Abstract
Portal vein thrombosis (PVT) and acute variceal bleeding (AVB) are frequent complications of cirrhosis. The efficacy, safety, and timing of anticoagulant treatment in cirrhotic patients with PVT and AVB are contentious issues. We aimed to establish the safety and efficacy of initiating nadroparin calcium–warfarin sequential (NWS) anticoagulation therapy early after esophageal variceal band ligation within PVT patients having cirrhosis and AVB. Cirrhotic patients having AVB and PVT who underwent EVL were included and randomly allocated to either the NWS therapy group (1-month nadroparin calcium by subcutaneous injection following 5-month warfarin through oral administration, n = 43) or the control group (without any anticoagulation therapy, n = 43). The primary endpoint was the rate of PVT recanalization. Secondary endpoints included major bleeding events mainly referring to variceal rebleeding (5-day failure, 14-day, 4-week, 6-week, and 6-month rebleeding rates) and mortality after EVL. The overall recanalization (complete and partial) rate in the NWS therapy group was significantly higher than that in the control group (67.4% vs. 39.5%, P = 0.009). Low Child–Pugh score (P = 0.039, OR: 0.692, 95% CI 0.488–0.982), D-dimer < 2.00 ug/mL (P = 0.030, OR: 3.600, 95% CI 1.134–11.430), and NWS anticoagulation therapy (P = 0.002, OR: 4.189, 95% CI 1.660–10.568) were the predictors of PVT recanalization through univariate analysis of binary logistic regression. NWS anticoagulation therapy (P = 0.003, OR: 4.506, 95% CI 1.687–12.037) was the independent factor of recanalization through multivariate analysis. Nobody bled except for variceal rebleeding. Five-day failure and 14-day rebleeding were zero. There were no significantly different in 4-week (2.3% vs. 4.7%, P = 1.000), 6-week (4.7% vs. 9.3%, P = 0.672) and 6-month rebleeding (18.6% vs. 20.9%, P = 0.787) between the two groups. There was no mortality during six months follow-up. Low serum albumin (P = 0.011, OR: 0.844, 95% CI 0.741–0.962), high MELD score (P = 0.003, OR: 1.564, 95% CI 1.167–2.097) and Child–Pugh score (P = 0.006, OR: 1.950, 95% CI 1.206–3.155) were predictors of rebleeding by univariate analysis of binary logistic regression analysis. The Child–Pugh score (7 [6–8] vs. 6 [5–7], P = 0.003) and albumin levels (33.93 ± 5.30 vs. 37.28 ± 4.32, P = 0.002) were improved in the NWS therapy group at six months. In PVT patients with cirrhosis and AVB, starting NWS anticoagulation therapy early after EVL was safe and effective. It has the potential to raise albumin levels and improve liver function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-neoplastic portal vein thrombosis (PVT) and acute variceal bleeding (AVB) are common complications of liver cirrhosis. AVB is a medical emergency with 15–20% mortality at 6 weeks [1], and 60–70% rebleeding within 2 years [2] without effective treatment and preventive measures. PVT can induce portal venous flow obstruction, resulting in higher portal hypertension and risk of rebleeding. A systematic review observed a higher rate of 5-day failure, 14-day, 6-week, and 1-year rebleeding in cirrhotic PVT patients [3]. Our team recently confirmed that PVT was associated with increased 14-day and 6-week rebleeding in AVB patients following esophageal variceal band ligation (EVL) [4].
Anticoagulation therapy was the first-line therapy for PVT, and studies have proved its safety and efficacy in cirrhotic patients [5,6,7]. However, early diagnosis and treatment are key factors for the successful management of PVT in cirrhosis [8, 9]; anticoagulation must always be started after implementing adequate prophylaxis for gastrointestinal bleeding [10]. The crucial question is whether initiating anticoagulation therapy early after EVL is safe in cirrhotic PVT patients with AVB.
Most published studies have used LMWH or warfarin as anticoagulant drugs. Previous research by our teams demonstrated that 6-month nadroparin calcium–warfarin sequential (NWS) anticoagulation therapy was safe and effective in cirrhotic PVT patients, with PVT repermeation rate increasing from 34.4 to 62.5% and albumin level increasing [5]. Nevertheless, it is unknown whether the strategy is proper in PVT patients with cirrhosis and AVB after EVL.
This study aims to confirm the safety and efficacy of starting nadroparin calcium warfarin sequential (NWS) anticoagulation therapy early after EVL in PVT patients with cirrhosis and AVB.
Methods
The present study was a multi-centric, single-blinded randomized controlled trial evaluating NWS anticoagulation therapy's safety and efficacy among PVT patients with cirrhosis and AVB. The study protocol was approved by the Medical Ethics Committee of Qilu Hospital of Shandong University. The informed consent was signed by all participants. The trial was registered on ClinicalTrials.gov as NCT04976543.
Patients and groups
All the cirrhotic patients admitted at Qilu Hospital of Shandong University, Liaocheng People’s Hospital, and Taian City Central Hospital combined with AVB and PVT were prospectively and consecutively estimated within this study. The inclusion criteria were as follows: liver cirrhosis (as determined by the clinical, laboratory, and imaging examinations or liver biopsies); age between 18 and 75 years; PVT diagnosed using abdominal contrast-enhanced computed tomography (CT) or contrast-enhanced magnetic resonance angiography (MRI) before endoscopy; endoscopy-proven esophageal variceal bleeding (EVB) and treated with EVL with or without gastric variceal obstruction. The exclusion criteria were: uncontrolled active bleeding, hepatocellular carcinoma or other extrahepatic malignancy, previous TIPS treatment, the cavernous transformation of the portal vein, platelet count < 10 × 10^9/L, creatinine > 170 µmol/L, Budd–Chiari syndrome, pregnancy or breast-feeding period and any on-going anticoagulation treatment.
Eligible patients from the three hospitals were randomly divided into two equal groups: the NWS and control groups. Randomized patients were grouped using a computer-based random number table procedure by a specialized researcher. The numbers produced were put in a sealed envelope and then successively sent to clinicians during enrollment. Patients and clinicians providing the interventions were not blinded, while clinicians assessing the imaging and analyzing the data were blinded to the group allocation and the coded data of the patients.
Procedure
To achieve hemodynamic stability, all AVB patients were immediately resuscitated. Meanwhile, the patients were given prophylactic antibiotics, proton pump inhibitors (PPIs), and vasoactive drugs during their admission. When the patients' hemodynamics were stable, they underwent contrast-enhanced CT or MRI to determine PVT, followed by esophagogastroduodenoscopy [11]. Close loop ligatures were recommended and performed by physicians with more than three years of experience with the endoscopic procedure.
EVL was performed using the Cook or Boston Scientific Medi-Tech. Operators decided the number of bands based on the conditions of varices, and N-butyl cyanoacrylate was injected into gastric varices if necessary. Endoscopic therapy was performed every 28 days until the varices were eradicated, they were unsuitable for medicine, they died, or they were converted to another treatment (i.e., liver transplantation or TIPS).
Carvedilol treatment began 24 h after EVL with an initial dose of 6.25 mg daily, and doses were gradually increased until 55 beats per minute (bpm) or a 25% decrease in heart rate was achieved. All patients in the NWS group began anticoagulation therapy 48 h after EVL and received a monthly subcutaneous injection of nadroparin calcium 1 mg/kg. Then oral warfarin was provided continuously for five months. Warfarin was initiated five days before nadroparin calcium was stopped. The international normalized ratio (INR) was monitored every 3–4 days, and the warfarin dose was carefully adjusted until 2–3. Anticoagulation therapy was stopped three days before the subsequent endoscopic treatment. Patients in the control group did not receive any anticoagulation therapy.
Data collection and definitions
Clinical variables such as patient demographics, past medical and surgical history, ascites, laboratory, imaging, and endoscopic data were collected during admission. Clinical ascites were either absent or present. Cirrhosis severity and liver function were assessed through the end-stage liver disease score (MELD) model and the Child–Pugh score based on formulas described previously [12, 13].
PVT was diagnosed based on the absence of blood flow in part or whole of the lumen of the splenoportomesenteric axis, with solid material in the vein by imaging (upper abdominal contrast-enhanced CT or MRI). The site of PVT (within the portal trunk, the portal branch, both in the trunk and branches) and the extent of the portal vein system (SV, SMV, or both) were recorded [14]. The vessel and residual patent lumen were outlined at the maximum thrombosis level for each venous segment. Commercial software was used to calculate the total and patent lumen area [15]. Thrombus occlusion was computed as a percentage of the entire lumen area. Complete thrombosis was defined as equal to or greater than 90% thrombotic material presence within vessels. Otherwise, it was described as partial thrombosis [5].
According to the Japanese Research Society for Portal Hypertension classification, forms of esophageal varices were classified as F1, F2, or F3. Red signs included red whale marks, cherry-red spots, or hematocysts, while bleeding signs included gushing bleeding, spurting bleeding, oozing bleeding, a red plug, or a white plug [16]. Gastric varices were classified depending on the method of Sarin et al. [17]. Other gastrointestinal lesions (i.e., peptic ulcer or portal hypertensive gastropathy) were recorded simultaneously.
Follow-up
Follow-up visits were scheduled at 1, 3, and 6 months or whenever portal hypertension recurred, containing clinical, biochemical, and imaging evaluations. After 6 months, liver function and imaging data were re-evaluated (end of the anticoagulation therapy). The follow-up began on the EVL date and ended in May 2022, or on the death day or the day of the last visit in the 6th month. When a severe bleeding episode occurred, the anticoagulation treatment was discontinued.
Endpoints and definitions
The primary endpoint was the overall recanalization rate, defined as the fraction of patients who had complete or partial recanalization. Portal vein recanalization was assessed through imaging. Complete recanalization was defined as the complete disappearance of the intravenous thrombus. Partial recanalization refers to reduced thrombus size ≥ 50% and thrombus without extending to other veins. No response or stable thrombosis was considered when the thrombus had the same volume, decreased < 50%, or increased < 30% on the cross-section. Progression was defined when the thrombus size increased by > 30% or extended to other segments [5]. The second endpoint was severe bleeding and death. Major bleeding was defined as fatal bleeding in a critical area or organ, such as the upper gastrointestinal (GI), pericardial, or intracranial regions, with the accompanied syndrome and/or a ≥ 2 g drop in hemoglobin or requiring transfusion of ≥ 2U red cells [18]. Variceal rebleeding was defined as any significant hematemesis or melena (i.e., with a ≥ 2 g drop in hemoglobin and proved by re-endoscopy) after a 24-h period of stable vital signs and post-treatment hemoglobin. Five-day failure was a composite outcome, including death, failure to control bleeding (FTCB), and rebleeding within five days. 14-day, 4-week, 6-week, and 6-month rebleeding was defined as rebleeding within 14 days, four weeks, six weeks, and six months after EVL, respectively. During the six-month follow-up, mortality was defined as death from any cause of illness related to liver dysfunction. Anticoagulation therapy initiated within 48 h of EVL was referred to as anticoagulation early.
Group size calculation
The overall recanalization rate of PVT at the sixth month was selected as the primary outcome of the computed group size. Twenty-seven patients in each group were required to detect a difference of 0.30 and achieve 80% power at a 5% significance level, assuming a recanalization rate of 70% in the anticoagulation group and 40% in the control group, depending on previous studies [19]. Thirty patients were included in each group considering a 10% dropout rate.
Statistical analysis
The statistical software IBM SPSS Statistical 21.0 was used for the analysis (SPSS, Chicago, IL, US). Intention-to-treat (ITT) and per-protocol (PP) analyses were used to compare the outcomes of the groups. ITT analysis was performed on all eligible patients, and PP analysis was performed on patients who completed the program.
Quantitative data were expressed as mean ± SD and were assessed through the Student’s t test. As appropriate, categorical variables were expressed as percentages and compared using the Chi-square or Fisher exact test. Univariate and multivariate logistic regression analyses were used to identify predictors of recanalization and rebleeding. All the tests were two-sided, and a P value < 0.05 was considered statistically significant.
Results
Patients
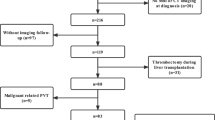
Recruitment was implemented from February 2020 to December 2021, and the final follow-up was completed in May 2022. A total of 148 patients were identified at the beginning. Sixty-two met the exclusion criteria, while 86 were included and randomly separated into two groups (Fig. 1). One patient dropped out of the NWS group at the second month, and another discontinued anticoagulation therapy due to intestinal perforation and underwent partial small intestinal resection at the third month. One patient in the control group rebled on day 22 and was admitted again but dropped out later. The other quit after the third month. Finally, 82 patients completed the trial (Fig. 1). Baseline characteristics were comparable between the groups through ITT analysis (Table 1).
Portal vein recanalization
The PVT outcomes in patients who dropped out were considered as no response for the NWS group and recanalization in the control group. In the ITT analysis, the overall recanalization (partial or complete) was observed in 29/43 (67.4%) patients in the NWS group compared with 17/43 (39.5%) within the control group. The difference was significant (P = 0.009). The PVT progression rate in the NWS group was 5/43 (11.5%), which was lower than the control group's rate of 12/43 (27.9%) (P = 0.058). In the PP analysis, the odds of overall recanalization were higher in the NWS therapy group (70.7% vs. 36.6%, P = 0.002), while the odds of PVT progression were lower (7.3% vs. 29.3%, P = 0.010) (Table 2).
Child–Pugh score (P = 0.039, odds ratio [OR]: 0.692; 95% confidence interval [CI]: 0.488–0.982), D-dimer < 2.00 ug/mL (P = 0.030, OR: 3.600; 95% CI 1.134–11.430), and NWS anticoagulation therapy (P = 0.002, OR: 4.189; 95% CI 1.660–10.568) were related to the portal vein recanalization (partial or complete) within the univariate analysis of binary logistic regression. In the multivariate analysis, only the NWS anticoagulation therapy (P = 0.003, OR: 4.506; 95% CI 1.687–12.037) was characterized as an independent predictor of recanalization (Table 3).
Safety
During the 6-month follow-up, all patients (n = 43) completed the nadroparin calcium therapy, and no patients experienced other sites of bleeding except for variceal rebleeding. Five-day failure and 14-day rebleeding were both zero. Four-week (2.3% vs. 4.7%, P = 1.000), six-week (4.7% vs. 9.3%, P = 0.672), and six-month rebleeding rates (18.6% vs. 20.9%, P = 0.787) rebleeding rates (18.6% vs.20.9%, P = 0.787) were all similar between the two groups. In the PP analysis, there were also no significantly different in the rates of four-week (2.4% vs. 2.4%, P = 1.000), six-week (4.9% vs. 7.3%, P = 1.000), and six-month rebleeding (14.6% vs. 19.5%, P = 0.557) between the two groups (Table 2). No patient died during the six months of follow-up.
Child–Pugh score (P = 0.006, OR: 1.950; 95% CI 1.206–3.155), MELD score (P = 0.003, OR: 1.564; 95% CI 1.167–2.097), and albumin levels (P = 0.011, OR: 0.844; 95% CI 0.741–0.962) were associated with rebleeding in the univariate analysis of binary logistic regression. However, in the multivariate analysis, we were unable to identify an independent predictor of rebleeding (Table 3).
Impact on liver function
Compared with the baseline, the Child–Pugh score at the sixth month was better in both the NWS therapy group (7 [6–8] vs. 6 [5–7], P = 0.003) and the control group (7 [6–8] vs. 7 [6–8], P = 0.267). However, the difference was significant only in the NWS therapy group. Similarly, albumin levels significantly increased only in the NWS therapy group (33.93 ± 5.30 vs. 37.28 ± 4.32, P = 0.002) compared to the control group (34.27 ± 4.61 vs. 35.57 ± 4.48, P = 0.199). However, the MELD score in both the NWS group (9 [8–10] vs. 9 [7–10], P = 0.104) and the control group (10 [9–12] vs. 9 [8–11], P = 0.124) showed no significant improvement (Fig. 2).
Discussion
Our findings show that starting nadroparin calcium anticoagulation therapy within 48 h of EVL is both safe and effective in PVT patients with cirrhosis and AVB. It also resulted in improved liver function. This is the first prospective randomized trial to investigate the effect of anticoagulation therapy on PVT in cirrhotic patients with AVB after EVL.
AVB is a severe complication of liver cirrhosis with a high rate of recurrence and death. For AVB, most adverse events (i.e., death, failure to control bleeding, and rebleeding) occur within the first five days and are defined as a 5-day failure [4]. Therefore, clinicians continue to face difficulties in initiating anticoagulation early in PVT patients with cirrhosis and AVB. We started anticoagulation as soon as 48 h after EVL, and none of the patients experienced 5-day failure. Another concerns with anticoagulant therapy in cirrhotic patients with AVB after EVL is that it may increase the risk of post-banding ulcer bleeding (PBUB), with a mean of 12 ± 5.6 days after variceal ligation [20]. In this study, no patients in the NWS group rebled within 14 days after EVL, indicating that nadroparin calcium anticoagulation therapy was safe. It was supported by a recent study, which pointed out that hepatocellular carcinoma and poor liver function were associated with a higher risk of PBUB [20]. Moreover, the rate of four-week, six-week, and 6-month rebleeding in the NWS group was also similar to the control group, depicting that NWS anticoagulation therapy was safe in PVT patients having cirrhosis and AVB.
Further analysis showed that Child–Pugh score, MELD score, and albumin levels were associated with 6-month rebleeding. The Child–Pugh and MELD scores are two of the most important scores, which are widely used to evaluate the severity of liver dysfunction and predict prognosis in cirrhosis [21,22,23]. Albumin is predominantly synthesized in the liver, with several functions such as anti-oxidant, anti-inflammation, modulation of hemostasis, etc. [24]. Hypoalbuminemia causes damage to the functions listed above, resulting in circulatory dysfunction, bacterial spontaneous peritonitis, and a high rate of variceal rebleeding. According to the findings, variceal rebleeding was more associated with liver function than anticoagulation therapy.
It is worth noting that rebleeding rates in this study were lower than that in our previous study [4]. It perhaps because first, the published article had shown that higher Child–Pugh score, lower albumin levels, and more bands were risk factors for 14-day and six-week rebleeding [4], while patients in this study had better liver function and fewer bands. Second, close-loop ligatures were recommended and performed by more experienced physicians in this study. Moreover, carvedilol was administrated to patients, which further decreased the rebleeding rates.
Despite the fact that variceal bleeding was serious and had a high mortality rate, no one died during the six-month follow-up period in this study. It was primarily due to our patients' relatively good liver function reserves, with 96.5% of them having Child–Pugh A/B. Furthermore, the treatment and preventive measures were effective, potentially lowering rebleeding and mortality rates. However, the Child–Pugh C patients were too few to evaluate the safety and efficacy of anticoagulation in this population. Consequently, future studies with more Child–Pugh C patients are required.
According to a previous study, the rate of spontaneous recanalization of PVT ranged from 25 to 48% [15, 25] and can increase to 51–83% under anticoagulant therapy [5, 7, 19]. In this study, the recanalization rate was 67.4% and 39.5% in the NWS and control groups. The anticoagulation group had a significantly higher rate of PVT recanalization, indicating that 6-month NWS therapy was effective. The PVT progression rate in the control group was as high as 27.9%, whereas it was only 11.6% in the NWS therapy group. The results were in line with the recent meta-analysis of Loffredo et al. [19], which reported a 9% rate of progression during therapy and 33% for untreated patients. Therefore, anticoagulation therapy becomes necessary.
Further analysis revealed that NWS anticoagulation therapy, lower Child–Pugh score, and D-dimer < 2 µg/mL were related to PVT recanalization, while NWS anticoagulation therapy was the independent predictor of PVT recanalization. Existing articles observed that Child–Pugh score and D-dimer were associated with PVT recanalization [5, 26], which were also observed in this study. Advanced liver disease [27] and an increased D-dimer concentration [28] were associated with a higher risk of PVT formation in liver cirrhosis. On the contrary, lower Child–Pugh scores and D-dimer were prone to recanalization among PVT patients.
Studies reported that anticoagulation therapy could increase albumin levels and improve the Child–Pugh score [5, 9], as observed in this study. It is direct evidence that anticoagulation therapy is effective in improving liver function. The result should be ascertained by more extensive prospective studies in PVT patients with cirrhosis in the future.
NWS therapy is safe and readily accepted by cirrhotic patients [5]. All AVB patients in the NWS therapy group completed 1 month of nadroparin calcium after EVL, with only one patient dropping out during the 5-month warfarin therapy. The findings suggested that NWS therapy could be easily accepted by AVB patients, resulting in high compliance.
Finally, it is interesting to note that comparable variceal rebleeding rates were found between the two groups during follow-up despite higher portal vein recanalization rates in NWS in this study. Similar results were found in recent research [25, 29]. A series of studies indicated that liver function was the most important predictor for rebleeding [4, 11, 30] rather than PVT. Though anticoagulation can improve liver function, the effect during 6th month was not obvious enough to decrease the risk of rebleeding. Furthermore, a large proportion of patients had partial portal vein recanalization, which had a limited effect on reducing portal vein hypertension and the risk of rebleeding. More research with a longer duration of follow-up and anticoagulation therapy is needed.
This research has some limitations. For starters, this study lacked a later anticoagulation therapy group. Anticoagulation was typically initiated following varices removal [31]. However, varices are easy to relapse, which made varices eradication difficult. Second, this study did not investigate direct oral anticoagulants (DOAC). DOAC have been licensed for atrial fibrillation and treatment/prophylaxis of venous thromboembolism, but no information is available on cirrhotic patients [10]. Third, the 6-month follow-up period was inadequate to assess the effect of anticoagulation therapy on rebleeding and the recurrence rate of PVT in patients who achieved recanalization after 6 months. A recent retrospective study of 122 cirrhotic PVT patients followed for a median of 44.1 (14–79.1) months discovered that the anticoagulation therapy group had a lower rate of variceal bleeding. [9]. In another retrospective study of 214 cirrhotic PVT patients, the median time of PVT recurrence following anticoagulation therapy discontinuation was 9.2 (6.4–11.3) months [29]. Further studies with more patients and a longer time of follow-up are necessary. Fourth, there were only three Child–Pugh C patients, and the safety and efficacy of NWS therapy in advanced cirrhotic patients had not been fully assessed. Future studies with more Child–Pugh C patients are needed.
Finally, starting NWS anticoagulation therapy within 48 h of EVL was safe and effective in PVT patients with cirrhosis and AVB. Furthermore, it may raise albumin levels and improve liver function. Future research with more advanced cirrhosis patients and a longer follow-up period are required.
References
Zhao Y, Ren M, Lu G et al (2020) The prognosis analysis of liver cirrhosis with acute variceal bleeding and validation of current prognostic models: a large scale retrospective cohort study. Biomed Res Int 2020:1–7
Kovacs TOG, Jensen DM (2019) Varices. Clin Liver Dis 23:625–642
Qi X, Su C, Ren W et al (2015) Association between portal vein thrombosis and risk of bleeding in liver cirrhosis: a systematic review of the literature. Clin Res Hepatol Gas 39:683–691
Gao Z, Zhao J, Liu X et al (2021) Portal vein thrombosis associated with high 14-day and 6-week rebleeding in patients after oesophageal variceal band ligation: a retrospective, multicentre, nested case-control study. Hepatol Int 15:1183–1195
Zhou T, Sun X, Zhou T et al (2020) Efficacy and safety of nadroparin calcium-warfarin sequential anticoagulation in portal vein thrombosis in cirrhotic patients: a randomized controlled trial. Clin Transl Gastroen 11:e228
Hayashi T, Takatori H, Horii R et al (2019) Danaparoid sodium-based anticoagulation therapy for portal vein thrombosis in cirrhosis patients. Bmc Gastroenterol 19:217
Noronha Ferreira C, Reis D, Cortez-Pinto H et al (2019) Anticoagulation in cirrhosis and portal vein thrombosis is safe and improves prognosis in advanced cirrhosis. Digest Dis Sci 64:2671–2683
Rodriguez-Castro KI, Vitale A, Fadin M et al (2019) A prediction model for successful anticoagulation in cirrhotic portal vein thrombosis. Eur J Gastroen Hepat 31:34–42
Scheiner B, Stammet PR, Pokorny S et al (2018) Anticoagulation in non-malignant portal vein thrombosis is safe and improves hepatic function. Wien Klin Wochenschr 130:446–455
EASL Clinical Practical Guidelines (2016) Vascular diseases of the liver. J Hepotol 64:179–202
de Franchis R, Bosch J, Garcia-Tsao G et al (2022) Baveno VII–Renewing consensus in portal hypertension. J Hepatol 76:959–974
Wiesner R (2001) MELD and PELD: application of survival models to liver allocation. Liver Transplant 7:567–580
Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R (1973) Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 60:646–649
Sarin SK, Philips CA, Kamath PS, Choudhury A, Maruyama H, Nery FG, Valla DC (2016) Toward a comprehensive new classification of portal vein thrombosis in patients with cirrhosis. Gastroenterology 151:574–577
Angelo Luca M, Settimo Caruso M, Mariapina Milazzo M et al (2012) Natural course of extrahepatic nonmalignant partial portal vein thrombosis in patients with cirrhosis. Radiology 265:124–132
Tajiri T, Yoshida H, Obara K et al (2010) General rules for recording endoscopic findings of esophagogastric varices (2nd edition). Digest Endosc 22:1–9
Sarin SK, Lahoti D, Saxena SP et al (1992) Prevalence, classification and natural history of gastric varices: a long-term follow-up study in 568 portal hypertension patients. Hepatology (Baltimore, MD) 16:1343–1349
Hanafy AS, Abd-Elsalam S, Dawoud MM (2019) Randomized controlled trial of rivaroxaban versus warfarin in the management of acute non-neoplastic portal vein thrombosis. Vasc Pharmacol 113:86–91
Loffredo L, Pastori D, Farcomeni A, Violi F (2017) Effects of anticoagulants in patients with cirrhosis and portal vein thrombosis: a systematic review and meta-analysis. Gastroenterology 153:480–487
Dueñas E, Cachero A, Amador A et al (2020) Ulcer bleeding after band ligation of esophageal varices: risk factors and prognosis. Digest Liver Dis 52:79–83
Krige J, Spence RT, Jonas E et al (2020) A new recalibrated four-category Child-Pugh score performs better than the original Child-Pugh and MELD scores in predicting in-hospital mortality in decompensated alcoholic cirrhotic patients with acute variceal bleeding: a real-world cohort analysis. Wrld J Surg 44:241–246
Tantai X, Liu N, Yang L et al (2019) Prognostic value of risk scoring systems for cirrhotic patients with variceal bleeding. World J Gastroentero 25:6668–6680
Fortune BE, Garcia-Tsao G, Ciarleglio M et al (2017) Child-Turcotte-Pugh class is best at stratifying risk in variceal hemorrhage. J Clin Gastroenterol 51:446–453
Carvalho JR, Machado MV (2018) New insights about albumin and liver disease. Ann Hepatol 17:547–560
Pettinari I, Vukotic R, Stefanescu H et al (2019) Clinical impact and safety of anticoagulants for portal vein thrombosis in cirrhosis. Am J Gastroenterol 114:258–266
Cui S, Shu R, Yan S et al (2015) Efficacy and safety of anticoagulation therapy with different doses of enoxaparin for portal vein thrombosis in cirrhotic patients with hepatitis B. Eur J Gastroentrol Hepat 27:914–919
Yan Zhang BXXW, Zhong-ji Meng YGZQ, Shang HLSW et al (2020) Prevalence and clinical significance of portal vein thrombosis in patients with cirrhosis and acute decompensation. Clin Gastroenterol H 18:2564–2572
Dai J, Qi X, Li H, Guo X (2015) Role of D-dimer in the development of portal vein thrombosis in liver cirrhosis: a meta-analysis. Saudi J Gastroentero 21:165–174
Naymagon L, Tremblay D, Zubizarreta N et al (2021) Safety, efficacy, and long-term outcomes of anticoagulation in cirrhotic portal vein thrombosis. Dig Dis Sci 66:3619–3629
Trebicka J, Gu W, Ibanez-Samaniego L et al (2020) Rebleeding and mortality risk are increased by ACLF but reduced by pre-emptive TIPS. J Hepatol 73:1082–1091
Lv Y, Qi X, He C et al (2018) Covered TIPS versus endoscopic band ligation plus propranolol for the prevention of variceal rebleeding in cirrhotic patients with portal vein thrombosis: a randomised controlled trial. Gut 67:2156–2168
Funding
There is no financial support of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Zhanjuan Gao, Shanshan Li, Jingrun Zhao, Jinhou Li, Yanjing Gao declare that there is no conflict of interest. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Human and animal rights
The study was approved by the local Ethical Committee.
Informed consent
Patients gave informed consent to participate.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, Z., Li, S., Zhao, J. et al. Anticoagulation therapy early is safe in portal vein thrombosis patients with acute variceal bleeding: a multi-centric randomized controlled trial. Intern Emerg Med 18, 513–521 (2023). https://doi.org/10.1007/s11739-023-03206-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-023-03206-x