Abstract
The aggregation behavior and flow characteristics of systems based on zwitterionic surfactant, erucyl amidopropyl betaine, silica and alumina nanoparticles in a wide range of surfactant concentrations from molecular to micellar solutions were studied using surface tensiometry, conductometry, dynamic and electrophoretic light scattering, and rheology techniques. The adsorption of zwitterionic surfactant molecules occurs on both positively and negatively charged surfaces via an electrostatic interaction mechanism. As a result, addition of a small amount silica nanoparticles (0.5–0.8 wt%) increases the surfactant solution's viscosity by more than two times.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanotechnology is one of the fastest growing fields in fundamental and applied science [1–3] due to the unique physical and chemical properties demonstrated at the nanoscale [4]. Nanoparticles of both metals and nonmetals are used, e.g., as semiconductors [5, 6], adsorbents for water purification [7], biosensors [8, 9] and components of anticancer drugs [10, 11].
Surfactants are widely used in the synthesis [12–14] and stabilization [9, 15, 16] of nanoparticle dispersions. A significant number of papers in the field of mixed surfactant–nanoparticle systems refer to the adsorption of anionic [17, 18], cationic [15, 17–20] and nonionic [17, 18, 21–23] surfactants on the nanoparticle's surface, where the surfactant aggregates are in the form of spherical micelles. As far as zwitterionic surfactants and cylindrical micelles are concerned, they are preferable in the synthesis of elongated metal nanoparticles [12, 13]. Therefore, the choice of surfactants is determined by matching micellar morphology with the form of the synthesised nanoparticles. On the other hand, there is a tendency for the modification of cylindrical micellar solutions based on anionic and cationic surfactants [24–26] and polymers [27, 28] by nanoparticle additives. Addition of nanoparticles results in increased viscosity via formation of additional topological links between the micelles, and generally affects the rheological properties of the solutions. However, there are no papers related to the study of nanoparticle addition to zwitterionic surfactant solutions, i.e., such as carboxybetaines. Important advantages of zwitterionic surfactants over other ionic amphiphiles are their high water solubility, low sensitivity to the presence of salts, high biodegradation rate and the ability to form cylindrical micelles in solution in the absence of counter ions (salts) [29]. Many of these properties are attractive for industrial applications. Long and flexible worm-like micelles form cross-links at high surfactant concentrations [30–34] resulting in an increase in solution viscosity. However, unlike polymer solutions, micellar solutions are dynamic systems. Thus, their properties can be controlled by external factors (pressure, temperature, and additives) [31, 35]. Therefore, this class of surfactants is of practical importance and is widely used in oil recovery [36] and cosmetics [37] as a template to create viscoelastic compositions and increase solution viscosity.
In this paper, the effect of silica and alumina nanoparticle addition on the aggregation and rheological properties of the zwitterionic surfactant, erucyl amidopropyl betaine (EAPB; Fig. 1), were studied in a wide range of surfactant concentrations from molecular to micellar solutions, including cylindrical micelles.
Experimental Section
Materials and Methods
LUDOX TM-40 colloidal silica—40 wt% suspension in H2O (Sigma Aldrich) and Nanobyk-3600 dispersion of alumina nanoparticles—50 wt% suspension in H2O (BYK USA, Inc.) were used as received. EAPB was synthesized by the Limited Liability Company “Scientific Research Institution of Surfactants” (Russia, Volgodonsk) and characterized by elemental analysis, and proton nuclear magnetic resonance ( 1H NMR) spectroscopy. Water used as a solvent was obtained using Direct-Q ultraviolet water purification equipment (Direct-Q 5 UV, EMD Millipore Corp.) with a resistivity 18.2 MΩ cm at 25 °C. All samples were prepared by mixing the nanoparticles dispersion with EAPB water solutions and equilibrated at 25 °C for at least 24 h before any measurement. EAPB was dissolved in water at 40 °C using a magnetic stirrer for 24 h.
Dynamic and Electrophoretic Light Scattering
Dynamic light scattering (DLS) is a well-established technique for determining the hydrodynamic radius R h and size distribution of aggregates which is based on the spherical approximation to the Stokes–Einstein relationship:
where D is the diffusion coefficient, k b is the Boltzmann constant, T is absolute temperature and η is the solvent (water) viscosity. Measurements were performed using a Zetasizer Nano (Malvern Instruments) with a He–Ne gas laser source (633 nm). The data was analyzed using the second-order cumulant expansion method. DLS results are averaged by the number of particles. Millipore filters with a pore diameter of 450 nm were used to remove impurities from the solutions prior to measurement.
The zeta potential of the samples was also measured using the Zetasizer Nano. The measurement is based on particle mobility in an electric field. Zeta potential was measured from electrophoretic mobility using the Helmholtz–Smoluchowski equation. All measurements were performed at least three times for each sample at 25 °C.
Rheological Measurements
Rheological measurements at static shear were conducted on a RheoStress 6000 rheometer (Thermo Scientific HAAKE, Germany) using two different measuring geometries. For low-viscosity solutions, a double coaxial cylinder gap was used (outer cylinder diameter is 21.7 mm, the inner cylinder diameter is 18 mm, and the height is 55 mm). Highly viscous samples were examined using a cone and plane cell with a 35-mm diameter and a cone angle of 2°. Experiments were undertaken within the stress range from 0.002 to 100 Pa. Solution viscosity η is defined as the proportionality coefficient between the applied stress σ and the shear rate (η = σ/γ). At low shear rates the viscosity reaches a plateau (not dependent on applied stress). This value is taken as the maximum Newtonian viscosity η 0 (viscosity at zero shear rate).
Surface Tension Measurement
Surface tension was measured using a K6 tensiometer (Krüss Instruments, Germany) using the Du Noüy ring detachment method. All experiments were performed at 25 °C. This method is based on measuring the ring detachment force F which is related to the surface tension γ using Eq. 2:
where R is the radius of the ring and f is an empirical correction factor which accounts for the shape of the liquid pulled up in the ring and the diameter of the wire.
Conductometry
The electrical conductivity data χ (µS cm−1) were collected with an InoLab Cond 7110 conductivity meter (WTW GmbH, Germany) at 25 °C.
Results and Discussion
Both electrostatic forces and hydrophobic effect can mediate surfactant–nanoparticles interactions. A survey of the literature revealed that the balance between the two mechanisms depends strongly on the particle charge and the nature of surfactant head group [38–42]. In the case of like charged partners, hydrophobic effects prevail, whereas, when oppositely charged nanoparticles and surfactants interact, electrostatic forces play a key role. Apart from these extreme cases, different types of intermediate interactions occur, depending on the nature of both partners.
The adsorption of EAPB on positively charged hydrophilic alumina nanoparticles (Al2O3) and negatively charged silica nanoparticles (SiO2) was studied by electrophoretic techniques and DLS. EAPB is a zwitterionic molecule, i.e., a molecule that has both a positive and negative charge over a wide pH range [29]. However, the measured zeta potential of EAPB in aqueous solution has a negative value equal to −20 mV. In our previous work [43], it was shown that EAPB has a neutral pH (6.5–7.2) over a wide range of solution concentrations. Addition of a small amount of nanoparticles (0.1 wt%) does not change the solution pH.
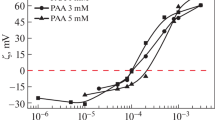
In the mixed EAPB–Al2O3 systems with a constant Al2O3 nanoparticles concentration (0.1 wt%) the dependence of particle zeta potential and hydrodynamic particle size as a function of surfactant concentration are shown in Fig. 2. All of the samples formed transparent dispersions up to an EAPB concentration of 0.0025 M. Charge reversal of the nanoparticle surface from +15 to −15 mV was observed with increasing EAPB concentration. The latter value is close to the zeta potential of pure EAPB, suggesting that the surfactant adsorbed on the particle surface due to electrostatic association of the negatively charged EAPB head group with the positively charged Al2O3 surface. In the absence of added surfactant, alumina nanoparticle aggregates with a hydrodynamic diameter of 130 nm are formed. This large size likely indicates the presence of clusters of nanoparticles. The increase in hydrodynamic diameter with increasing EAPB concentration (~15 nm) suggests the formation of surfactant bilayer-like vesicular structures on the aggregate's surface.
In the case of negatively charged SiO2 nanoparticles (0.1 wt%), an increase in zeta potential from −55 to −30 mV was observed with increasing EAPB concentration, as shown in Fig. 3. All samples with silica nanoparticles were transparent at all surfactant/nanoparticle concentration ratios tested. The negative magnitude was maintained due to the adsorption of the EAPB by hydrophobic association. An increase in the hydrodynamic diameter by 3–4 nm with increasing surfactant concentration is observed, which indicates the presence of an adsorbed surfactant layer on the particle surface. The dependence is in good agreement with the four-step adsorption isotherm [44, 45]. A sharp increase in the zeta potential and the aggregate's hydrodynamic diameter by 4 nm is observed with increasing surfactant concentration over the range from 0 to 0.2 mM. This is equivalent to monolayer adsorption of surfactant molecules on the nanoparticle surface, assuming that the radius of EAPB micelles is 2.9 nm [35]. A further increase in the EAPB concentration is followed by a small change in the zeta potential, which corresponds to surfactant monolayer saturation. Finally, the curve reaches a plateau region with a constant hydrodynamic diameter and zeta potential value, in which no further adsorption is observed.
Further experiments were carried out with fixed surfactant concentration at 20 mM, where worm-like EAPB micelles are formed [43]. The zeta potential and hydrodynamic diameter were measured with increasing SiO2 particle concentration from 0.1 to 0.4 wt%, as shown in Fig. 4. These systems are stable with no phase separation observed over the entire concentration range. The zeta potential decreases with increasing nanoparticle concentrate, close to that of pure SiO2 nanoparticles due to the increase in the surface being free from adsorbed surfactant molecules [46]. Thus, a denser packing of surfactant molecule and a higher particle surface saturation are observed in the low SiO2 concentration region. In the absence of nanoparticles, 25 nm surfactant aggregates are formed, which increase in size with increasing SiO2 concentration. Bimodal particle size distributions are not observed in the system, which indicates the formation of particles of the same type. And the presence of the plateau indicates that this is the saturation concentration of aggregates by nanoparticles.
Surface tensiometry and conductometry techniques were applied to study the self-organization of EAPB in the presence of SiO2 nanoparticles. Both methods are widely used to determine the critical micelle concentration (CMC) in surfactant solutions. Previously, the self-organization of EAPB in aqueous solution has been studied and the CMC values determined [43]. Our current focus is on the effect of silica nanoparticle additives on the EAPB self-organization in water. The CMC of EAPB is insignificantly increased when silica nanoparticles are added (0.1 wt%), but the minimum surface tension is higher than in the individual EAPB solution, as shown in Fig. 5. This suggests surfactant concentration at the water/air interface is lower for the EAPB–SiO2 system than for pure EAPB and the surfactant molecules are more favorably adsorbed at the nanoparticle surface than at the water/air interface.
The CMC values obtained by conductometry are in good agreement with the tensiometry data, as shown in Fig. 6 and Table 1. It is important to note that the individual SiO2 nanoparticles have no surface activity, since no decrease in the surface tension of the SiO2 solutions is observed over a wide range of nanoparticle concentrations (0.1–5 wt%).
Thus, interactions between EAPB molecules and silica and alumina nanoparticles have been shown at a low surfactant concentration. Further experiments were conducted at higher surfactant concentrations where a viscoelastic network of long, worm-like micelles were formed [35]. One of the main mechanisms mediating the increase in the viscosity of micellar solutions is the formation of rod-like micelles. Sphere-to-rod transitions of micelles can be induced by different additives, such as electrolytes, hydrophobic solutes and nanoparticles, with their mechanisms markedly differing. The most general explanation is that the additives cause the neutralization and dehydration of head groups, thereby allowing for closer packing.
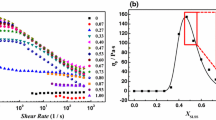
In our work, we found that increasing the concentration of alumina nanoparticles to 0.3 wt% at a constant surfactant concentration of 0.0625 M resulted in the suspension formation. Therefore, the modification of highly concentrated EAPB solutions with nanoparticle addition was conducted using only the silica nanoparticles. The dependence of viscosity on shear rates in the mixed EAPB–SiO2 solutions is shown in Fig. 7. The experiment was carried out at the constant surfactant concentration (0.0625 M) by varying the concentration of the SiO2 particles from 0.3 to 0.8 wt%. The selected range is determined by the reversible phase separation observed at higher nanoparticle concentrations (C >1 wt%).
That the viscosity increased by more than two times is evidence of interactions between nanoparticles and worm-like micelles due to the formation of mixed aggregates. A similar effect was previously observed in the mixed systems based on worm-like micelles of cetyltrimethylammonium bromide (CTAB) and silica nanoparticles with a modified surface [25, 26]. The viscosity increase was due to cross-linking between worm-like micelles and nanoparticles. The adsorption of the end-cup molecules of worm-like micelles on the nanoparticles surface is an energetically favourable process driven by decreasing free energy [25].
Conclusion
The interaction between the zwitterionic surfactant erucyl amidopropyl betaine (EAPB) and negatively charged silica and positively charged alumina nanoparticles has been studied. The adsorption of surfactant molecules on nanoparticles affects its self-aggregation characteristics, which is reflected in a slight increase of the CMC values and a higher minimum surface tension. The increase in viscosity of EAPB solutions with small addition of silica nanoparticles confirms the interaction between the components and surfactant adsorption on SiO2 nanoparticles.
References
Manthe RL, Foy SP, Krishnamurthy N, Sharma B, Labhasetwar V (2010) Tumor ablation and nanotechnology. Mol Pharm 7:1880–1898
Sládková M, Vlcˇková B, Pavel I, Šišková K, Šlouf M (2009) Surface-enhanced Raman scattering from a single molecularly bridged silver nanoparticle aggregate. J Mol Struct 924–926:567–570
Thong-On B, Rutnakornpituk B, Wichai U, Rutnakornpituk M (2012) Magnetite nanoparticle coated with amphiphilic bilayer surfactant of polysiloxane and poly(poly(ethylene glycol) methacrylate). J Nanopart Res 14:953–964
Russel WB, Saville DA, Schowalter WR (1989) Colloidal dispersions. Cambridge University Press, Cambridge
Hung C-Y, Marshall AF, Kim D-K, Nix WD, Harris JS Jr, Kiehl RA (1999) Strain directed assembly of nanoparticle arrays within a semiconductor. J Nanopart Res 1:329–347
Schroder DK (1998) Semiconductor material and device characterization. Wiley, New York
Ali I (2012) New generation adsorbents for water treatment. Chem Rev 112:5073–5091
De M, Ghosh PS, Rotello VM (2008) Applications of nanoparticles in biology. Adv Mater 20:4225–4241
Fernandes SC, de Souza FD, de Souza BS, Nome F, Vieira IC (2012) Gold nanoparticles dispersed in zwitterionic surfactant for peroxidase immobilization in biosensor construction. Sens Actuators B 173:483–490
Alexis F, Pridgen EM, Langer R, Farokhzad OC (2010) Nanoparticle technologies for cancer therapy. Handb Exp Pharmacol 197:55–86
Brannon-Peppas L, Blanchette JO (2012) Nanoparticle and targeted systems for cancer therapy. Adv Drug Deliv Rev 64:206–2123
Gupta VKN, Mehra A, Thaokar R (2012) Worm-like micelles as templates: formation of anisotropic silver halide nanoparticles. Colloids Surf A 393:73–80
Salkar RA, Jeevanandam P, Kataby G, Aruna ST, Koltypin Yu, Palchik O, Gedanken A (2000) Elongated copper nanoparticles coated with a zwitterionic surfactant. J Phys Chem B 104:893–897
Zi X, Wang R, Liu L, Dai H, Zhang G, He H (2011) Cetyltrimethylammonium bromide assisted preparation and characterization of pd nanoparticles with spherical, worm-like, and network-like morphologies. Chin J Catal 32:827–835
Gao GM, Zou H-F, Liu DR, Miao LN, Ji GJ, Gan SC (2009) Influence of surfactant surface coverage and aging time on physical properties of silica nanoparticles. Colloids Surf A 350:33–37
Praus P, Dvorský R, Horínková P, Pospíšil M, Kovár P (2012) Precipitation, stabilization and molecular modeling of ZnS nanoparticles in the presence of cetyltrimethylammonium bromide. J Colloid Interface Sci 377:58–63
Kumar P, Bohidar HB (2010) Aqueous dispersion stability of multi-carbon nanoparticles in anionic, cationic, neutral, bile salt and pulmonary surfactant solutions. Colloids Surf A 361:13–24
Kumar S, Aswal VK, Kohlbrecher J (2012) Size-dependent interaction of silica nanoparticles with different surfactants in aqueous solution. Langmuir 28:9288–9297
He Sh, Chen H, Guo Z, Wang B, Tang Ch, Feng Yu (2013) High-concentration silver colloid stabilized by a cationic gemini surfactant. Colloids Surf A 429:98–105
Xu F, Zhang Q, Gao Z (2013) Simple one-step synthesis of gold nanoparticles with controlled size using cationic gemini surfactants as ligands: effect of the variations in concentrations and tail lengths. Colloids Surf A 417:201–210
Chen C-N, Huang C-T, Tseng WJ, Wei M-H (2010) Dispersion and rheology of surfactant-mediated silver nanoparticle suspensions. Appl Surf Sci 257(2):650–655
Santra S, Tapec R, Theodoropoulou N, Dobson J, Hebard A, Tan W (2001) Synthesis and characterization of silica-coated iron oxide nanoparticles in microemulsion: the effect of nonionic surfactants. Langmuir 17:2900–2906
Sharma KP, Aswal VK, Kumaraswamy G (2010) Adsorption of nonionic surfactant on silica nanoparticles: structure and resultant interparticle interactions. Phys Chem B 114:10986–10994
Bandyopadhyay R, Sood AK (2005) Effect of silica colloids on the rheology of viscoelastic gels formed by the surfactant cetyl trimethylammonium tosylate. J Colloid Interface Sci 283:585–591
Helgeson ME, Hodgdon TK, Kaler EW, Wagner NJ, Vethamuthu M, Ananthapadmanabhan KP (2010) Formation and rheology of viscoelastic “double networks” in wormlike micelle—nanoparticle mixtures. Langmuir 26:8049–8060
Nettesheim F, Liberatore MW, Hodgdon TK, Wagner NJ, Kaler EW, Vethamuthu M (2008) Influence of nanoparticle addition on the properties of wormlike micellar solutions. Langmuir 24:7718–7726
Kamibayashi M, Ogura H, Otsubo Y (2008) Shear-thickening flow of nanoparticle suspensions flocculated by polymer bridging. J Colloid Interface Sci 321:294–301
Yang J, Zhao J-J, Han C-R, Duan JF (2014) Keys to enhancing mechanical properties of silica nanoparticle composites hydrogels: the role of network structure and interfacial interactions. Compos Sci Technol 95:1–7
Bluestein BR, Hilton CL (1982) Amphoteric surfactants. Marcel Dekker, New York
Cates ME (1987) Reptation of living polymers: dynamics of entangled polymers in the presence of reversible chain-scission reactions. Macromolecules 20:2289–2296
Cates ME, Candau SJ (1990) Statics and dynamics of worm-like surfactant micelles. J Phys Condens Matter 2:6869–6892
Cates ME, Turner MS (1990) Flow-induced gelation of rodlike micelles. Europhys Lett 11(7):681–686
Magid LJ (1998) The surfactant-polyelectrolyte analogy. J Phys Chem B 102:4064–4074
Rehage H, Hofman H (1991) Viscoelastic surfactant solutions: model systems for rheological research. Mol Phys 74:933–973
Kumar R, Kalur GC, Ziserman L, Danino D, Raghavan SR (2007) Wormlike micelles of a C22-tailed zwitterionic betaine surfactant: from viscoelastic solutions to elastic gels. Langmuir 23:12849–12856
Maitland GC (2000) Oil and gas production. Curr Opin Colloid Interface Sci 5:301–311
Ridout G, Hinz RS, Hostynek JJ, Reddy AK, Wiersema RJ, Hodson CD, Lorence CR, Guy RH (1991) The effects of zwitterionic surfactants on skin barrier function. Fundam Appl Toxicol 16:41–50
Chang SY, Zheng N-Y, Chen C-Sh, Chen C-D, Chen Y-Y, Wang CRC (2007) Analysis of peptides and proteins affinity-bound to iron oxide nanoparticles by MALDI MS. J Am Soc Mass Spectrom 18:910–918
Schulze C, Schaefer UF, Ruge CA, Wohlleben W, Lehr C-M (2011) Interaction of metal oxide nanoparticles with lung surfactant protein A. Eur J Pharm Biopharm 77:376–383
Nam J, Won N, Bang J, Jin H, Park J, Jung S, Jung S, Park Y, Kim S (2013) Surface engineering of inorganic nanoparticles for imaging and therapy. Adv Drug Deliv Rev 65:622–648
Wang Z, Lam A, Acosta E (2013) Suspensions of iron oxide nanoparticles stabilized by anionic surfactants. J Surfactants Deterg 16:397–407
Atta AM, Al-Lohedan HA (2014) Influence of nonionic rosin surfactants on surface activity of silica particles and stability of oil in water emulsions. J Surfactants Deterg 17:1043–1053
Gaynanova GA, Valiakhmetova AR, Kuryashov DA, Kudryashova YuR, Lukashenko SS, Syakaev VV, Latypov ShK, Bukharov SV, Bashkirtseva NYu, Zakharova LYa (2013) The self-organization and functional activity of binary system based on erucyl amidopropyl betaine—alkylated polyethyleneimine. Chem Phys Lett 588:145–149
Fan A, Somasundaran P, Turro NJ (1997) Adsorption of alkyltrimethylammonium bromides on negatively charged alumina. Langmuir 13:506–510
Somasundaran P, Fuerstenau DW (1966) Mechanisms of alkyl sulfonate adsorption at the alumina-water interface. J Phys Chem 70:90–96
Wang W, Gu B, Liang L, Hamilton WA (2004) Adsorption and structural arrangement of cetyltrimethylammonium cations at the silica nanoparticle-water interface. J Phys Chem B 108:17477–17483
Acknowledgments
We are grateful to the Russian Foundation for Basic Research (Grant No. 15-43-02490) for financial support.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Gaynanova, G.A., Valiakhmetova, A.R., Kuryashov, D.A. et al. Mixed Systems Based on Erucyl Amidopropyl Betaine and Nanoparticles: Self-Organization and Rheology. J Surfact Deterg 18, 965–971 (2015). https://doi.org/10.1007/s11743-015-1743-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-015-1743-1