similar foaming ability (about 18 cm) as the culture broth. In contrast, the cell sediments and the other components showed very limited foaming ability of only 1 cm.
Foam stability is shown in Fig. 3b. The foam generated from the constituted rhamnolipid solution manifested the same stability as the culture broth in the absence of whatever cells or other components, while the “R-free” and other two solutions, i.e., “Cell” and “Others”, showed much lower foam stability with a half-time of below 5 min.
It therefore seems that rhamnolipid is the major contributing component for the severe foaming in fermentation.
Contribution of Rhamnolipid Content and Agitation to Foaming in Fermentation
The impact of rhamnolipid concentration on foaming was evaluated in the classic Bikerman foam test where the precipitated rhamnolipid was used as the foaming solution. As shown in Fig. 4, the foaming ability increased with the rhamnolipid concentration, reaching a steady-state foam height at 5 g/L of rhamnolipid concentration. In contrast, the foam stability continuously increased with rhamnolipid concentration, achieving about 35 min at a concentration of 40 g/L.
The effect of agitation was then evaluated using 20 g/L of precipitated rhamnolipid as the foaming solution in the 10-L bioreactor. As shown in Fig. 5, the half-time and water content increased with the agitation while the bubble size showed a decreasing trend. When the agitation was set at a high stirring speed of 300 rpm, the generated foam showed a much higher stability of 60 min and a high water content of about 5 %. In contrast, the foam generated under no stirring presented a low stability of about 20 min and a low water content of 1.83 % (Fig. 5a, b). According to the morphologic observation, aeration alone (i.e., no stirring) produced large and transparent bubbles (Fig. 5c), while the extra stirring generated much finer and uniform bubbles (Fig. 5d).
Impact of stirring speed on the foam stability (a), water content and bubble size (b), and foam texture of rhamnolipid fermentation broth without (c) or with (d) stirring at 300 rpm. The precipitated rhamnolipid at 20 g/L was used as the foaming agent. Scale bars in c, d represent 5 and 1 mm, respectively
It seems that vigorous stirring together with rhamnolipid concentration could largely increase foaming.
Comparison of Rhamnolipid Foaming Behavior with Other Chemical Surfactants
Surfactants SDS and polysorbate (Tween 20), representing strong foaming and weak foaming agents, respectively, were used for evaluation of the foam properties in a Bikerman foam column under the same concentrations of 10 and 20 g/L. Surfactants showed the same foaming ability at 10 g/L (Table 3), and the further increase of concentration to 20 g/L showed no significant difference (data not shown). However, the three surfactants had very different foam stability (Fig. 6a). At the same concentration of 10 g/L, SDS manifested the highest foam stability of about 30 min while Tween 20 and rhamnolipid sustained a low foam stability of 9–12 min. The further increase of concentration to 20 g/L enhanced the foam stability by around 20–40 % for each surfactant.
To confirm the impact of stirring (or agitation) on the foam stability, the foam property was assayed in a Bikerman foam column agitated at the bottom (300 rpm). As shown in Fig. 6b, rhamnolipid exhibited a foam stability as almost high as SDS at a concentration of 10 g/L and largely surpassed SDS at a concentration of 20 g/L. By comparing Fig. 6b with Fig. 6a, it is evident that agitation enhanced the foam stability for all the three surfactants, especially for rhamnolipid. Rhamnolipid showed the strongest impact of increasing surfactant concentration on foam stability among the three surfactants. For example, when the concentration increased from 10 to 20 g/L, the foam stability of rhamnolipid increased from 36 to 57 min while those of both Tween 20 and SDS had slight increment (less than 7 min).
It seems that rhamnolipid exhibits low foam stability (cf. Tween 20) under no stirring but could significantly aggravate foaming issues under stirring. The increasing concentration further exacerbated the foam stability.
Discussion
The problem of foaming is serious issue in the fermentation of rhamnolipid, as reflected by the almost equivalent overflowing foam rate to the input air flow rate (Table 1). To reduce the foam formation during fermentation, a very low air rate (0.03 vvm) was applied and a large pressure of 0.09 MPa was further required to avoid the foam overflowing from the tank [29]. Even the use of a large foam storage tank for foam drainage could not solve the problem generated by the high foam stability. The commonly used mechanical foam breaker (stirring impeller) has a very limited defoaming capability in rhamnolipid fermentation [2, 30] and, unexpectedly, aggravated the already difficult foam control, forming a dense airy emulsion which was hard to break down [2]. Such severe foaming problems have largely inhibited the production of rhamnolipid as well as its industrial applications [11].
Severe foaming in fermentation largely depends on the accumulation of rhamnolipid. According to our findings, rhamnolipid, the main product in the fermentation broth, was demonstrated to be the dominating component for severe foaming (Fig. 3). This biosurfactant, particularly at a high concentration, could generate highly stable foam by forming abundant micelles which would intensively block the Plateau channels and resist the foam drainage as well as foam coalescence [31, 32]. The identified contribution of rhamnolipid to severe foaming during fermentation is totally the opposite to a previous claim according to which cells, rather than rhamnolipids, were a dominant component for such severe foaming [17]. This inconsistence might be due to the different cells used in each study; however, according to our own observation, the lack of rinsing of isolated cells might more likely be the case. As noticed, similar foaming occurred on the suspended cells directly precipitated from culture broth (data not shown) but not on the cells subjected to an extra rinse with water (Fig. 3). Hence, the rhamnolipid residues attached on the cell surfaces or blocked inside might explain the previously reported foaming ability of the cells [17]. Cell together with other components (like proteins) in the fermentation culture broth could, to some limited extent, synergistically improve the foam stability via blocking the Plateau channels with surfactant micelles [33].
Agitation was demonstrated to strengthen the foam stability of rhamnolipid in the present study (Fig. 4), explaining its negative influence on foam control during fermentation. As noticed, the bubbles formed via vigorous stirring feature wet films and a small and narrowly distributed bubble diameter (Fig. 5d). The vigorous agitation supplies a high shear force, producing fine bubbles and/or breaking the occasionally formed large bubbles into small ones according to the theory of secondary foam formation [34, 35]. Such a fine and wet film could greatly increase the foam stability to about 60 min (Figs. 4 and 5) by prolonging the time for foam coalescence and drainage [36]. Hence, the stirring in the foam tank does not completely break the foam, but actually aggravates the difficult foam control by generating secondary foam with higher stability.
Interestingly, and according to the Bikerman foaming test, rhamnolipid had low foam stability. In fact, rhamnolipid exhibited a foam stability as low as Tween 20, a poor foaming surfactant (Fig. 6a), corresponding well with the previous findings [23, 24]. This could be attributed to the low HLB of rhamnolipid (Table 2), which indicates low hydrophilicity, and thus it could not effectively resist the foam drainage. Also, the thin adsorption monolayer of rhamnolipid at the foam film surface [37, 38] could also result in low foam stability. Nevertheless, the existence of agitation turned rhamnolipid into a highly foaming surfactant and even made it surpass SDS at a concentration of 20 g/L (Fig. 6b). The significant increase in foam stability of rhamnolipid with agitation and concentration might be due to its capability of carrying more water (increased from 2 to 5 % with agitation) (Fig. 5b). Foams with higher water content are usually more stable. In contrast, foams generated by SDS and Tween 20 experienced very limited improvement in their water content (about 1 %) in the presence of agitation (data not shown). The mechanism of foam stabilization by the different surfactants under agitation remains unclear and will need further study.
Hence, although the widely used mechanical foam breaker performs well in the foam control of regular fermentation [39], it should be avoided in rhamnolipid fermentation. Instead, the reduction of agitation in either bioreactor or foam tank would be a suitable approach to reduce foam stability and thus facilitate foam control.
In conclusion, rhamnolipid, though having low foam stability as compared with conventional surfactants, could be the main agent in causing severe foaming during fermentation, while the other components in the broth manifested very limited impact. Vigorous agitation could largely aggravate foaming issues, and this effect was more significant in rhamnolipid fermentation. The correct interpretation of the high foam stability would be very helpful for developing an effective solution in the near future.
References
Jadhav M, Kalme S, Tamboli D, Govindwar S (2011) Rhamnolipid from Pseudomonas desmolyticum NCIM-2112 and its role in the degradation of Brown 3REL. J Basic Microbiol 51:385–396
Müller MM, Hörmann B, Syldatk C, Hausmann R (2010) Pseudomonas aeruginosa PAO1 as a model for rhamnolipid production in bioreactor systems. Appl Microbiol Biotechnol 87:167–174
Reis RS, Pereira AG, Neves BC, Freire DM (2011) Gene regulation of rhamnolipid production in Pseudomonas aeruginosa—a review. Bioresour Technol 102:6377–6384
Sha R, Jiang L, Meng Q, Zhang G, Song Z (2012) Producing cell-free culture broth of rhamnolipids as a cost-effective fungicide against plant pathogens. J Basic Microbiol 52:458–466
Long X, Zhang G, Han L, Meng Q (2013) Dewatering of floated oily sludge by treatment with rhamnolipid. Water Res 47:4303–4311
Long X, Zhang G, Shen C, Sun G, Wang R, Yin L, Meng Q (2013) Application of rhamnolipid as a novel biodemulsifier for destabilizing waste crude oil. Bioresour Technol 131:1–5
Lang S, Wullbrandt D (1999) Rhamnose lipids–biosynthesis, microbial production and application potential. Appl Microbiol Biotechnol 51:22–32
Mukherjee S, Das P, Sen R (2006) Towards commercial production of microbial surfactants. Trends Biotechnol 24:509–515
Chen S-Y, Wei YH, Chang JS (2007) Repeated pH-stat fed-batch fermentation for rhamnolipid production with indigenous Pseudomonas aeruginosa S2. Appl Microbiol Biotechnol 76:67–74
Timmis KN, McGenity T, Van Der Meer J, De Lorenzo V (2010) Handbook of hydrocarbon and lipid microbiology. Springer, Berlin Heidelberg
Pansiripat S, Pornsunthorntawee O, Rujiravanit R, Kitiyanan B, Somboonthanate P, Chavadej S (2010) Biosurfactant production by Pseudomonas aeruginosa SP4 using sequencing batch reactors: effect of oil-to-glucose ratio. Biochem Eng J 49:185–191
Wu JY, Yeh KL, Lu WB, Lin CL, Chang JS (2008) Rhamnolipid production with indigenous Pseudomonas aeruginosa EM1 isolated from oil-contaminated site. Bioresour Technol 99:1157–1164
Zhao F, Shi R, Zhao J, Li G, Bai X, Han S, Zhang Y (2015) Heterologous production of Pseudomonas aeruginosa rhamnolipid under anaerobic conditions for microbial enhanced oil recovery. J Appl Microbiol 118:379–389
Neto DC, Meira JA, de Araújo JM, Mitchell DA, Krieger N (2008) Optimization of the production of rhamnolipids by Pseudomonas aeruginosa UFPEDA 614 in solid-state culture. Appl Microbiol Biotechnol 81:441–448
Camilios-Neto D, Bugay C, de Santana-Filho AP, Joslin T, de Souza LM, Sassaki GL, Mitchell DA, Krieger N (2011) Production of rhamnolipids in solid-state cultivation using a mixture of sugarcane bagasse and corn bran supplemented with glycerol and soybean oil. Appl Microbiol Biotechnol 89:1395–1403
Zhu L, Yang X, Xue C, Chen Y, Qu L, Lu W (2012) Enhanced rhamnolipids production by Pseudomonas aeruginosa based on a pH stage-controlled fed-batch fermentation process. Bioresour Technol 117:208–213
Sodagari M, Ju LK (2014) Cells were a more important foaming factor than free rhamnolipids in fermentation of Pseudomonas aeruginosa E03-40 for high rhamnolipid production. J Surfactants Deterg 17:573–582
Salwa M, Asshifa MN, Amirul A, Yahya AR (2009) Different feeding strategy for the production of biosurfactant from Pseudomonas aeruginosa USM AR2 in modified bioreactor. Biotechnol Bioprocess Eng 14:763–768
Zhang Q, Ju LK (2011) Rhamnolipids as affinity foaming agent for selective collection of β-glucosidase from cellulase enzyme mixture. Enzyme Microb Technol 48:175–180
Hori K, Marsudi S, Unno H (2002) Simultaneous production of polyhydroxyalkanoates and rhamnolipids by Pseudomonas aeruginosa. Biotechnol Bioeng 78:699–707
Chayabutra C, Wu J, Ju LK (2001) Rhamnolipid production by Pseudomonas aeruginosa under denitrification: effects of limiting nutrients and carbon substrates. Biotechnol Bioeng 72:25–33
Wu J, Ju LK (1998) Extracellular particles of polymeric material formed in n-hexadecane fermentation by Pseudomonas aeruginosa. J Biotechnol 59:193–202
Urum K, Pekdemir T, Ross D, Grigson S (2005) Crude oil contaminated soil washing in air sparging assisted stirred tank reactor using biosurfactants. Chemosphere 60:334–343
Urum K, Pekdemir T (2004) Evaluation of biosurfactants for crude oil contaminated soil washing. Chemosphere 57:1139–1150
Tang X, Zhu Y, Meng Q (2007) Enhanced crude oil biodegradability of Pseudomonas aeruginosa ZJU after preservation in crude oil-containing medium. World J Microbiol Biotechnol 23:7–14
Long X, Meng Q, Sha R, Huang Q, Zhang G (2012) Two-step ultrafiltration of rhamnolipids using PSU-g-PEG membrane. J Membr Sci 409:105–112
Sha R, Meng Q, Jiang L (2012) The addition of ethanol as defoamer in fermentation of rhamnolipids. J Chem Technol Biotechnol 87:368–373
Zhang JC, Zhang L, Wang XC, Zhang L, Zhao S, Yu JY (2011) A surface rheological study of polyoxyethylene alkyl ether carboxylic salts and the stability of corresponding foam. J Dispers Sci Technol 32:372–379
Gong Z, Peng Y, Wang Q (2015) Rhamnolipid production, characterization and fermentation scale-up by Pseudomonas aeruginosa with plant oils. Biotechnol Lett 37:1–6
Lee BS, Kim EK (2004) Lipopeptide production from Bacillus sp. GB16 using a novel oxygenation method. Enzyme Microb Technol 35:639–647
Bikerman JJ (1965) Foams and emulsion—formation, properties, and breakdown. Ind Eng Chem 57:56–62
Murray BS, Ettelaie R (2004) Foam stability: proteins and nanoparticles. Curr Opin Colloid Interface Sci 9:314–320
Mulligan CN, Wang S (2006) Remediation of a heavy metal-contaminated soil by a rhamnolipid foam. Eng Geol 85:75–81
Gutwald S, Mersmann A (1997) Mechanical foam breaking – a physical model for impact effects with high speed rotors. Chem Eng Technol 20:76–84
Pelton R (2002) A review of antifoam mechanisms in fermentation. J Ind Microbiol Biotechnol 29:149–154
Sarachat T, Pornsunthorntawee O, Chavadej S, Rujiravanit R (2010) Purification and concentration of a rhamnolipid biosurfactant produced by Pseudomonas aeruginosa SP4 using foam fractionation. Bioresour Technol 101:324–330
Helvacı Ş, Peker S, Özdemir G (2004) Effect of electrolytes on the surface behavior of rhamnolipids R1 and R2. Colloid Surface B 35:225–233
Cohen R, Exerowa D (2007) Surface forces and properties of foam films from rhamnolipid biosurfactants. Adv Colloid Interface Sci 134:24–34
Chen CY, Baker SC, Darton RC (2006) Continuous production of biosurfactant with foam fractionation. J Chem Technol Biotechnol 81:1915–1922
Acknowledgments
We gratefully acknowledge the financial support of this study by the National High-Technology R&D Program (863 program) of China (No. SS2014AA022104) and National Natural Science Foundation of China (No. 21406195).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
About this article
Cite this article
Long, X., Sha, R., Meng, Q. et al. Mechanism Study on the Severe Foaming of Rhamnolipid in Fermentation. J Surfact Deterg 19, 833–840 (2016). https://doi.org/10.1007/s11743-016-1829-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-016-1829-4