Abstract
Background and aims
The association between prognosis of variceal bleeding and portal vein thrombosis (PVT) is unclear. In this multicentre study, we determined the effect of PVT on rebleeding and mortality in patients with acute variceal bleeding (AVB) after oesophageal variceal band ligation (EVL).
Methods
Cirrhotic patients with AVB who had undergone EVL were included. The patients were allocated to either the PVT group or the control cirrhotic group (CCG) based on the presence or absence of PVT. One-year rebleeding episodes and mortality after EVL were recorded.
Results
A total of 218 cirrhotic patients with AVB from 3 centres were included. Patients with PVT had a higher rate of 14-day and 6-week rebleeding than those without PVT (14-day: 8.26% vs. 1.83%, p = 0.03; 6-week: 11.92% vs. 1.83%, p = 0.003). The rates of 5-day failure (3.67% vs. 0.92%, p = 0.175), 1-year rebleeding (21.10% vs. 20.18%, p = 0.867), and 14-day, 6-week, and 1-year mortality were similar between the groups (14-day: 3.67% vs. 0.92%, p = 0.175; 6-week: 3.67% vs. 0.92%, p = 0.175; 1-year: 3.67% vs. 1.83%, p = 0.408). The Child–Pugh class [p = 0.022, hazard ratio (HR): 1.453; 95% confidence interval (CI) 1.056–1.998], PVT (p = 0.050, HR: 4.622, 95% CI 0.999–21.395), albumin < 30 g/L (p = 0.023, HR: 5.886, 95% CI 1.272–27.245), and number of bands (p = 0.010, HR: 1.207, 95% CI 1.046–1.393) were identified as the predictors for 14-day rebleeding; the multivariate analysis revealed only the number of bands (p = 0.009, HR: 1.247, 95% CI 1.056–1.473) as the independent factor. PVT (p = 0.012, HR: 6.732, 95% CI 1.519–29.835) and albumin < 30 g/L (p = 0.027, HR: 3.643, 95% CI 1.160–11.441) were identified as predictors for 6-week rebleeding; however, only PVT (p = 0.015, HR: 6.380, 95% CI 1.427–28.515) was found to be the independent factor in the multivariate analysis. Further analysis showed that superior mesenteric vein (SMV) thrombosis is the only risk factor predicting 6-week rebleeding in patients with PVT (p = 0.032, HR: 3.405, 95% CI 1.112–10.429).
Conclusions
PVT was associated with high 14-day and 6-week rebleeding in patients after EVL. SMV thrombosis was the only risk factor for 6-week rebleeding in patients with PVT. High albumin levels may serve as a protective factor for the 14-day and 6-week rebleeding risk. PVT was not responsible for mortality after EVL during 1-year follow-up.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute variceal bleeding (AVB) is a serious complication of liver cirrhosis, with a high risk of rebleeding and mortality. Despite the appropriately administered resuscitation measures such as oxygen inhalation, fluid resuscitation, blood transfusions, and medication administration, along with balloon tamponade or emergency endoscopic treatment, or even transjugular intrahepatic portosystemic stent-shunt (TIPSS) or surgery, when necessary, the in-hospital rebleeding and mortality rates were reported to be 20.3% and 10.6%, respectively, in a study[1]. The mortality rate at 6 weeks was reported to be 10–20% [2], and the 1-year AVB recurrence rate was reported to be approximately 60% in patients without prophylactic treatment [3]. Studies have recommended using oesophageal variceal band ligation (EVL) for acute EV bleeding [2, 4].
Portal vein thrombosis (PVT) is a critical and frequent complication of liver cirrhosis. It is defined as the formation of thrombus in the portal vein trunk involving right and/or left branches, which may extend to the superior mesenteric vein (SMV) and splenic vein (SV) [5].The prevalence of nonmalignant PVT increases with the degree of liver failure, usually ranging from 0.6 to 23 [6, 7]. In patients with cirrhosis, PVT is closely associated with static portal blood flow from advancing portal hypertension (PH) [8]. Abdominal surgery and invasive procedures, especially splenectomy and endoscopic sclerotherapy, can effectively promote the development of PVT [7].
Hepatic vein pressure gradient (HVPG) is the gold standard for diagnosing PH [9]. It helps in determining the risk of the development of varices and first or recurrent bleeding [10]. A HVPG of ≤ 12 mmHg indicates no bleeding risk [11]. In patients who have had an episode of variceal bleeding, HVPG ≥ 20 mmHg indicates the risk of early rebleeding and death [10]. However, the procedure for HVPG measurement is invasive and expensive and is associated with potential complications. A recent study reported that HVPG increased from 5 (0–12) mmHg to 8 (0–14) mmHg after portal vein embolisation in patients with liver cancer. Similarly, elevated HVPG can be caused by PVT-induced obstruction of portal venous flow, often influencing the risk stratification of variceal bleeding.
The association between variceal bleeding prognosis and PVT is poorly understood. Lee et al. [12] concluded that PVT did not considerably affect the incidence of rebleeding. Amitrano et al. [13] highlighted that PVT favoured the relapse of oesophageal varices, but rebleeding can be effectively prevented using standard scheduled band ligations. However, other studies have reported that PVT increased the risk of 5-day failure and results in elevated rebleeding risk at 14 days and 6 weeks in cirrhotic patients with AVB [14,15,16,17]. Zhang et al. [7] reported that the 1-year mortality rate did not differ significantly between patients with and without PVT. Trebicka et al. [17] found that although PVT increased the 6-week and 1-year mortality rate, it was not an independent risk factor.
Limited data are available to confirm that PVT is associated with a high rate of rebleeding and poor outcomes for AVB after EVL, independent of the severity of liver disease. Moreover, no consensus is available regarding an effective treatment for patients with both AVB and PVT.
In this retrospective multicentre study, we determined the effect of PVT on rebleeding and mortality in patients with AVB after EVL. Additionally, we explored the risk factors for rebleeding and death in these patients.
Methods
Patients
This retrospective analysis included data of consecutive patients with AVB treated at Qilu Hospital of Shandong University Chinese People’s Liberation Army No. 960 Hospital and Liaocheng People’s Hospital between January 2016 and December 2019. Eligibility criteria for the patients were: (1) liver cirrhosis (diagnosed by clinical presentations, laboratory tests, images examination, or liver biopsies [18]), (2) age between 18 and 80 years, (3) endoscopy-proven variceal bleeding (VB) and treatment with EVL and/or gastric variceal obturation, and (4) completion of at least 6 weeks of follow-up. The time frame for an acute bleeding episode should be 120 h (5 days), according to Baveno V criteria [19]. Patients with previous treatment with EVL plus nonselective beta‑blockers (NSBBs); TIPSS placement or liver transplantation; hepatocellular carcinoma or other extrahepatic malignancy; myeloproliferative disease and non-cirrhotic PH; and treatment with anticoagulation were excluded. The included patients were allocated to either the PVT group or the control cirrhotic group (CCG) based on the presence or absence of PVT. When a patient with PVT was included, the next cirrhotic patient admitted and comparable for age (± 5 years), sex, and Child–Pugh class was chosen as ‘control’. Prophylactic antibiotics, vasoactive drugs, and proton pump inhibitors (PPIs) were administered to all the patients with cirrhosis presenting with upper gastrointestinal (GI) bleeding at admission. The patients were advised to continue using PPI even after discharge.
Clinical and laboratory data
Demographic data of the included patients, such as age, sex, Child–Pugh score, and primary cause of cirrhosis, were obtained. In addition, details on the history of splenectomy or partial splenic embolism (PSE) and AVB were obtained. Clinical ascites was classified as absent or present. Routine blood tests, including platelet count, hemoglobin, prothrombin time, serum albumin, serum creatinine, international normalised ratio (INR), d-dimer, and bilirubin, were performed at admission. The model for end-stage liver disease score (MELD) was calculated according to the United Network for Organ Sharing formula [20]. Packed red blood cell transfusion and albumin infusion were conservatively performed before the first EVL until the target levels for hemoglobin (70–80 g/L) and albumin (≥ 30 g/L) were achieved.
Endoscopic data
EVL was performed in all the patients as soon as the hemodynamic stability was achieved. Endoscopic tissue adhesives were administered to patients with gastroesophageal varices, if required. According to the Japanese Research Society for Portal Hypertension classification, endoscopic findings of esophagogastric varices were recorded as follows: (1) location: locus superior (Ls) varices, locus medialis (Lm) varices, and locus inferior (Li) varices; (2) form: F1, F2, or F3; (3) red signs: red whale marks, cherry red spots, or haematocysts; (4) bleeding signs: gushing bleeding, spurting bleeding, oozing bleeding, red plug, or white plug [21]. Gastric varices were classified according to the method of Sarin et al. [22]. Tissue adhesives, such as N-butyl cyanoacrylate, are recommended for acute bleeding combined with gastric varices. In the included patients, other gastrointestinal lesions, such as peptic ulcer and portal hypertensive gastropathy, were also recorded. EVL was performed using Cook or Boston Scientific Medi-Tech. The operator decided the number of bands based on varices with signs of bleeding and red plug. EVL was performed every 14–21 days until eradication, evidence of varices not suitable for banding, death, or conversion treatment (liver transplantation, splenectomy, or PSE).
Assessment of PVT
PVT was diagnosed using contrast-enhanced computed tomography scanning or magnetic resonance angiography before endoscopy. Diagnosis was based on the absence of blood flow in part or whole of the lumen of the splenoportomesenteric axis, with the presence of solid material in the vein. Based on the location, PVT was classified into three types [23]: type 1, PVT only in the portal trunk; type 2, PVT only in the portal branch; and type 3, PVT both in the trunk and branches. The degree of portal venous system occlusion was classified as occlusive (no visible flow in the PV lumen in the imaging) or non-occlusive (visible flow in the PV lumen in the imaging). The extent of portal vein system occlusion was classified as SV, SMV, or both [23]. In addition, the PVT duration was classified as chronic or recent. Chronic PVT was defined as previously diagnosed PVT (> 3 months) or portal cavernoma, whereas recent PVT was defined as the latest first PVTs detected (< 3 months), with no indication of chronic PVT [7].
Study endpoints and definitions
The primary endpoint was rebleeding after the first EVL, and the secondary endpoint was all-cause mortality. Failure to control bleeding (FTCB) was defined as the inability to achieve a 24-h bleed-free period after endoscopic treatment. Rebleeding was defined as any significant upper GI bleeding (i.e. with a ≥ 2 g drop in haemoglobin and need for re-endoscopy) occurring after a 24-h period of stable vital signs and hemoglobin post-treatment. The 5-day failure was a composite outcome, which included death, FTCB, and rebleeding within 5 days. Moreover, 14-day, 6-week, and 1-year rebleeding was defined as FTCB or rebleeding within 14 days, 6 weeks, and 1 year of EVL, respectively. Mortality was defined as death from all causes of illness related to liver dysfunction after EVL.
Follow-up
The date of entry was the date of first EVL. The patients were regularly followed up for 1 year until death, conversion treatment (liver transplantation, splenectomy, or PSE), or end of follow-up. None of the patients with PVT were prescribed anticoagulant therapy during the follow-up period to avoid an enhanced bleeding risk.
Statistical analysis
All data were analysed using IBM SPSS Statistical 21.0 (SPSS, Chicago, IL, US). Quantitative data are expressed as mean ± SD and were compared using the Student’s t test. Categorical variables were assessed using the Chi-square test. The Kaplan–Meier analysis was used to estimate the cumulative risk of rebleeding and probability of survival, whereas the log-rank test was used to compare differences between the groups. Univariate and multivariate Cox regression analyses were used to identify independent predictors for 14-day and 6-week rebleeding. Potential risk factors and factors with p < 0.1 in the univariate Cox regression analysis were included in the multivariate analysis to analyse the hazard ratios (HRs). All tests were two sided, and a p value < 0.05 was considered statistically significant.
Results
Baseline characteristics and PVT prevalence in patients with AVB at admission
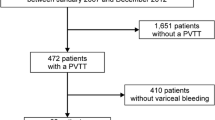
A total of 1376 patients were diagnosed as having liver cirrhosis with AVB. As shown in Fig. 1, 744 patients met the exclusion criteria and the remaining 632 patients were included in the study. Of the included patients, 109 (17.25%) patients had PVT. In addition, 109 patients were allocated to the CCG based on admission time, age, sex, and Child–Pugh class. Baseline characteristics of the included patients are summarised in Table 1. PVT occurred more commonly in the portal vein trunk (71.56%) and was often non-occlusive (89.91%) and recent (84.40%). Most PVTs were resident (59.63%), whereas some extended into the SMV (27.52%), SV (7.34%), or both mesenteric and splenic veins (5.50%). Importantly, a history of splenectomy or PSE was significantly more common in patients with PVT (36.70% vs. 15.60%; p = 0.000). However, no significant difference was observed in the AVB history (45.87% vs. 36.68%; p = 0.169) and incidence of ascites (66.97% vs. 65.14%; p = 0.775) between the groups. On comparing the endoscopic features at admission, we noted that the PVT group had significantly more Ls varices (55.05% vs. 33.94%; p = 0.002) and number of bands (11.41 3.29 vs. 9.84 3.73; p = 0.001).
Rebleeding and mortality after EVL
Patients with PVT had a higher rate of 14-day and 6-week rebleeding than those without PVT (14-day rebleeding: 8.26% vs. 1.83%, p = 0.03; 6-week rebleeding: 11.92% vs. 1.83%, p = 0.003; Table 2). Similarly, the cumulative risk of rebleeding was significantly higher in the PVT group at 14 days [p by log-rank test = 0.031; HR: 4.622, 95% confidence interval (CI) 0.999–21.395, p = 0.050] and 6 weeks (p by log-rank test = 0.004; HR: 6.732, 95% CI 1.519–29.835, p = 0.012; Fig. 2A, B). The rates of 5-day failure (3.67% vs. 0.92%, p = 0.175); 1-year rebleeding (21.10% vs. 20.18%, p = 0.867); 14-day, 6-week, and 1-year mortality (14-day: 3.67% vs. 0.92%, p = 0.175; 6-week: 3.67% vs. 0.92%, p = 0.175; 1-year: 3.67% vs. 1.83%, p = 0.408) were similar between the groups (Table 2).
Of the patients with rebleeding at 6 weeks, 2 patients had bleeding due to FTCB within 24 h of EVL and 1 patient died. Seven patients had postbanding ulcer bleeding (PBUB) between 3 and 10 days, two patients received a second EVL, 1 patient was transferred to the intensive care unit (ICU) for further treatment, and four patients were administered antibiotics, vasoactive drugs, and PPIs (of whom 3 died). Two patients had bleeding from gastric varices 20 days after EVL and received tissue adhesives. Two patients had oesophageal varice rebleeding 30 days after EVL and received a second EVL. Two patients had bleeding, but they refused a second endoscopy; the cause of rebleeding in these patients was unclear, and 1 of the patients died on day 3. The possible causes of rebleeding and mortality within 6 weeks of EVL are summarised in Table 3.
Risk factors for 14-day and 6-week rebleeding
The Child–Pugh class (p = 0.022, HR: 1.453, 95% CI 1.056–1.998), PVT (p = 0.050, HR: 4.622, 95% CI 0.999–21.395), albumin < 30 g/L (p = 0.023, HR: 5.886, 95% CI 1.272–27.245), and number of bands (p = 0.010, HR: 1.207, 95% CI 1.046–1.393) were identified as the predictors for 14-day rebleeding. The multivariate analysis revealed the number of bands (p = 0.009, HR: 1.247, 95% CI 1.056–1.473) as an independent factor (Table 4). PVT (p = 0.012, HR: 6.732, 95% CI 1.519–29.835) and albumin < 30 g/L (p = 0.027, HR: 3.643, 95% CI 1.160–11.441) were identified as predictors for 6-week rebleeding, whereas PVT (p = 0.015, HR: 6.380, 95% CI 1.427–28.515) was found to be an independent factor in the multivariate analysis (Table 5). Albumin appeared to be a protective factor for the 14-day and 6-week rebleeding risk.
Characteristics of the patients with 6-week rebleeding (n = 13) and no rebleeding (n = 96) in the PVT group were further analysed. The primary clinical and biochemical parameters, including MELD score and endoscopic data, were similar between the groups, except for the SMV thrombosis rate, which was significantly higher in patients with rebleeding (p = 0.020; Table 6). SMV thrombosis was found to be the only factor predicting 6-week rebleeding in patients with PVT in the univariate Cox regression analysis (p = 0.029, HR: 3.484, 95% CI 1.139–10.655), as well as in the multivariate Cox regression analysis (p = 0.032, HR: 3.405, 95% CI 1.112–10.429; Table 7).
Discussion
Non-neoplastic PVT is a common complication of liver cirrhosis. A study reported that 17.3% of patients with AVB have PVT [16],which is in line with our results (17.25%). In our study, non-occlusive thrombosis was present in 89.91% (98/109) patients with PVT. Moreover, PVT had mostly occurred recently, and 90.83% of PVTs had occurred in the portal vein trunk alone or both in the trunk and branches.
For the first time, we comprehensively analysed the effect of PVT on rebleeding and mortality in patients with AVB after EVL. We found that PVT is associated with high 14-day and 6-week rebleeding after EVL. SMV thrombosis was found to be the only risk factor for 6-week rebleeding in patients with PVT.
Only a few studies have reported that the 5-day failure and 14-day and 6-week rebleeding rates are higher in cirrhotic patients with PVT than in those without PVT [12, 14, 16, 24]. However, those studies did not exclude patients with hepatocellular carcinoma, and the number of patients with PVT was small. In this multicentre study, we enrolled 109 cirrhotic AVB patients with PVT and excluded patients with hepatocellular carcinoma or those with a treatment history of EVL + NSBBs. Our results also revealed higher rates of 5-day failure and 14-day, 6-week, and 1-year rebleeding in patients with PVT, although only the 14-day and 6-week rebleeding rates were found to be significantly higher.
VB is a clinical emergency requiring immediate and intensive intervention. The first 5 days is considered the most crucial period for prognosis because most adverse events occur during this time [24]. All unfavourable outcomes (i.e. death, FTCB, and rebleeding within 5 days) were included in a complex variable defined as the 5-day failure. Amitrano et al. found that PVT is an independent predictor for the 5-day failure [16]. Although we observed a higher rate of 5-day failure in the PVT group, the difference was not significant because of a few events. This finding indicated that the combination treatment with endoscopy and pharmacologic agents is effective for AVB with PVT and helps achieve control of initial bleeding in 96.33% patients.
Baveno VI Consensus Workshop recommends 6-week mortality as the primary endpoint for AVB studies. Lee et al. found that [12] 70% of the patients died 6 weeks after initial EV bleeding because of rebleeding and that most instances (71.43%) of rebleeding occurred within 14 days of EV bleeding cessation. This result is similar to those of other studies [15, 16]. In our study, 73% (11/15) rebleeding occurred within 14 days of 6 weeks after the initial bleeding cessation. Rebleeding after EVL was mostly due to post-ligation ulcer (7/11), which occurred within 3–10 days of EVL. The incidence of PBUB was 3.21% in total, which is in line with a previously reported incidence rate of 2.6–7.3% [25]. Despite the low incidence, PBUB was difficult to manage and resulted in high mortality (42.86% in our study). Of the seven patients with PBUB, two patients received a second EVL, and one patient was transferred to the ICU for further treatment; all of these patients survived. Furthermore, of the four patients who were administered antibiotics, vasoactive drugs, and PPIs, three patients died. These results indicate that a second EVL and intensive care may be effective.
The Child–Pugh score, albumin < 30 g/L, PVT, and number of bands were associated with 14-day rebleeding, and the number of bands was the only risk factor predicting 14-day rebleeding (p = 0.009). This finding is in accordance with previous studies that have revealed a greater risk of early rebleeding in patients with a greater number of bands [12, 26] and can be ascribed to two reasons: first, placing more bands involves more surface area of mucosal injury and postbanding ulcers, causing a high risk of early rebleeding; second but more importantly, a greater number of bands indicates more extensive varices, with signs of bleeding and red plug, which indirectly reflects higher PH and risk of rebleeding in the future.
The most important and clinically relevant findings of this study were that PVT and albumin < 30 g/L were predictors for 6-week rebleeding and that PVT was an independent predictor. Published studies have reported that the serum bilirubin levels [27], size of varices [27], hemoglobin [17], PVT [16, 17, 28], and Child–Pugh class C [15, 16] are the predictors significantly associated with rebleeding after EVL. However, in our study, no significant differences were observed between rebleeding and no rebleeding in terms of the aforementioned risk factors, except PVT. Additionally, other parameters such as etiology, ascites, and serum creatinine had no significant effect on the incidence of rebleeding.
Importantly, in our study, a significantly higher rate of splenectomy or PSE history was observed in the PVT group (p = 0.000), whereas the history of AVB was similar between the groups. Previously, AVB was treated with a combination of endoscopy and pharmacological agents. Patients with splenomegaly and associated hypersplenism with a Child–Pugh A/B classification are typically treated with splenectomy or PSE later [29]. The enhanced rate of splenectomy or PSE history in the PVT group suggested higher PH, with splenomegaly and the associated hypersplenism as the characteristic features [30]. Both static portal blood flow from advancing PH and splenectomy can increase the risk of PVT development in patients with cirrhosis [7, 8].
Interestingly, we found that albumin < 30 g/L was a rebleeding predictor, although hypoalbuminemia was corrected to an extent. Hypoalbuminemia was assumed to be an indicator of the severity of liver dysfunction [24]. It indicated that 6-week rebleeding after EVL is related more to the liver disease than to the bleeding severity. Albumin is predominantly synthesised in the liver. Treatment with albumin has been widely used in liver cirrhosis due to its oncotic properties to prevent circulatory dysfunction. Albumin has other important functions, such as binding capacity and antioxidant and anti-inflammatory properties. It also regulates hemostasis, vasodilatation, and acid–base homeostasis [31]. In our study, albumin appeared to protect against 6-week rebleeding risk after EVL. Bacterial infection is a critical determinant of rebleeding in cirrhosis as it increases the portal pressure through vasoactive substances [32]. The effect of albumin on systemic inflammation can help reduce bleeding by preventing bacterial infection. Recently, Wang et al. [32] found that albumin infusion is associated with a low risk of rebleeding and in-hospital deaths in cirrhosis with acute gastrointestinal bleeding; however, more studies are required to confirm this finding.
On further analysis, we found that the absence of SMV thrombosis is the only independent predictor for 6-week rebleeding in patients with PVT. To the best of our knowledge, this study is the first to describe the prevalence of SMV thrombosis and its association with 6-week rebleeding in patients with AVB after EVL. This may be because obstruction of portal venous inflow was only partial in most of our patients (89.91%). The development of extensive portosystemic collaterals may limit PH exacerbation and reduce liver blood perfusion. However, in the setting of SMV thrombosis, the blood flow into the liver is reduced, which induces intestinal edema, bacterial translocation, and liver dysfunction, ultimately leading to an increased rebleeding rate after EVL. Even TIPSS also had a high failure and shunt dysfunction in this population [33]. Anticoagulation therapy is the preferred first-line therapy. Importantly, occlusive PVT did not increase the risk of rebleeding in our study (p = 0.760), possibly because of the presence of a cavernoma and/or venous collateralisation. However, more studies are required to validate this finding.
The synthesis of most factors involved in coagulation and fibrinolysis process is known to be impaired in patients with cirrhosis due to reduced liver function and platelet count secondary to PH in these patients. The typical markers of hepatic coagulopathy, including reduced platelet count (and function) and elevated INR, were found to have no influence on early rebleeding in the studies by Lee and Drolz [12, 27]. These findings are in line with our results and imply that the coagulopathy status may play a minor role in early rebleeding. However, most clinicians are unsure whether the anticoagulation therapy is safe for patients with cirrhosis. A series of studies were conducted recently, and most of these studies found that the anticoagulation therapy in patients with cirrhosis is safe as well as effective (i.e. 62.5–70% of patients reach partial or complete recanalisation, the incidence of PH complications and rate of thrombosis progression are reduced, liver function is improved, and survival is prolonged), if VB prophylaxis is performed well [34,35,36,37,38]. Data regarding the role of anticoagulant therapy and EVL-associated bleeding complications are unavailable to date. Therefore, a consensus regarding the efficacy of anticoagulation therapy for AVB combined with PVT is lacking, and more studies on this aspect are warranted.
Studies have reported that PVT is not associated with long-term (1-year) rebleeding [13] or increased mortality [7, 17, 39] among patients with cirrhosis. In this study, we found no significant difference in 1-year rebleeding between the groups, and the finding could be attributed to several factors. First, obstruction of portal venous inflow is only partial in most patients, and its impact on exacerbating PH and reducing liver blood perfusion requires a precise assessment. Second, approximately 30–50% of patients with PVT can achieve spontaneous recanalisation, as revealed in a series of studies [6, 35, 40], and the actual impact of PVT on clinical outcome in the long term remains unclear. The mortality rates were similar between the groups in our study. According to a systematic review by Qi et al. [41], liver dysfunction plays a crucial role in the prediction of survival in cirrhotic patients with PVT.
In our study, the rates of rebleeding and mortality at 6 weeks were lower than those reported in previous studies [4, 17]. This finding could be attributed to the following reasons: first, new medications and techniques have been developed recently to effectively decrease PH and prevent rebleeding; second and more importantly, it was the first EVL for our patients; more than half of our patients had never had a bleeding before and 90% of the patients were having a Child–Pugh class A or B; third, patients with hepatocellular carcinoma, which has been reported to be associated with the prognosis of patients who have experienced an episode of variceal bleeding [26, 42,43,44]were excluded from our study. Rebleeding occurred in 21.10% and 20.18% of patients with PVT and CCG, respectively, at 1 year. These rates are higher than those (11.9% vs. 9.5%) reported by Amitrano et al. [13]. The higher rate could be because of the following reasons: our study included a small number of patients with FTCB; NSBBs were not used in patients because it could increase the risk of PVT in cirrhotic patients; and anticoagulant therapy was not administered to patients with PVT as it could elevate the risk of bleeding. According to a systematic review and meta-analysis, the use of NSBBs increases the PVT risk by 4.62 folds in patients with cirrhosis [45].
Our study has several strengths. In this multicentre study, we enrolled 109 cirrhotic AVB patients with PVT and excluded those with a history of EVL + NSBBs or hepatocellular carcinoma. To clarify whether PVT is associated with poor outcomes independent of the severity of liver disease, the control group patients were matched to the study group patients in terms of Child–Pugh class, age, and sex. Subsequently, the baseline values of bilirubin, albumin, Child–Pugh score and MELD score, which are the possible independent predictors for poor outcomes [41], were similar between the groups. Therefore, our result indicating the association of PVT with a high rate of 6-week rebleeding after EVL appears convincing. Moreover, we identified the prevalence and characteristics of AVB patients with PVT and discovered SMV thrombosis as an independent risk factor for 6-week rebleeding in the PVT group. Furthermore, albumin appeared to serve as a protective factor for the 6-week rebleeding risk. Albumin infusion may improve the prognosis of patients with AVB after EVL.
This study has certain limitations. First, the retrospective study design, which has its inherent disadvantages. However, our study comprised a large number of patients and procedures from three teaching hospitals. All data were documented in the patient data management systems in a prospective manner and extracted later for the analysis. Second, although the ‘control’ group was selected on the basis of admission time, comparable age, sex, and Child–Pugh class, we cannot completely rule out the selection bias. However, the inclusion of a larger number of CCG patients would increase the complexity of the study. Third, the incidence of rebleeding and death was low, which could have reduced the statistical power. Therefore, more multicentre, prospective studies are warranted for further analysis. Finally, the determining factor of VB was PH. It is a dynamic pathology regulated by complex interactions among the injured hepatocytes, sinusoidal endothelial cells, Kupffer cells, and hepatic stellate cells, which impact the sinusoidal calibre. Inflammation may be a key mediator [46]. However, we did not study the effect of inflammation on rebleeding because of the low infection rate in the patients possibly due to the use of antibiotics.
In conclusion, PVT was associated with a high rate of 14-day and 6-week rebleeding in patients after EVL. SMV thrombosis was the only risk factor for 6-week rebleeding in patients with PVT. High albumin levels possibly served as a protective factor for the 14-day and 6-week rebleeding risk. PVT was not responsible for mortality after EVL during the 1-year follow-up. Further studies are warranted to investigate the role of anticoagulant therapy and EVL-associated bleeding complications and the benefit of albumin infusion in reducing rebleeding risk in patients with AVB after EVL.
Data availability
All date generated or analyzed during this study are included in this published article.
References
Tantai X, Liu N, Yang L, Wei Z, Xiao C, Song Y, et al. Prognostic value of risk scoring systems for cirrhotic patients with variceal bleeding. World J Gastroenterol 2019;25(45):6668–6680
de Franchis R. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol 2015;63(3):743–752
Lv Y, Qi X, He C, Wang Z, Yin Z, Niu J, et al. Covered TIPS versus endoscopic band ligation plus propranolol for the prevention of variceal rebleeding in cirrhotic patients with portal vein thrombosis: a randomised controlled trial. Gut 2018;67(12):2156–2168
Kovacs TOG, Jensen DM. Varices. Clin Liver Dis 2019;23(4):625–642
Turon F, Hernández-Gea V, García-Pagán JC. Portal vein thrombosis. Curr Opin Organ Transplant 2018;23(2):250–256
Nery F, Chevret S, Condat B, de Raucourt E, Boudaoud L, Rautou PE, et al. Causes and consequences of portal vein thrombosis in 1,243 patients with cirrhosis: results of a longitudinal study. Hepatology 2015;61(2):660–667
Yan Z, Bao-yan X, Xian-bo W, Xin Z, Yan H, Jinjun C, et al. Prevalence and clinical significance of portal vein thrombosis in patients with cirrhosis and acute decompensation. Clin Gastroenterol Hepatol 2020;18:2564–2572
Intagliata NM, Caldwell SH, Tripodi A. Diagnosis, development, and treatment of portal vein thrombosis in patients with and without cirrhosis. Gastroenterology 2019;156(6):1582–1599
Razek AA, Massoud SM, Azziz MR, El-Bendary MM, Zalata K, Motawea EM. Prediction of esophageal varices in cirrhotic patients with apparent diffusion coefficient of the spleen. Abdom Imaging 2015;40(6):1465–1469
Bochnakova T. Hepatic venous pressure gradient. Clin Liver Dis (Hoboken) 2021;17(3):144–148
Vorobioff JD, Groszmann RJ. Hepatic venous pressure gradient measurement in pre-primary and primary prophylaxis of variceal hemorrhage. Ann Hepatol 2013;12(1):22–29
Lee S, Lee S, Lee T, Lee T, Chang C, Chang C. Independent factors associated with recurrent bleeding in cirrhotic patients with esophageal variceal hemorrhage. Digest Dis Sci 2009;54(5):1128–1134
Amitrano L, Guardascione MA, Scaglione M, Menchise A, Martino R, Manguso F, et al. Splanchnic vein thrombosis and variceal rebleeding in patients with cirrhosis. Eur J Gastroenterol Hepatol 2012;24(12):1381–1385
Chen PH, Chen WC, Hou MC, Liu TT, Chang CJ, Liao WC, et al. Delayed endoscopy increases re-bleeding and mortality in patients with hematemesis and active esophageal variceal bleeding: a cohort study. J Hepatol 2012;57(6):1207–1213
Yang M, Chen H, Lee H, Lin C. Risk factors and survival of early bleeding after esophageal variceal ligation. Hepatogastroenterology 2007;54(78):1705
Amitrano L, Guardascione MA, Manguso F, Bennato R, Bove A, Denucci C, et al. The effectiveness of current acute variceal bleed treatments in unselected cirrhotic patients: refining short-term prognosis and risk factors. Am J Gastroenterol 2012;107(12):1872–1878
Trebicka J, Gu W, Ibanez-Samaniego L, Hernandez-Gea V, Pitarch C, Garcia E, et al. Rebleeding and mortality risk are increased by ACLF but reduced by pre-emptive TIPS. J Hepatol 2020;73(5):1082–1091
Razek AAKA, Khashaba M, Abdalla A, Bayomy M, Barakat T. Apparent diffusion coefficient value of hepatic fibrosis and inflammation in children with chronic hepatitis. Radiol Med (Torino) 2014;119(12):903–909
de Franchis R. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 2010;53(4):762–768
Wiesner R. MELD and PELD: application of survival models to liver allocation. Liver Transplant 2001;7(7):567–580
Tajiri T, Yoshida H, Obara K, Onji M, Kage M, Kitano S, et al. General rules for recording endoscopic findings of esophagogastric varices (2nd edition). Digest Endosc 2010;22(1):1–9
Sarin SK, Lahoti D, Saxena SP, Murthy NS, Makwana UK. Prevalence, classification and natural history of gastric varices: a long-term follow-up study in 568 portal hypertension patients. Hepatology (Baltimore, MD) 1992;16(6):1343–1349
Sarin SK, Philips CA, Kamath PS, Choudhury A, Maruyama H, Nery FG et al. Towards a comprehensive new classification of portal vein thrombosis in patients with cirrhosis. Gastroenterology 2016;151(4):574–577
D’Amico G. Upper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicators. Hepatology 2003;38(3):599–612
Tierney A. Interventions and outcomes of treatment of postbanding ulcer hemorrhage after endoscopic band ligation: a single-center case series. Gastrointest Endosc 2013;77:136–140
Dueñas E, Cachero A, Amador A, Rota R, Salord S, Gornals J, et al. Ulcer bleeding after band ligation of esophageal varices: risk factors and prognosis. Digest Liver Dis 2020;52(1):79–83
Drolz A, Schramm C, Seiz O, Groth S, Vettorazzi E, Horvatits T, et al. Risk factors associated with bleeding after prophylactic endoscopic variceal ligation in cirrhosis. Endoscopy 2021;53(3):226–234
EASL Clinical Practical Guidelines. Vascular diseases of the liverq. J Hepatol 2016;64:179–202
Mercado MA. Surgical treatment for portal hypertension. Br J Surg 2015;102(7):717–718
Leung JC, Loong TC, Pang J, Wei JL, Wong VW. Invasive and non-invasive assessment of portal hypertension. Hepatol Int 2018;12(S1):44–55
Carvalho JR, Verdelho Machado M. New insights about albumin and liver disease. Ann Hepatol 2018;17(4):547–560
Wang Z, Xie Y, Lu Q, Yan H, Liu X, Long Y, et al. The impact of albumin infusion on the risk of rebleeding and in-hospital mortality in cirrhotic patients admitted for acute gastrointestinal bleeding: a retrospective study of a single institute. Bmc Gastroenterol 2020;20(1):198–206
Xingshun Qi CH, Wengang G, Zhanxin Y, Jianhong W, Zhengyu W, Jing N, et al. Transjugular intrahepatic portosystemic shunt for portal vein thrombosis with variceal bleeding in liver cirrhosis: outcomes and predictors in a prospective cohort study. Liver Int 2016;36:667–676
Leonardi F, Maria ND, Villa E. Anticoagulation in cirrhosis: a new paradigm? Clin Mol Hepatol 2017;23(1):13–21
Zhou T, Sun X, Zhou T, Li Y, Chen X, Cheng B, et al. Efficacy and safety of nadroparin calcium-warfarin sequential anticoagulation in portal vein thrombosis in cirrhotic patients: a randomized controlled trial. Clin Transl Gastroenterol 2020;11(9):e228
Artaza T, Lopes M, Romero M, Gómez A, de la Cruz G, Sánchez JJ, et al. Efficacy and safety of anticoagulation in non-malignant portal vein thrombosis in patients with liver cirrhosis. Gastroenterol Hepatol 2018;41(10):611–617
Hayashi T, Takatori H, Horii R, Nio K, Terashima T, Iida N, et al. Danaparoid sodium-based anticoagulation therapy for portal vein thrombosis in cirrhosis patients. Bmc Gastroenterol 2019;19(1):217–227
Noronha Ferreira C, Reis D, Cortez-Pinto H, TatoMarinho R, Gonçalves A, Palma S, et al. Anticoagulation in cirrhosis and portal vein thrombosis is safe and improves prognosis in advanced cirrhosis. Digest Dis Sci 2019;64(9):2671–2683
Berry K, Taylor J, Liou IW, Ioannou GN. Portal vein thrombosis is not associated with increased mortality among patients with cirrhosis. Clin Gastroenterol Hepatol 2015;13(3):585–593
Luca A, Caruso S, Milazzo M, Marrone G, Mamone G, Crinò F, et al. Natural course of extrahepatic nonmalignant partial portal vein thrombosis in patients with cirrhosis. Radiology 2012;265(1):124–132
Qi X, Dai J, Yang M, Ren W, Jia J, Guo X. Association between portal vein thrombosis and survival in non-liver-transplant patients with liver cirrhosis: a systematic review of the literature. Gastroenterol Res Pract 2015;2015:1–7
Zhao Y, Ren M, Lu G, Lu X, Yin Y, Zhang D, et al. The prognosis analysis of liver cirrhosis with acute variceal bleeding and validation of current prognostic models: a large scale retrospective cohort study. Biomed Res Int 2020;2020:1–7
Thomopoulos K, Theocharis G, Mimidis K, Lampropouloukaratza C, Alexandridis E, Nikolopoulou V. Improved survival of patients presenting with acute variceal bleeding prognostic indicators of short- and long-term mortality. Digest Liver Dis 2006;38(12):899–904
Abdel Razek AAK, El-Serougy LG, Saleh GA, Shabana W, Abd E-W. Liver imaging reporting and data system version 2018: what radiologists need to know. J Comput Assist Tomogr 2020;44(2):168–177
Xu X, Guo X, De Stefano V, Silva-Junior G, Goyal H, Bai Z, et al. Nonselective beta-blockers and development of portal vein thrombosis in liver cirrhosis: a systematic review and meta-analysis. Hepatol Int 2019;13(4):468–481
Mehta G, Gustot T, Mookerjee RP, Garcia-Pagan JC, Fallon MB, Shah VH, et al. Inflammation and portal hypertension—the undiscovered country. J Hepatol. 2014;61(1):155–63
Acknowledgements
We are sincerely grateful to the physicians and all co-medical staff.
Funding
There is no financial support of this study.
Author information
Authors and Affiliations
Contributions
Professor YG designed and administrated the study. ZG conducted the study, collected and interpreted the data, drafted the manuscript. J-RZ interpreted the result and supervised the study. XL and SL administrated the study. MW collected the date. All authors had access to the study data and had reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Zhanjuan Gao, Jingrun Zhao, Xiaofeng Liu, Senlin Li, Minghui Wang,Yanjing Gao declare that there is no conflict of interest.
Ethical approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975. Each institution approved the protocol, and the data of all patients were recorded in a standard form. Because of retrospective, we did not have consent to participate, but all the patients information will be kept strictly confidential.
Animal research
This article does not contain any studies with animal subjects.
Informed consent
Informed consent for publication was obtained from all participants (Zhanjuan Gao, Jingrun Zhao, Xiaofeng Liu, Senlin Li, Minghui Wang, Yanjing Gao).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gao, Z., Zhao, J., Liu, X. et al. Portal vein thrombosis associated with high 14-day and 6-week rebleeding in patients after oesophageal variceal band ligation: a retrospective, multicentre, nested case–control study. Hepatol Int 15, 1183–1195 (2021). https://doi.org/10.1007/s12072-021-10224-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-021-10224-4