Abstract
Osteoporosis is characterized by low bone mass and qualitative structural abnormalities of bone tissue, leading to increased bone fragility that results in fractures. Pharmacological therapy is aimed at decreasing the risk of fracture, mainly correcting the imbalance between bone resorption and formation at the level of bone remodeling units. Anabolic therapy has the capability to increase bone mass to a greater extent than traditional antiresorptive agents. The only currently available drug licensed is parathyroid hormone 1–34 (teriparatide); new drugs are on the horizon, targeting the stimulation of bone formation, and therefore improving bone mass, structure and ultimately skeletal strength. These are represented by abaloparatide (a 34-amino acid peptide which incorporates critical N-terminal residues, shared by parathyroid hormone and parathyroid hormone-related protein, followed by sequences unique to the latter protein) and romosozumab (an antibody to sclerostin). In the future, the availability of new anabolic treatment will allow a more extensive utilization of additive and sequential approach, with the goal of both prolonging the period of treatment and, more importantly, avoiding the side effects consequent to long-term use of traditional drugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Osteoporosis definition and its burden
Osteoporosis is characterized by low bone mass (simply quantified by bone mineral density—BMD—measurement) and qualitative structural abnormalities of bone tissue (not easily evaluated by current techniques); therefore, both quantitative and qualitative skeletal alterations lead to increased bone fragility resulting in fractures [1]. Mistakenly often considered an inevitable consequence of aging, osteoporosis with its ominous consequences of fractures represents a substantial and ever-growing burden on the health-care systems in many countries around the world.
Traditionally, typical osteoporotic fractures are considered as those occurring at the hip, vertebrae and forearm. A recent study, however, demonstrates that 70% of fractures occur in non-hip and non-vertebral sites in postmenopausal women receiving therapy for osteoporosis in a primary care setting [2], thus emphasizing the role of other sites, such as for example ribs, pelvis, and humerus [3].
The number of fractures in the world has been estimated to be about 9 million per year, more than one-third of those occurring in Europe [4]. Figure 1 illustrates the behavior of hip fractures in Italy during an 8-year period (2007–2014); it can be observed that while there is a trend of fractures to plateau until the age range 80–85 years, there is a continuous trend to increase in the oldest population. This has obvious important consequences from both an economic and health policy point of view.
Osteoporosis is associated with significant morbidity, mortality and reduced quality of life [5] leading to an increase in health-care resource utilization [6]. Indeed, the cost of managing osteoporosis is very high, and in 2010 it was estimated to be around €37 billion. When considering quality-adjusted life years (QALYs) (which reflects a societal perspective on burden of disease), the amount is estimated to be around 1,165,000 QUALYS. Considering both the cost of managing osteoporosis and the cost of QALYs lost, the expenditure of osteoporosis amounted to €98 billion in Europe in 2010 [7].
It is also important to put such costs in the context of other non-communicable diseases. Indeed, if we consider another parameter, i.e., DALYS (total disability-adjusted life years, expressing the years of life lost due to a fracture and the disability in those who survive), the cost amounted to 5.8 million in 2010. These figures are greater than those of other diseases such as hypertension and rheumatoid arthritis. Moreover, fractures due to osteoporosis are responsible for more deaths and morbidity than any cancer type, other than lung cancer [4, 6, 7].
Standard therapies for osteoporosis
The mainstays of treatment (and prevention) of osteoporosis are represented by fall avoidance [8], weight-bearing exercise [9], and adequate calcium and vitamin D intake [10,11,12]. These general measures can be effective [13], especially in elderly subjects confined to nursing homes, and the vitamin D deficient; however, medical therapy is needed when a previous fracture has already occurred.
Pharmacological therapy is aimed at decreasing the risk of fracture, mainly correcting the imbalance between bone resorption and formation at the level of bone remodeling units [14]. Indeed, there are a number of available drugs that have been shown to reduce the risk of future fractures based on both experimental and clinical data. These pharmacological agents can be broadly divided into two subgroups: those decreasing bone resorption (acting on osteoclasts) and those increasing skeletal formation (acting on osteoblasts).
Antiresorptive agents [such as estrogens, bisphosphonates, selective estrogen receptor modulator and the monoclonal antibody to receptor activator of nuclear factor kappa-B ligand (denosumab)] reduce the rate of bone resorption, followed by a decrease in the rate of bone formation due to the coupling between these two processes; after about 6 months, a new equilibrium between the two phases of bone remodeling is reached, although at a lower rate. These changes are associated with increases of bone mineral density, and maintenance of some improvement of structural and material properties of bone leading to reduction of bone fragility. Long-term increases in bone mass are largely secondary to an increase in mineralization density, which is a consequence of reduced bone turnover [15].
Anabolic therapy has the ability to increase bone mass to a greater extent than traditional antiresorptive agents. The only currently available drug licensed is parathyroid hormone (PTH) 1–34 (teriparatide). It stimulates bone formation, particularly in those skeletal segments rich in trabecular bone such as vertebrae, thus reducing both vertebral and non-vertebral fractures [16]. PTH improves bone quality and strength by inducing more favorable changes in microarchitectural features such as connectivity, density and geometric properties compared to antiresorptive agents [17, 18].
In addition, owing to its ability to primarily stimulate bone formation, PTH 1–34 should be considered a first-line therapy in glucocorticoid-induced osteoporosis, which is associated with reduced bone formation [19, 20]. Studies comparing antiresorptive drugs vs PTH 1–34 have indeed demonstrated the superiority of teriparatide both in terms of improvements in bone strength [21] and reduction in vertebral fractures [22].
Available treatments for osteoporosis are efficacious; however, there are some drawbacks to be considered. For example, when we prescribe an antiresorptive therapy we can only increase bone mineral density up to a certain point; indeed, owing to a coupling between bone formation and resorption, there is no possibility of “de novo” synthesis of bone by osteoblasts [23]. This concept is unquestionably true for bisphosphonates (risedronate, alendronate and zoledronate), but may be challenged with the use of the antibody against the receptor activator of nuclear factor kappa-B ligand. In fact, the studies carried out so far have demonstrated a continuous increase of bone mineral density with a cumulative 8-year gains of 18.4 and 8.3% at the lumbar spine and total hip, respectively, implying that other mechanisms should be involved [24]. Secondly, when we use teriparatide, (which primarily stimulates bone formation), an increase in osteoclastic activity can be observed after a certain period; this foretells the closure of the so-called anabolic window, thus limiting further accrual of bone mass [25]. Finally, clinicians face a number of issues, such as, for example, cost, and those related to long-term treatment of a disease chronic in nature (compliance, side effects) [26]. It is in this context that new drugs are on the horizon, mainly targeting the stimulation of bone formation (rather than decreasing bone resorption), therefore improving bone mass, structure and ultimately skeletal strength.
Abaloparatide
This is a 34-amino acid peptide that incorporates critical N-terminal residues, shared by PTH and PTH-related protein (PTHrP) followed by sequences unique to PTHrP. Preclinical studies have clearly demonstrated that both peptides activate the same receptor; however, their kinetics are different determining dissimilar responses. It has been hypothesized that the two molecules favor different receptor conformations with abaloparatide binding with high affinity, but only for a short period of time, thus limiting the duration of signaling compared with teriparatide.
Miller and coworkers [27] report on the efficacy and safety of abaloparatide administered in a daily dose of 80 μg subcutaneously for 18 months. A total of 2463 patients were initially randomized, and 1901 completed the investigation. Women were subdivided into three groups receiving subcutaneous injection of placebo, abaloparatide 80 μg or open label teriparatide 20 μg, respectively. In the modified intention-to-treat population analysis (defined as the intention-to-treat analysis in participants who had both pretreatment and post-baseline X-rays), new vertebral fractures occur less frequently compared with placebo (0.58 vs 4.22%; RR 0.14, 95% CI 0.05–0.39, p < 0.001). In the teriparatide group, new morphometric vertebral fractures were detected in six women (RR 0.20, CI 0.08–0.47, p < 0.001 vs placebo). Somehow, similar results were obtained considering non-vertebral fractures; the Kaplan–Meier estimated event rate for non-vertebral fracture is lower with abaloparatide with respect to the placebo group [2.7% in patients treated with abaloparatide vs 4.7% in placebo-treated patients; hazard ratio: 0.57 (95 CI 0.32–1.00) p < 0.049]. There were no significant differences with respect to the teriparatide group (HR 0.79, CI 0.43–1.45, p = 0.44), though the Kaplan–Meier estimated event rates in this last group were not significantly different from placebo-treated patients (HR 0.72, CI. 0.42–1.22, p = 0.22).
Considering bone mineral density, mean improvements with abaloparatide are significantly greater than those with teriparatide at the total hip and femoral neck at 6, 12 and 18 months (p < 0.001). Regarding lumbar spine there is a statistical significant difference (p < 0.001) at 6 and 12 months.
It is also important to underline the changes in bone turnover markers in patients treated with abaloparatide or teriparatide. Indeed, there is a similar increase of serum procollagen type I N-terminal propeptide (P1NP), and less increase in serum carboxy-terminal cross-linking telopeptide of type I collagen (βCTX) in abaloparatide-treated patients compared to those treated with teriparatide (Fig. 2). This finding might be ascribed to the differential binding to PTH type 1 receptor, thus determining a more transient stimulation and lower expression of osteoblast-derived RANK ligand. This very initial uncoupling of bone formation and resorption may justify the very rapid increase of bone mass with consequent rapid protection against future fracture. This could be particularly important in the immediate period after a fracture occurs; indeed, a number of studies have documented that the risk is higher soon after the initial event and then declines with time [28].
Behavior of biochemical markers of bone formation (serum procollagen type I N-terminal propeptide, P1NP) and resorption (serum carboxy-terminal cross-linking telopeptide of type I collagen, βCTX) following treatment with teriparatide, abaloparatide and romosozumab. The figure is redrawn based on the data presented in [27] and [34]
Finally, concerning safety, there were more withdrawals from the study in abaloparatide-treated group (9.9 vs 6.8% in the teriparatide and 6.1% in the placebo groups, respectively) because of adverse events mainly represented by nausea, dizziness, headache and palpitations. This negative aspect, if confirmed in future studies, implies a need for stringent medical surveillance.
Data from this original investigation have been subsequently re-analyzed, demonstrating that abaloparatide maintains its effect in postmenopausal women with osteoporosis, regardless of age, previous fracture or basal BMD value [29].
Romosozumab
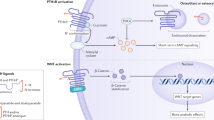
An important component of the Wnt signaling pathway, a well-known metabolic route to drive osteoblast proliferation and commitment, is represented by sclerostin. This is a glycoprotein mainly secreted by osteocytes (and to a lesser extent by cementocytes and mineralized hypertrophic chondrocytes) which is a potent inhibitor of osteoblastogenesis. Sclerostin secreted from osteocytes reaches the bone surface through the canaliculi, where it binds to co-receptors LRP5 and LRP6. It prevents co-receptor localization with frizzled protein and Wnt, thereby decreasing osteoblastogenesis and bone formation [15, 30].
The link between sclerostin and bone formation is very well illustrated by two rare genetic decreases. Both sclerosteosis (due to a loss of function mutation) and Van Buchem disease (caused by a deletion downstream of sclerostin gene, which includes sclerostin protein) are characterized by high bone mass. In this context, some scientists suggest that the effect of parathyroid hormone therapy in humans is mediated, at least in part, by a decrease in serum sclerostin levels [31, 32]. In addition, genetic studies have shown that polymorphism in the sclerostin gene is associated with low bone mineral density in older men and women, further emphasizing a causal link between modified sclerostin expression and bone mineral density.
These observations led to an exploration of the pharmacological inhibition of sclerostin by a monoclonal antibody in various animal models of bone disease. Data obtained in experimental animals show a consistent effect of sclerostin antibody (from now on called romosozumab) to increase bone formation, bone mass and strength at various skeletal sites [33].
Romosozumab has been evaluated in a double-blind study at a monthly dose of 210 mg versus placebo in more than 7000 post-menopausal osteoporotic patients [34]. At 12 months of treatment, romosozumab was associated with a risk of new vertebral fracture that was 73% lower than the risk observed in the placebo group (16 of 3321 patients in the romosozumab group vs 59 of 3322 in the placebo group; risk ratio 0.25, confidence interval 0.16–0.47, p < 0.0001). Non-vertebral fractures occurred in 56 patients of the romosozumab group compared with 75 in the placebo group (p = 0.10).
After 12 months of romosozumab therapy, the positive percent changes of BMD therapy were 13.3, 6.9 and 5.9 at the lumbar spine, total hip and femoral neck, respectively.
Concerning markers of bone turnover, the serum level procollagen type I N-terminal propeptide peaks at day 14, while the levels of βCTX decrease, reaching a maximum decline at day 14, and then remain below the level of the placebo group during the whole treatment period.
Figure 2 illustrates the behavior of P1NP and βCTX following 12 months administration of teriparatide, abaloparatide [27] and romosozumab [34]. Although these are not head-to-head studies, and we are aware that investigations undertaken in different populations cannot be precisely compared, it is clear that romosozumab acutely (i.e., 1 month) determines a rapid positive biochemical balance that is instead delayed with the other two bone forming agents.
Regarding the safety profile, all adverse events are balanced between active treatment and placebo. Surprisingly, one atypical femoral fracture and two cases of osteonecrosis of the jaw were observed in the romosozumab group, challenging the notion that these adverse events were only detected with the use of potent inhibitors of bone resorption. Indeed, this probably reflects their spontaneous occurrence in the real world.
A recent paper [35] investigated the effect of romosozumab administration in postmenopausal women with low bone mass by analyzing lumbar spine and hip volumetric BMD by quantitative computerized tomography. The most important finding from this study is a significant and rapid improvement in bone density and mass at both skeletal sites. Furthermore, the beneficial effects after 12 months of therapy are observed at both trabecular and cortical compartments, each resulting in improved mechanical bone strength.
Other drugs
Two additional drugs have been tested in human studies, but they have not reached approval by registrative authorithies.
The first one, blosozumab, is a monoclonal antibody against sclerostin; it has been tested as a potential anabolic therapy for osteoporosis in a double-blind phase 2 clinical trial in postmenopausal women with low bone mineral density [36, 37]. No phase 3 trials are underway.
The second drug, odanacatib, is a reversible inhibitor of cathepsin K, an enzyme expressed almost exclusively in osteoclasts. Interestingly, mutations in the gene encoding for this enzyme are the cause of pycnodysostosis, a disease characterized by increased density of bone of the entire skeleton. This pharmaceutical compound has been tested in a phase 3 trial, showing fracture risk reduction comparable to registered inhibitors of bone resorption [38]. However, due to incomplete evaluation of adverse events, this drug is not yet approved and therefore not yet commercialized.
Sequential and combination therapy
In the real world, patients with osteoporosis are usually treated with calcium (preferably by diet or alternatively by supplements), vitamin D and an antiresorptive or anabolic agents. Concerning the last type of treatment, more than 50% of teriparatide prescriptions are initiated in those already treated with an antiresorptive agent (in the great majority of cases, bisphosphonates or denosumab) in the USA [39] and an even smaller proportion in Europe, probably depending on reimbursability; therefore, the combination therapy is at present a rare option.
However, changes of bone mineral density with sequential or combination therapy are of particular interest, both from a pathophysiological and from a therapeutic point of view. Owing to space constraints, we will focus on bone mineral density changes obtained at the total hip (the skeletal site most important from a clinical and socioeconomic perspective) without considering the lumbar spine.
After switching from an antiresorptive drug to teriparatide, BMD values tend to decrease for the first 12 months, after which there is a tendency to increase in the next 12 months of hormone treatment [40] On the other hand, the results are quite different if the patients are pretreated with denosumab; indeed, in the last situation, there is a constant finding of total hip BMD values below the initial starting point.
Considering an additive instead of a sequential approach, one study shows that total hip BMD increases are greater in those patients who add teriparatide to ongoing alendronate therapy compared with those switched to teriparatide [41]. Similar results are also observed when analyzing the hip by quantitative computed tomography [42] or finite element analysis [43] also resulting in an increase in hip strength of the cortical compartment.
There are very few studies looking at the effect of sequential or additive therapy on bone histologic parameters. Dempster and coworkers compared short-term bone formation response to teriparatide in treatment-naïve patients and in patients on prior and ongoing alendronate therapy. In both groups, an increase in bone formation is observed after 7 weeks, although quantitatively lower in those pretreated with bisphosphonates. Concerning cortical porosity, this is higher in the treatment naïve than in those previously treated [18].
In this context, it is important to report the long-term studies published by Leder and coworkers showing that combination therapy (denosumab and teriparatide) determines the most important gain in terms of areal BMD at the lumbar spine, total and femur neck and distal shaft [44]. These gains were consolidated by adding a further 2 years of denosumab treatment [45]. More importantly, these large gains obtained after 4 years of intensive therapy are lost if patients are left untreated [46], thus reinforcing the concept that after discontinuation of teriparatide and denosumab therapy there is a rapid decline of BMD, which can lead the patient to the pretreatment fracture risk [47].
Finally, it should be kept in mind that in the investigations conducted by Leder and coworkers, there was not fracture end points, but only bone mineral density data. Notwithstanding, the findings of these studies are important to guide the sequence of therapy, especially in clinical conditions in which a rapid increase of bone mineral density is needed, such as for example in patients on long-term steroid therapy [19].
Conclusions
The forthcoming arrival of new anabolic drugs will not only increase the portfolio of compounds available to treat bone fragility, but will also probably change our way of prescribing drugs for the most common metabolic bone disease. Indeed, unlike the majority of chronic diseases, which are generally treated by a combination of drugs, the current practice in the field of osteoporosis is the administration of only one drug at a fixed dose. However, the chronic nature of osteoporosis will force doctors to find alternative solutions by adding and switching therapies with the goal of both prolonging the period of treatment and, more importantly, avoiding the side effects consequent to long-term use of drugs.
References
Papapoulos SE (2015) Anabolic bone therapies in 2014: new bone-forming treatments for osteoporosis. Nat Rev Endocrinol 11:69–70. doi:10.1038/nrendo.2014.214
Freemantle N, Cooper C, Roux C et al (2010) Baseline observations from the POSSIBLE EU® study: characteristics of postmenopausal women receiving bone loss medications. Arch Osteoporos 5:61–72. doi:10.1007/s11657-010-0035-7
Holloway KL, Henry MJ, Brennan-Olsen SL et al (2016) Non-hip and non-vertebral fractures: the neglected fracture sites. Osteoporos Int 27:905–913. doi:10.1007/s00198-015-3322-8
Hernlund E, Svedbom A, Ivergård M et al (2013) Osteoporosis in the European Union: medical management, epidemiology and economic burden: a report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 8(1–2):136. doi:10.1007/s11657-013-0136-1
Romagnoli E, Carnevale V, Nofroni I et al (2004) Quality of life in ambulatory postmenopausal women: the impact of reduced bone mineral density and subclinical fractures. Osteoporos Int 15:975–980. doi:10.1007/s00198-004-1633-2
Romagnoli E, Carnevale V, Calandra P et al (2003) Impact of fractures on health care in a major university hospital in Rome. Aging Clin Exp Res 15:505–511. doi:10.1007/BF03327374
Kanis JA, Cooper C, Rizzoli R et al (2017) Identification and management of patients at increased risk of osteoporotic fracture: outcomes of an ESCEO expert consensus meeting. Osteoporos Int 28(7):2023–2034. doi:10.1007/s00198-017-4009-0
Salked G, Cameron ID, Cumming RG et al (2000) Quality of life related to fear of falling and hip fracture in older women: a time trade off study. Br Med J 320(7231):341–346
Pfeifer M, Sinaki M, Geusens P et al (2004) Musculoskeletal rehabilitation in osteoporosis: a review. J Bone Miner Res 19:1208–1214. doi:10.1359/JBMR.040507
Boonen S, Lips P, Bouillon R et al (2007) Need for additional calcium to reduce the risk of hip fracture with vitamin D supplementation: evidence from a comparative metaanalysis of randomized controlled trials. J Clin Endocrinol Metab 92(4):1415–1423. doi:10.1210/jc.2006-1404
Romagnoli E, Pepe J, Piemonte S et al (2013) Management of endocrine disease: value and limitations of assessing vitamin D nutritional status and advised levels of vitamin D supplementation. Eur J Endocrinol 169:59–69. doi:10.1530/EJE-13-0435
Cipriani C, Pepe J, Piemonte S et al (2014) Vitamin D and its relationship with obesity and muscle. Int J Endocrinol. doi:10.1155/2014/841248
Murad MH, Drake MT, Mullan RJ et al (2012) Comparative Effectiveness of drug treatments to prevent fragility fractures: a systematic review and network meta-analysis. J Clin Endocrinol Metab 97(6):1871–1880. doi:10.1210/jc.2011-3060
Riggs BL, Parfitt AM (2005) Drugs used to treat osteoporosis: the critical need for a uniform nomenclature based on their action on bone remodeling. J Bone Miner Res 20:177–184. doi:10.1359/JBMR.041114
Minisola S (2014) Romosozumab: from basic to clinical aspects. Expert Opin Biol Ther 14(9):1225–1228. doi:10.1517/14712598.2014.920815
Neer RM, Arnaud CD, Zanchetta JR et al (2001) Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Eng J Med 344(19):1434–1441. doi:10.1056/NEJM200105103441904
Dempster DW, Zhou H, Recker RR et al (2016) A longitudinal study of skeletal histomorphometry at 6 and 24 months across four bone envelopes in postmenopausal women with osteoporosis receiving teriparatide or zoledronic acid in the SHOTZ trial. J Bone Miner Res 31:1429–1439. doi:10.1002/jbmr.2804
Moreira CA, Dempster DW (2017) Histomorphometric changes following treatment for osteoporosis. J Endocrinol Invest. doi:10.1007/s40618-017-0662-6
Hansen KE, Wilson HA, Zapalowski C et al (2011) Uncertainties in the prevention and treatment of glucocorticoid-induced osteoporosis. J Bone Miner Res 26:1989–1996. doi:10.1002/jbmr.362
Mazziotti G, Formenti AM, Adler RA et al (2016) Glucocorticoid-induced osteoporosis: pathophysiological role of GH/IGF-I and PTH/VITAMIN D axes, treatment options and guidelines. Endocrine 54:603–611. doi:10.1007/s12020-016-1146-8
Farahmand P, Marin F, Hawkins F et al (2013) Early changes in biochemical markers of bone formation during teriparatide therapy correlate with improvements in vertebral strength in men with glucocorticoid-induced osteoporosis. Osteoporos Int 24:2971–2981. doi:10.1007/s00198-013-2379-5
Saag KG, Shane E, Boonen S et al (2007) Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Eng J Med 357:2028–2039. doi:10.1056/NEJMoa071408
Harslǿf T, Langdhal BL (2016) New horizons in osteoporosis therapies. Curr Opin Pharmacol 28:38–42
Papapoulos SE, Lippuner K, Roux C et al (2015) The effect of 8 or 5 years of denosumab treatment in postmenopausal women with osteoporosis: results from the FREEDOM Extension study. Osteopros Int 26(12):2773–2783. doi:10.1007/s00198-015-3234-7
Papapoulos SE, Makras P (2008) Selection of antiresorptive or anabolic treatments for postmenopausal osteoporosis. Nat Clin Pract End Metab 4(9):514–523. doi:10.1038/ncpendmet0941
Adler RA (2016) Osteoporosis treatment: complexities and challenges. J Endocrinol Invest 39(7):719–720. doi:10.1007/s40618-016-0437-5
Miller PD, Hattersley G, Riis BJ et al (2016) Effect of Abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: a randomized clinical trial. JAMA 316(7):722–733. doi:10.1001/jama.2016.11136
Johnell O, Kanis JA, Odén A et al (2004) Fracture risk following an osteoporotic fracture. Osteoporos Int 15:175. doi:10.1007/s00198-003-1514-0
Cosman F, Hattersley G, Hu MY et al (2017) Effects of Abaloparatide-SC on fractures and bone mineral density in subgroups of postmenopausal women with osteoporosis and varying baseline risk factors. J Bone Miner Res 32:17–23. doi:10.1002/jbmr.2991
Costa AG, Bilezikian JP, Lewiecki EM (2014) Update on romosozumab: a humanized monoclonal antibody to sclerostin. Expert Opin Biol Ther 14(5):697–707. doi:10.1517/14712598.2014.895808
Drake MT, Srinivasan B, Mödder UI et al (2010) Effects of parathyroid hormone treatment on circulating sclerostin levels in postmenopausal women. J Clin Endocrinol Metab 95(11):5056–5062. doi:10.1210/jc.2010-0720
Piemonte S, Romagnoli E, Bratengeier C et al (2012) Serum sclerostin levels decline in post-menopausal women with osteoporosis following treatment with intermittent parathyroid hormone. J Endocrinol Invest 35:866–868. doi:10.3275/8522
Ke HZ, Richards WG, Li X, Ominsky MS (2012) Sclerostin and Dickkopf-1 as therapeutic targets in bone diseases. Endocr Rev 33(5):747–783. doi:10.1210/er.2011-1060
Cosman F, Crittenden DB, Adachi JD et al (2016) Romosozumab treatment in postmenopausal women with osteoporosis. N Eng J Med 375(16):1532–1543
Genant HK, Engelke K, Bolognese MA et al (2017) Effects of Romosozumab compared with teriparatide on bone density and mass at the spine and hip in postmenopausal women with low bone mass. J Bone Miner Res 32:181–187. doi:10.1002/jbmr.2932
McColm J, Hu L, Womack T, Tang CC, Chiang AY (2014) Single- and multiple-dose randomized studies of blosozumab, a monoclonal antibody against sclerostin, in healthy postmenopausal women. J Bone Miner Res 29:935–943. doi:10.1002/jbmr.2092
Recknor CP, Recker RR, Benson CT (2015) The effect of discontinuing treatment with Blosozumab: follow-up results of a phase 2 randomized clinical trial in postmenopausal women with low bone mineral density. J Bone Miner Res 30:1717–1725. doi:10.1002/jbmr.2489
Bone HG, Dempster DW, Eisman JA et al (2015) Odanacatib for the treatment of postmenopausal osteoporosis: development history and design and participant characteristics of LOFT, the long-term odanacatib fracture trial. Osteoporos Int 26(2):699–712. doi:10.1007/s00198-014-2944-6
Bonafede MM, Shi N, Bower AG et al (2015) Teriparatide treatment patterns in osteoporosis and subsequent fracture events: a US claims analysis. Osteoporos Int 26:1203–1212. doi:10.1007/s00198-014-2971-3
Cosman F, Nieves JW, Dempster DW (2017) Treatment sequence matters: anabolic and antiresorptive therapy for osteoporosis. J Bone Miner Res 32:198–202. doi:10.1002/jbmr.3051
Cosman F, Wermers RA, Recknor C et al (2009) Effects of Teriparatide in postmenopausal women with osteoporosis on prior alendronate or raloxifene: differences between stopping and continuing the antiresorptive agent. J Clin Endocrinol Metab 94(10):3772–3780. doi:10.1210/jc.2008-2719
Boonen S, Marin F, Obermayer-Pietsch B et al (2008) Effects of previous antiresorptive therapy on the bone mineral density response to 2 years of teriparatide treatment in postmenopausal women with osteoporosis. J Clin Endocrinol Metab 93(3):852–860. doi:10.1210/jc.2007-0711
Cosman F, Keaveny TM, Kopperdahl D et al (2013) Hip and spine strength effects of adding versus switching to teriparatide in postmenopausal women with osteoporosis treated with prior alendronate or raloxifene. J Bone Miner Res 28:1328–1336. doi:10.1002/jbmr.1853
Leder BZ, Tsai JN, Uihlein AV et al (2014) Two years of denosumab and teriparatide administration in postmenopausal women with osteoporosis (the DATA extension study): a randomized controlled trial. J Clin Endocrinol Metab 99(5):1694–1700. doi:10.1210/jc.2013-4440
Leder BZ, Tsai JN, Uihlein AV et al (2015) Denosumab and teriparatide transitions in postmenopausal osteoporosis (the DATA-switch study): extension of a randomised controlled trial. Lancet 386(9999):1147–1155. doi:10.1016/S0140-6736(15)61120-5
Leder BZ, Tsai JN, Jiang LA, Lee H (2017) Importance of prompt antiresorptive therapy in postmenopausal women discontinuing teriparatide or denosumab: the Denosumab and Teriparatide follow-up study (DATA-follow-up). Bone 98:54–58. doi:10.1016/j.bone.2017.03.006
Aubry-Rozier B, Gonzalez-Rodriguez E, Stoll D, Lamy O (2016) Severe spontaneous vertebral fractures after denosumab discontinuation: three case reports. Osteoporos Int 27(5):1923–1925. doi:10.1007/s00198-015-3380-y
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Prof. S. Minisola served as speaker for Abiogen, Amgen, Bruno Farmaceutici, Diasorin, Eli Lilly and Fujii. He also served on the Advisory Board of Abiogen. He received consultancy from Bruno Farmaceutici.
Statement of human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent is not required.
Funding
There was no specific funding for this work.
Rights and permissions
About this article
Cite this article
Minisola, S., Cipriani, C., Occhiuto, M. et al. New anabolic therapies for osteoporosis. Intern Emerg Med 12, 915–921 (2017). https://doi.org/10.1007/s11739-017-1719-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-017-1719-4