Abstract
Background
There currently is no consensus on how to accurately predict early rebleeding and death after a major variceal bleed. This study investigated the relative predictive performances of the original Child–Pugh (CP), model for end-stage liver disease (MELD) and a four-category recalibrated Child–Pugh (rCP).
Methods
This prospective study included all adult patients admitted to Groote Schuur Hospital with acute esophageal variceal bleeding secondary to alcoholic cirrhosis, between January 2000 and December 2017. CP and rCP grades and MELD score were calculated on admission, and the predictive ability in discriminating in-hospital rebleeding and death was compared by area under receiver-operating characteristic (AUROC) curves.
Results
During the study period, 403 consecutive adult patients were treated for bleeding esophageal varices of whom 225 were secondary to alcoholic cirrhosis. Twenty-four (10.6%) patients were CP grade A, 88 (39.1%) grade B and 113 (50.2%) grade C on hospital admission. MELD scores ranged from 6 to 40. Thirty-one (13.8%) patients rebleed, and 41 (18.2%) patients died. There was no difference in the discriminatory capacity of the CP (AUROC 0.59, 95% CI 0.50–0.670) and MELD (AUROC 0.62, 95% CI 0.51–0.73) to predict rebleeding (p = 0.72), or between the Child–Pugh (AUROC 0.75, 95% CI 0.71–0.81) and MELD (AUROC 0.71, 95% CI 0.62–0.80) to predict death (p = 0.35). The rCP classification (A–D) had a significantly improved discriminatory capacity (AUROC 0.83 95% CI 0.77–0.89) compared to the CP score (A–C) and MELD to predict death (p = 0.004).
Conclusion
A recalibrated Child–Pugh score outperforms the original Child–Pugh grade and MELD score in predicting in-hospital death in patients with bleeding esophageal varices secondary to alcoholic cirrhosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Variceal bleeding is a serious complication of portal hypertension, with a mortality rate of 20% at first presentation [1, 2]. Endoscopic control of bleeding is the emergency treatment of choice and may include injection sclerotherapy (IST) and/or variceal banding (VBL) [3, 4]. Although advances in treatment have reduced overall mortality, uncontrolled and recurrent bleeding and hepatic decompensation remain the commonest causes of in-hospital mortality [5, 6].
The Child–Pugh (CP) and MELD scores are widely used to assess severity of liver dysfunction and predict patient risk for in-hospital rebleeding and death [7,8,9]. The original classification was created by Child and Turcotte to predict mortality in patients who had portacaval shunt surgery and was subsequently modified by Pugh et al. to assess outcome in patients who underwent an esophageal transection to control bleeding varices [10, 11]. The MELD score was subsequently developed to assess outcome after a radiologically placed transjugular intrahepatic portosystemic shunt [9]. However, the predictive abilities of these scores in patients with variceal bleeding are variable with current evidence limited by small sample sizes, referral bias, dissimilar study end points, differences in patient selection and techniques of endoscopic intervention, and inconsistent definitions of rebleeding [12]. In order to overcome the limitations of the arbitrary cutoff grading points in the existing CP classification, we propose a four-category rCP with revised cutoff levels (A = 5, 6, 7; B = 8, 9, 10; C = 11, 12, 13; D = 14, 15). The objective of this study was to compare the relative ability of CP, MELD and the proposed rCP to predict in-hospital rebleeding and death following the index admission for acute variceal bleeding in patients with portal hypertension secondary to alcoholic liver cirrhosis.
Materials and methods
Patient population
Patient information was obtained from a prospectively collected database which includes all adult patients admitted to the Groote Schuur Hospital Surgical Gastroenterology Unit with endoscopically proven acute esophageal variceal bleeding from January 1, 2000 to December 30, 2017. Patient records were de-identified for the purpose of this study. In order to assess risk factors in a defined population and to minimize possible confounding variables, only those patients with bleeding esophageal varices due to alcohol-related cirrhosis who were treated with endoscopic therapy were analyzed. All endoscopies were performed by the staff in the Surgical Gastroenterology Unit. Patients with non-alcoholic cirrhosis or other causes of portal hypertension were excluded from the analysis to maintain a homogenous cohort. The diagnosis of cirrhosis was established by clinical evaluation, laboratory data, findings on radiological imaging including ultrasound and portal venous Doppler assessment and, in selected patients, liver biopsy and hepatic vein wedge pressure measurements. Cirrhosis was considered to be alcohol related if patients gave a history of sustained heavy alcohol consumption over several years. Blood samples for complete blood and platelet counts, bilirubin, INR, albumin and creatinine levels were taken immediately on admission before patients underwent their initial endoscopic intervention. CP grade was categorized according to the Pugh modification [11] and the revised cutoff levels of the rCP score (A = 5, 6, 7; B = 8, 9, 10; C = 11, 12, 13; D = 14, 15), and the MELD score was calculated by United Network of Organ Sharing (UNOS) adjustments [13]. The study design and analysis were approved by the appropriately convened Departmental and Institutional Ethics and Research Committees. Data validation and quality-control procedures followed accepted international Good Clinical Practice guidelines, and the study was conducted in accordance with the Declaration of Helsinki.
Therapeutic intervention for variceal bleeding
The management of acute variceal bleeding in our unit, including endoscopic hemostatic techniques, has been described previously [14,15,16]. All patients admitted with hematemesis underwent appropriate resuscitation and medical management which included intravenous cefuroxime and somatostatin or octreotide as an initial intravenous bolus followed by a continuous infusion for 72 h. Urgent upper gastrointestinal endoscopy was performed as soon as safely possible. Variceal bleeding was treated with either IST or VBL if bleeding esophageal varices were found. Where initial endoscopy failed to control active bleeding, a Sengstaken–Blakemore or Minnesota tube was placed for temporary control during resuscitation and endoscopy repeated when appropriate.
Definitions and outcomes
The primary end points of the study were variceal rebleeding and death during the index hospital admission. Time zero was defined as time of admission. Uncontrolled variceal bleeding was defined as continued bleeding despite optimal medical management, endoscopic therapy and balloon tamponade. Rebleeding was defined as any episode of upper gastrointestinal bleeding that occurred after the initial bleeding episode had been successfully controlled by endoscopic therapy, or bleeding that occurred subsequently between scheduled treatment sessions. Mortality was defined as death from any cause.
Statistical analysis
Categorical variables were reported as frequency (percentage) and continuous variables as mean ± standard deviations. Receiver-operating characteristic (ROC) curve analysis was performed to compare the ability of the CP, rCP and MELD scores to predict the risk of in-hospital rebleeding and death. Areas under the ROC curves (AUROC) with 95% confidence intervals (CIs) were reported. We compared the performance of the two scoring systems by using the DeLong tests. A p value <0.05 was considered statistically significant. Statistical analyses were performed using Stata 14.0, StataCorp LP.
Results
Patient characteristics
During the 216-month study period, 403 consecutive adult patients were treated for bleeding esophageal varices. Patients with non-alcoholic causes of portal hypertension (n = 166) or who had received endoscopy elsewhere and were referred for TIPS or liver transplantation (n = 12) were excluded from the study. Data in the remaining 225 patients with alcoholic cirrhosis and proven esophageal variceal bleeding who received only endoscopic therapy for bleeding during their index admission to hospital form the basis of this study. Baseline characteristics are shown in Table 1. The 225 patients [175 (77.8%) male, 50 (22.2%) female] had a median age of 50 years (IQR 43–57) and underwent a total of 261 endoscopic treatments. Thirty-three (14.7%) patients were CP grade A, 79 (35.1%) were grade B, and 113 (50.2%) were grade C. MELD scores ranged from 3 to 40. Fifty-five (14.7%) patients were rCP grade A, 87 (48.4%) were grade B, 61 (27.1%) were grade C, and 22 (9.8%) were grade D. (Table 2). MELD scores ranged from 6 to 10 in 68 (30.2%), 11 to 20 in 111 (49.3%), 21 to 30 in 39 (17.3) and 31 to 40 in 7 (3.1%) patients (Table 3).
Outcome
In-hospital rebleeding
Thirty-one (13.8%) patients had either uncontrolled bleeding (n = 12) during the first endoscopic procedure and required balloon tamponade and subsequent endoscopy or required repeat endoscopic intervention for recurrent bleeding (n = 19) during the index admission. Median length of hospital stay was 5 days (range 1–129 days).
Overall mortality
Overall, 41 (18.2%) patients died. The mortality rate was higher in patients who rebleed (4/31, 21.1%) and in those who required balloon tamponade (8/12, 66.7%). In terms of the CP score, mortality in grades A–C was 0%, 7.5% and 32.7%, respectively, while, for the rCP, mortality was 0%, 8%, 31.1% and 68.1% for grades A–D, respectively (Table 3). Mortality was 8.8% for MELD 6–10, 15.3% for 11–20, 35.9% for 21–30 and 57.1% for 31–40. Liver failure (n = 29, 70.7%) was the most common cause of death.
Predictive performance
In-hospital rebleeding
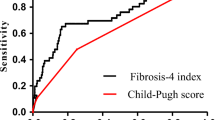
Overall, predictive ability for rebleeding was poor with no significant difference in performance between the three scoring systems (AUROC 0.59, 95% CI 0.50–0.67 for CP, AUROC 0.62, 95% CI 0.51–0.73 for MELD and AUROC 0.57, 95% CI 0.47–0.67 for rCP, p = 0.72) (Fig. 1).
Mortality
There was no difference in the ability to predict death between the CP and MELD scores CP (AUROC 0.75, 95% CI 0.71–0.81 and 0.71, 95% CI 0.62–0.80, respectively, p = 0.35). The rCP had significantly improved discriminatory capacity (AUROC 0.83, 95% 0.77–0.89) compared to the CP and MELD to predict death (p = 0.004) (Fig. 2).
Discussion
An accurate, objective and reproducible scoring system that predicts outcome and identifies high-risk patients who have portal hypertension and bleeding esophageal varices is a key component for determining clinical strategy and underpinning overall patient management [17]. In this study, the ability of a new rCP score to predict esophageal variceal rebleeding and death was compared to the original CP and MELD scores in an alcoholic cirrhotic population in whom the index variceal bleed was treated with emergency endoscopic intervention. The new rCP score demonstrated a significantly improved ability to predict death, compared to both the CP and MELD scores, but ultimately it was no better in predicting rebleeding. Importantly, although the initial endoscopic intervention was effective in controlling acute variceal bleeding from esophageal varices in most patients, one in 20 patients had uncontrolled bleeding and one in 12 had recurrent variceal bleeding during the index admission. The mortality rate in this cohort of decompensated alcohol-related cirrhotic patients was 18.2%, which is less than the 23.6% [18] reported by D’Amico et al., similar to the 18.4% documented by Thomopoulos [19], but higher than the 17.5% [20], 15.3% [21] and 14.5% [22] mortality rates reported by other authors, a difference which may reflect the alcoholic population in our study.
Although several prognostic models have been proposed to predict mortality in patients with cirrhosis, the CP and MELD scores have remained the most consistent and widely used scoring systems in both clinical practice and research and are superior to other predictive systems in determining mortality within the first 6 weeks after a major variceal bleed [23]. The advantages of the CP score are its simplicity and easily obtained variables and convenience as a prognostic tool, and calculations can be done at the bedside using mental arithmetic compared to the elaborate computations necessary for the MELD score, which require an online calculator. However, subjective assessment of the degree of ascites and encephalopathy, arbitrary cutoff values for bilirubin, albumin and INR and suboptimal differentiation between mild and severe disease of patients in grade C may limit the clinical application of the CP system [24, 25]. Also, assigning equal weights to all variables in the CP score may not be appropriate [25].
In contrast, the MELD score is a prospectively developed and validated indicator of the severity of end-stage liver disease that utilizes quantitative, objective measures, including bilirubin, creatinine and INR values to predict mortality. Also, variables are weighted to account for relative importance in reflecting severity of liver disease. The MELD scoring system therefore provides a more refined, granular grading system with a score ranging from 6 to 40, and as such it has replaced the CP score for prioritization for liver transplantation [13]. Caveats of the MELD score include a cap at a maximum value of 40 points, and the maximum assigned value of serum creatinine is capped at 4 even when the measured serum level is higher. In addition, the MELD score does not include independent prognostic indicators that correlate with severity of portal hypertension such as the degree of ascites and hepatic encephalopathy.
Our four-category RCP scoring system provides a refined stratification of patients in the C category of the original CP, in that it allocates patients with scores of 14 and 15 to a category D. Our results show that the rCP may be better than the CP and MELD scores at predicting in-hospital death, but not rebleeding, in patients who present with variceal bleeding secondary to alcoholic liver cirrhosis. These findings may not necessarily change management practices, but they are important for better prognostication prospectively as well as retrospectively for quality improvement initiatives and standardized audits.
Our study has the limitations of a retrospective analysis. The study duration is over an 18-year period during which improvements in supportive care, vasoactive drugs and antibiotic therapy have occurred. A major strength of this study is that it was conducted in a single center in a well-defined population of consecutive patients using a standard endoscopic technique and was supervised by the same group of investigators during the study period. The robustness of this study is enhanced by the prospective data collection, restriction of subjects to alcoholic cirrhotic patients and the complete follow-up of the cohort. The use of rebleeding and death as the main outcomes provided consistent and objective end points.
Despite substantial improvements over the past two decades in the overall survival after variceal bleeding, mortality during the index admission remains discouragingly high, especially in alcoholic patients who have limited liver reserve and present with active bleeding. Identification and knowledge of accurate prognostic data predicting early rebleeding can ideally provide a powerful tool to identify at an early stage those patients in whom conventional treatment is likely to be unsuccessful and who require accelerated and escalated intervention. The course and outcome of bleeding esophageal varices due to chronic liver disease are notoriously difficult to predict, and an important component of future medical care therefore is the acquisition and application of robust, reliable and validated prognostic tools to predict the likely outcome in individual patients. In order to improve the accuracy of prognostic models, better prognostic variables that are central to the disease process are required [8].
In the present analysis, the rCP classification system used scores stratified into four categories with three-point cutoffs, which demonstrated an improved ability to predict in-hospital mortality, but not rebleeding, compared to existing scoring systems. Future prospective studies incorporating and evaluating the full spectrum of prognostic factors including clinical variables, renal function and liver biochemistry, endoscopic findings and portal pressures are necessary to devise a new validated variceal rebleeding risk score which will be a valuable addition to improving the effective management of patients with portal hypertension and bleeding esophageal varices. This proposed modified and recalibrated CP model will need to be validated in prospective studies including a wider spectrum beyond alcoholic cirrhosis.
References
Garcia-Tsao G, Bosch J (2010) Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med 62:823–832
Hernández-Gea V, Berbel C, Baiges A, García-Pagán JC (2018) Acute variceal bleeding: risk stratification and management (including TIPS). Hepatol Int 12(Supplement 1):81–90
de Franchis R (2015) Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol 63:743–752
Augustin S, González A, Genescà J (2010) Acute esophageal variceal bleeding: current strategies and new perspectives. World J Hepatol 27(2):261–274
Amitrano L, Guardascione MA, Manguso F et al (2012) The effectiveness of current acute variceal bleed treatments in unselected cirrhotic patients: refining short-term prognosis and risk factors. Am J Gastroenterol 107:1872–1878
Augustin S, Altamirano J, Gonzalez A et al (2011) Effectiveness of combined pharmacologic and ligation therapy in high-risk patients with acute esophageal variceal bleeding. Am J Gastroenterol 106:1787–1795
Fortune BE, Garcia-Tsao G, Ciarleglio M et al (2017) Child-Turcotte-Pugh class is best at stratifying risk in variceal hemorrhage: analysis of a US multicenter prospective study. J Clin Gastroenterol 51:446–453
Christensen E (2004) Prognostic models including the Child-Pugh, MELD and Mayo risk scores -where are we and where should we go? J Hepatol 41:344–350
Malinchoc M, Kamath PS, Gordon FD et al (2000) A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology 31:864–871
Child CG, Turcotte JG (1964) Surgery and portal hypertension. The liver and portal hypertension. Saunders, Philadelphia, pp 50–64
Pugh RN, Murray-Lyon IM, Dawson JL et al (1973) Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 60:646–649
Augustin S, Millan L, Gonzalez A et al (2011) Prognostic evaluation of patients with acute variceal bleeding. Dis Markers 31:155–164
Trieu JA, Bilal M, Hmoud B (2018) Factors associated with waiting time on the liver transplant list: an analysis of the United Network for Organ Sharing (UNOS) database. Ann Gastroenterol 31:84–89
Krige JE, Kotze UK, Bornman PC, Shaw JM, Klipin M (2006) Variceal recurrence, rebleeding, and survival after endoscopic injection sclerotherapy in 287 alcoholic cirrhotic patients with bleeding esophageal varices. Ann Surg 244:764–770
Krige JEJ. Beningfield SJ (2018) Endoscopic therapy in the management of esophageal varices: injection sclerotherapy and variceal ligation. In: Nyhus, Baker, Fischer (ed) Mastery of surgery, 7th edn. Philadelphia, pp 1384–1398
Krige JEJ, Bornman PC (2007) Endoscopic therapy in the management of esophageal varices: injection sclerotherapy and variceal ligation. In: Blumgart L (ed) Surgery of the liver, biliary tract and pancreas, 4th edn. Saunders, Elsevier, Philadelphia, pp 1579–1593
Al Sibae MR, Cappell MS (2011) Accuracy of MELD scores in predicting mortality in decompensated cirrhosis from variceal bleeding, hepatorenal syndrome, alcoholic hepatitis, or acute liver failure as well as mortality after non-transplant surgery or TIPS. Dig Dis Sci 56:977–987
D’Amico G, De Franchis R (2003) Upper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicators. Hepatology 38:599–612
Thomopoulos K, Theocharis G, Mimidis K et al (2006) Improved survival of patients presenting with acute variceal bleeding. Prognostic indicators of short- and long-term mortality. Dig Liver Dis 38:899–904
Chalasani N, Kahi C, Francois F et al (2003) Improved patient survival after acute variceal bleeding: a multicenter, cohort study. Am J Gastroenterol 98:653–659
Boix J, Lorenzo-Zuniga V, Moreno de Vega V et al (2007) Sclerotherapy and esophageal variceal bleeding: time to forget it, or not? Endoscopy 39:478
Carbonell N, Pauwels A, Serfaty L et al (2004) Improved survival after variceal bleeding in patients with cirrhosis over the past two decades. Hepatology 40:652–659
Reverter E, Tandon P, Augustin S et al (2014) A MELD-based model to determine risk of mortality among patients with acute variceal bleeding. Gastroenterology 146:412–419
Augustin S, Muntaner L, Altamirano JT et al (2009) Predicting early mortality after acute variceal hemorrhage based on classification and regression tree analysis. Clin Gastroenterol Hepatol 7:1347–1354
Kaplan DE, Dai F, Skanderson M, Aytaman A et al (2016) Recalibrating the Child-Turcotte-Pugh Score to improve prediction of transplant-free survival in patients with Cirrhosis. Dig Dis Sci 61:3309–3320
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Krige, J., Spence, R.T., Jonas, E. et al. A New Recalibrated Four-Category Child–Pugh Score Performs Better than the Original Child–Pugh and MELD Scores in Predicting In-Hospital Mortality in Decompensated Alcoholic Cirrhotic Patients with Acute Variceal Bleeding: a Real-World Cohort Analysis. World J Surg 44, 241–246 (2020). https://doi.org/10.1007/s00268-019-05211-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-019-05211-8