Abstract
Climate change has an increasingly negative impact on the availability of water for agriculture. Therefore, enhancing water use efficiency under poor irrigation conditions has become a major objective. Here, we investigate the effects of moderate water deficit on tomato plant growth, physiology, fruit yield and quality and expression of carotenoid biosynthesis genes. The treatments control (irrigated) and water deficit were applied from the fruit fixation stage forward and the observations were recorded when the fruits were mature. The results revealed adaptive responses to moderate water deficit, in which plants showed a reduction in leaf water potential, leaf gas exchange and growth variables but an increase in intrinsic and instantaneous water use efficiencies. These adaptive responses were also observed in the activities of antioxidant enzymes in leaves and fruits, which did not show significant differences between treatments. Analysis of abscisic acid (ABA)-regulated carotenoid biosynthesis genes in fruits showed that most of them were either negatively or negligibly regulated by water deficit, except for the carotene isomerases, and their responses were altered by changes in carotenoid metabolites. Principal component analysis (PCA) revealed that 9-cis-epoxycarotenoid dioxygenase 5 (LeNCED), phytoene synthase (PSY) and beta-carotene hydroxylase 1 (CRTR-B1) and 2 (CRTR-B2) were the genes that most contributed to the variations observed in the expression patterns between treatments. Overall, these findings contribute to a further understanding of tomato plant and fruit responses to moderate water deficit.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water resources have been increasingly impacted by global climate change. Therefore, studies on irrigation and the rational use of water are critical to reduce consumption and improve the efficiency of water use in agriculture (Chaves and Oliveira 2004). Water deficit alters the water balance of cells and tissues, which negatively affects many biological processes (Dai 2013; Siddiqui et al. 2015; Zhou et al. 2015; Zandalinas et al. 2018).
The acclimatization of plants to water deficit depends on a series of events that cause adaptive changes in plant growth and physiological-biochemical processes, including modifications of plant structure, growth rate, osmotic potential and antioxidant defenses (Anjum et al. 2011). Abiotic stresses such as drought increase the generation of free reactive oxygen species that attack biological structures, damage DNA, and stimulate amino acid and protein oxidation and lipid peroxidation (Jaleel et al. 2009). Plant responses to water deficit may allow a balance between growth and defense mechanisms, but this is still poorly understood (Claeys and Inzé 2013).
The narrow genetic variations in crops and the complex nature of drought-tolerance traits make the improvement of drought-tolerant varieties challenging (Hill et al. 2013; Langridge and Reynolds 2015). The observed susceptibility to water deficit calls for the improvement of more tolerant varieties that can cope with lower water availability without compromising crop yield and quality (Mickelbart et al. 2015). In this context, genetic diversity can be considered as a valuable genetic resource to improve crop productivity under unfavourable environments (Conti et al. 2019).
Drought tolerance relies on the plant's ability to capture water through the roots and to prevent water loss from leaves. Since the favorable effects of each of these processes are difficult to separate, the strategy to improve drought tolerance is related to the increase of water uptake by the root system (Yamaguchi and Sharp 2010; Slovak et al. 2016).
To obtain the necessary knowledge for the development of varieties with greater tolerance to water deficit, it is important to associate physiological analyses with the identification of the main genes involved in drought tolerance (Zhu et al. 2016). In this context, transcriptome analysis is an important approach to identify genes and understand the molecular mechanisms associated with drought tolerance (Fan et al. 2015; Sprenger et al. 2016).
An important regulator of plant response to water deficit is abscisic acid (ABA), which is also involved in regulating fruit maturation; however, a comprehensive investigation of its influence on maturation processes is still lacking. As ABA induces the transcription of genes involved in pigment metabolism, including carotenoids (Taiz et al. 2017), studies demonstrate that the PSY gene, a precursor of the carotenoid biosynthesis pathway, plays a role in stress-induced ABA formation, thus suggesting that the first committed step in carotenogenesis is a limiting step in ABA biosynthesis (Cidade et al. 2012).
Tomato is an economically relevant vegetable crop worldwide (Perveen et al. 2015; Raja et al. 2020) whose domestication caused a greater susceptibility to water deficit (Kissoudis et al. 2016; Mareri et al. 2016), as is the case of cv. Santa Clara (Oliveira et al. 2021) as compared to the wild species Solanum pennelli (Bolger et al. 2014). Pervez et al. (2009) showed that cultivated tomato seedlings are sensitive to drought, compromising the productivity of an entire harvesting season. Other studies reported that water deficit significantly affected fruit production of cultivated tomato, however, increasing irrigation status to a specific level did not positively influence production, which remained stable (Wahb-Allah et al. 2011; SHARMA et al. 2015). In addition to yield, water deficit affects the fruit volume, diameter and composition in terms of the content of total soluble solids (SSC) and carotenoids (Sivakumar and Srividhya 2016). Regarding the content of carotenoids, the effects of water deficit range from negative to zero or positive, depending on the stress intensity and duration, genotype and stage of development of the plant and fruit (Ripoll et al. 2014, 2016). All of these parameters are useful indicators of drought tolerance. In tomato, the mechanisms of drought tolerance are still not well characterized and only a small number of genes associated with this trait have been identified (Iovieno et al. 2016; Arms et al. 2017).
The present study aimed to investigate the effects of moderate water deficit, applied at the fruit development stage, on plant growth, physiology, yield and quality of fruits, as well as on the expression of carotenoid biosynthesis genes in fruits of tomato (Solanum lycopersicum L.).
Materials and methods
Plant material and experimental conditions
The experiment was conducted under greenhouse conditions at the Universidade Estadual de Santa Cruz, located in the municipality of Ilhéus, Bahia (14°47′00" S, 39°02′00" W), from November 14, 2018, to March 8, 2019, with average temperatures in the period ranging from 24 to 27 °C. Seedlings of tomato (Solanum lycopersicum L.) cv. Santa Clara was propagated by the seminal route and transplanted when they reached the stage of 4–6 true leaves in 11 L pots containing a mixture of soil and washed sand in a 2:1 ratio, and cultivated under adequate conditions of water availability and nutrients until the beginning of treatments. At the fruit fixation stage, which occurred 42 days after transplanting, the plants were subjected to two water treatments as described by Kusvuran and Dasgan (2017), with the following adaptations: (i) control, with irrigation at 100% of the substrate field capacity (FC; cm−3 cm−3) when the water content decreased to 75% of the FC and (ii) moderate water deficit, with irrigation of 40% of the total irrigation volume applied in the control treatment. The plants in the water deficit treatment were irrigated whenever the control treatment plants were irrigated. The water treatments were extended until the fruit maturation stage, totaling 72 days. Twenty-five plants were used per treatment and the decrease in water content in the containers was monitored by gravimetry.
The near-isogenic lines (NILs) in cv. Micro-Tom carrying the mutations yellow flesh (r), Beta-carotene (B) and old-gold crimsom (og) (Carvalho et al. 2011; Sestari et al. 2014) were used to further assess the impact of water deficit on the regulation of carotenoid metabolism. The r allele eliminates fruit carotenoids by disrupting the activity of the fruit-specific phytoene synthase (PSY1), whereas the B and og alleles produce fruits with high contents of, respectively, beta-carotene or lycopene, by enhancing or disrupting, respectively, the activity of the fruit-specific lycopene beta-cyclase (Cyc-B). ‘Micro-Tom’ plants were subjected to the same experimental conditions as described above for ‘Santa Clara’.
Physiological and plant growth analyses
The predawn leaf water potential (Ψw) was determined using a Model 1000 Pressure Chamber (PMS Instrument Company, USA) on the second or third fully expanded and mature leaf from the plant apex during the fruit harvesting period in five replications per treatment. Measurements were made when the fruits reached the breaker stage (B), breaker + 7 days (B + 7), and breaker + 14 days (B + 14), from 1:00 am to 3:00 am. Relative leaf water content (RWC) was determined according to procedures described by Smart and Bingham (1974), from ten leaf discs per repetition collected in parallel to the Ψw measurements. Leaf gas exchanges were evaluated between 8:00 am and 9:00 am with a Li-6400 (Li-Cor Inc., Nebraska, USA) portable photosynthesis meter, under artificial saturating light of 1000 µmol photons m−2 s−1 and atmospheric CO2 concentration of 380 ± 10 µmol mol−1, concomitantly with the days of Ψw measurements with the same five repetitions per treatment. Water use efficiency (WUE) was expressed in two ways: instantaneous water use efficiency (A/E), which was obtained by the ratio between the net photosynthetic rate (A) and the transpiration rate (E), and intrinsic water use efficiency (A/gs), which was calculated by the ratio between the net photosynthetic rate (A) and stomatal conductance to water vapor (gs). The carboxylation efficiency (A/Ci) was obtained by the ratio between the net photosynthetic rate (A) and the intercellular concentration of CO2 (Ci). The a and b chlorophyll indices were determined using a portable Falker ClorofiLOG® 1030.
Plant growth variables were analyzed at the beginning and end of treatments application by measuring stem diameter, total plant height, dry biomass of roots, stems and leaves by drying in a forced air circulation oven at 70 °C until reaching constant weight, number of leaves per plant, leaf area per plant and root volume. The productive efficiency of fruits was calculated by the ratio of the average dry biomass of fruits per plant and the average dry biomass of the fruits of their corresponding treatment. Biomass data were used to calculate leaf area, root volume and number of leaves per plant, mass ratio of roots, stems, leaves, leaf area, leaf area ratio and specific leaf mass, in addition to the relative growth rate (RGR) and net assimilation rate (NAR) according to Hunt's (1990) methodology. Biomass allocation was calculated as the ratio of the dry mass of the respective plant part to the final total dry mass. For these calculations, five plants were collected at the time of transplantation and at the end of the experiment.
Fruit yield and quality phenotyping
Fruits were evaluated in relation to the number of days from transplanting until the fixation stage and from transplanting to the breaker stage. Fruits were collected seven days after the start of the breaker stage (B + 7) and phenotyped for characteristics related to fresh weight, obtained using a precision analytical balance; to equatorial lower and upper polar diameters, measured using a manual caliper; to pH, determined with the aid of a digital pH meter (PHS-3E-BI; Ion, Araucária, Brazil); and to the total soluble solids content (SSC), obtained using an analog refractometer (0 to 32% Brix; Akso, RHB32, São Leopoldo, Brazil).
Antioxidant enzyme activities
Freeze-dried samples of mature leaves and fruits from three biological replicates per treatment collected at stage B + 7 were used for the analysis of the activities of antioxidant enzymes. The leaf and pericarp tissues of these samples were macerated in liquid nitrogen in the presence of 0.7% (w/w) of polyvinylpyrrolidone (PVP) to prevent oxidation of the material. Subsequently, 40 mg of the macerate was homogenized in 800 µl of extraction buffer, specific for each enzymatic assay, and vortexed. Then, the samples were sonicated in a GEX 130 probe ultrasonicator (Cole-Parmer, Vernon Hills, USA) under an amplitude of 70%, 8 five-second pulses, with intervals of 10 s, and subjected to centrifugation at 14,000 rpm for 10 min at 4 °C. Finally, the supernatant was collected to be used in the analysis of superoxide dismutase (SOD), ascorbate peroxidase (APX), guaiacol peroxidase (GPX) and phenylalanine ammonia-lyase (PAL) activities, as described by Gonçalves et al. (2016). The enzymatic assays were performed in quadruplicate for each biological sample in the microplate spectrophotometer (SPECTRAMax Paradigm, Molecular Devices, Sunnyvale, USA).
In silico analyses
RNA-Seq data (Illumina, San Diego, USA) obtained by Mou et al. (2015) were used to generate comparative gene expression profiles of the carotenoid biosynthesis genes in fruits of Solanum lycopersicum L. treated with ABA and its biosynthesis inhibitor nordihydroguaiaretic acid (NDGA). Fruits were harvested at the mature green (MG) stage and treated with either 25 µl of aqueous exogenous ABA solution (10 mM), aqueous solution of NDGA, or distilled water (control), by injecting the respective solutions into each fruit from the pedicle using a microsyringe (Mou et al. 2015). Fruits were kept in the dark at 20 °C and 90% RH and samples of the 9th day after the treatments were used for RNA sequencing.
RNA extraction, cDNA synthesis and quantitative real-time PCR (RT-qPCR)
Total RNA from B + 7 stage fruits was isolated using the reagent TRIzol®, following the manufacturer's instructions. The quality and integrity of isolated RNA were evaluated in 1% agarose gel and quantified with the aid of NANOdrop (Thermo Scientific™, 2000/2000c, Wilmington, USA). RNA samples were treated with RNAse-free DNAse I (Invitrogen, California, USA) after the extraction process, following the manufacturer's instructions. Reverse transcription reaction for cDNA synthesis was performed using the Applied biosystems high-capacity kit (Thermo ScientificTM, Carlsbad, USA), as per the manufacturer's instructions.
Gene expression analysis via RT-qPCR was carried out using oligonucleotides specifically designed for the amplification of carotenoid biosynthesis genes based on their available sequences in the Sol Genomics database (https://solgenomics.net/). The reference genes ACT (Solyc03g078400), GAPDH (Solyc05g014470), PP2Ac1 (Solyc05g006590) and RPL2 (Solyc10g006580) were amplified along with the target genes as endogenous controls to normalize expressions between different samples. The best reference gene was selected using the program NormFinder (Andersen et al. 2004) (https://moma.dk/normfinder-software). Table S1 shows the sequences of endogenous target and control genes. For gene expression quantification, the comparative method of Ct: 2−ΔΔCt (Livak and Schmittgen 2001) was used with data from at least three biological replicates that were individually validated using the Ampliqon RealQPlus 2 × Master Mix Green Low Rox™ kit. Control reactions devoid of cDNA (NTC) were also used in all analyses.
Statistical analyses
The experiment was carried out in a completely randomized design with 25 replications and two treatments. Initially, data were evaluated in relation to their normal distributions by the Shapiro–Wilk test and homogeneities of variance by Levene's F test. Then, statistical comparisons were performed between treatments through analysis of variance (ANOVA) and the Mann–Whitney test for variables that did not meet the assumptions of normality and/or homogeneity, and between the three temporal observations of the variables under study through pairing and solution of the Student's t test and Wilcoxon test for variables that were not compliant with the assumptions. Multivariate analyses were performed using Wilks' Lambda test, based on assumptions of normality, homogeneity of covariance-variance matrices using Box's M test, absence of multivariate outliers by calculating the Mahalanobis distance and absence of multicollinearity using Pearson's correlation analysis, and by the Pillai Trace multivariate test when any of these assumptions was violated. Multivariate factor analyses were performed using the principal components method and their adequacy was verified by the Kaiser–Meyer–Olkin (KMO) statistic and the Bartlett sphericity test. The heatmaps of gene expression and FPKM (Fragments Per Kilobase Million) data were plotted using the “ComplexHeatmap” package. All statistical analyses were performed in R software (R Development Core Team) and in IBM SPSS Statistics Software®.
Results
Impact of water deficit on plant water status and photosynthetic aspects
No significant differences for Ψw, A, gs, Ci/Ca, E, A/gs and A/Ci were observed among the three evaluation periods (B, B + 7 and B + 14) within the treatments, indicating that their respective values in the different water regimes had been previously established (Table 1). A significant difference among the evaluation periods was only observed for A/E. Between treatments, except for A and A/Ci, each of the gas exchange variables analyzed did not show significant differences in all three evaluation periods (Table 1). Thus, to investigate the effects of water contrasts, subsequent analyses were performed with the temporal means of the variables that showed a difference, at the level of 5%, between treatments.
Water deficit resulted in lower Ψw values throughout the evaluation periods when compared to the control treatment, with mean values of − 0.45 to − 0.65 MPa and − 0.28 to − 0.33 MPa, respectively. The Ψw differed between treatments, although this difference was not significant in the B stage due to a greater variance of values in plants under water deficit conditions (Fig. 1a). The RWC had no significant effect between treatments (P ≤ 0.05), ranging from 79 to 82% in the control treatment and from 75 to 79% in the water deficit treatment (Fig. 1b).
Predawn leaf water potential and relative leaf water content of Solanum lycopersicum L. cv. Santa Clara is subjected to different water regimes. A predawn leaf water potential. The points represent the mean of four to five replications and the bars represent the standard error of the mean in each temporal observation. *5% significance by Student's t test. B Relative leaf water content (%). The columns represent the mean of four replications and the bars represent the standard error of the mean in each temporal observation
The A, gs and E values of plants under water deficit were significantly reduced compared to the control treatment (Table 1). These gas exchange variables were related with Ψw, as expected, revealing that the lower the Ψw, the lower the values of the gas exchange variables (Fig. 2). In mean values, A ranged from 13.05 to 16.73 μmol CO2 m−2 s−1 in the control treatment and from 5.04 to 9.52 μmol CO2 m−2 s−1 in the water deficit treatment (Fig. 2a). For gs, the water potentials provided mean values of 0.14 to 0.52 mol H2O m−2 s−1 in control plants and 0.06 to 0.09 mol H2O m−2 s−1 in plants under water deficit conditions, showing a smaller variation when compared to the control (Fig. 2b). In relation to E, these mean values ranged from 3.13 to 7.95 mmol H2O m−2 s−1 in control plants, while in plants under water deficit these values ranged from 1.16 to 2.45 mmol H2O m−2 s−1 (Fig. 2c).
Gas exchange in Solanum lycopersicum L. cv. Santa Clara under different water regimes. A Net photosynthetic rate. B Stomatal conductance to water vapor. C Transpiration rate. The points represent the mean of the plants in the three stages of evaluation (B, B + 7 and B + 14) with four replications per treatment and the bars represent the standard error of the mean
Homogeneous groups were formed between the water regimes with the interrelationships of the gas exchange variables and Ψw. For A with Ψw, the multivariate analysis via Pillai Trace revealed a significant simultaneous effect (P ≤ 0.05) with the treatments. For gs and E, the correlation with Ψw was not significant; however, the effects of treatments on these variables, by the multivariate test by Lambda Wilks, were significant (Fig. 2).
Concerning the leaf chloroplast pigments, the obtained a and b Falker chlorophyll indices (FCI) were lower in the control treatment compared to the mean values found in the water deficit treatment. In the control treatment, the chlorophyll a concentration ranged from 26.9 to 29.9 mg dm−3, while in plants under water deficit conditions they ranged from 26.6 to 31.8 mg dm−3 (Fig. 3a). Chlorophyll b concentration ranged from 8.8 to 12.2 mg dm−3 and from 10.4 to 15.2 mg dm−3 for control and water deficit treatments, respectively (Fig. 3b). The chlorophyll a/b ratio was higher in the control (2.4 to 3.1 mg dm−3) than in the water deficit treatment (2.1 to 2.6 mg dm−3) (Fig. 3c). Although the concentrations of these pigments were not significantly different between treatments, their simultaneous relationships with Ψw show an effect by the Lambda Wilks test.
Chlorophyll a and b index in Solanum lycopersicum L. cv. Santa Clara subjected to different water regimes. A Chlorophyll a index. B Chlorophyll b index. C Chlorophyll a/b ratio. The points represent point estimates of chloroplast pigments and means of temporal measurements of water potentials that differed between treatments at the significance level of 5%
Impact of water deficit on plant growth and fruit-related variables
The stem diameter and total plant height did not show significant differences at the beginning of the treatments (Table 2), indicating that the plants were in similar conditions for conducting the different water regimes. At the end of the analyses, the effects of water deficit on these variables were significantly pronounced. Among the studied variables, the number of leaves and fruits per plant, leaf, stem and total biomass and relative growth rate showed significant differences between treatments.
Although a significant fruit yield reduction per plant was observed in the water deficit treatment, the productive efficiency in this treatment did not differ significantly from the control treatment (Table 2). The leaf, stem and root biomass allocation patterns were similar between treatments, but the statistical similarity was only observed for root allocation (Fig. 4). The leaf biomass was higher in plants under water deficit conditions, while the stem biomass was higher in the control treatment.
Biomass allocation in leaves, stems and roots in Solanum lycopersicum L. cv. Santa Clara under different water regimes. It was calculated as the ratio of the dry mass of the respective plant part to the final total dry mass. Treatments followed by the same letters do not differ statistically from each other by Student's t test (P ≤ 0.05)
Table 3 shows the data obtained for the fruit-related variables. Considering all the studied variables, only fresh biomass, dry biomass, upper polar diameter and equatorial fruit diameter differed significantly between treatments.
Exploratory multivariate factor analysis revealed the interdependent relationship of variables that translate the fruit quality under different water regime conditions. Figure 5 shows that the biomass variables have a low correlation with all other variables, in which 27.8% of total variance are shared for the formation of the second factor, while the variables UPD, LPD, ED, pH and SSC share 55.8% of the total variance for the formation of the first factor by principal component analysis. The variable SSC followed by pH had the least influence in determining the first factor. Thus, the diameters, fresh and dry weight biomass of fruits can be used as the only qualitative factors to assess a possible association between water treatments.
Impact of water deficit on antioxidant enzyme activities
Enzyme activities tended to be higher in leaves of plants under water deficit treatment, although significant differences were observed only for APX and PAL (Fig. 6). On the other hand, there were no significant differences between treatments for any of the enzymatic activities evaluated in fruits. SOD and GPX activities were slightly higher in fruits of plants under water deficit conditions, whereas the opposite was observed for the activities of APX and PAL.
Antioxidant enzyme activities in leaves and fruits of Solanum lycopersicum L. cv. Santa Clara subjected to different water regimes. A Superoxide dismutase (SOD) activity. B Ascorbate peroxidase (APX) activity. C Guaiacol peroxidase (GPX) activity. D Phenylalanine ammonia lyase (PAL) activity. Means followed by the same letters do not differ by Student's t test (P ≤ 0.05)
Impact of water deficit on the expression of carotenoid biosynthesis genes
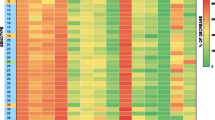
According to the RNA-Seq data obtained by Mou et al. (2015), 18 genes of the carotenoid biosynthesis pathway were differentially expressed during the maturation of tomato fruits. Concerning the effects of ABA or NDGA on the carotenoid pathway, these data showed that PSY, CRTR-B1, isopentenyl diphosphate isomerase (IPI), zeta-carotene desaturase (ZDS), carotene isomerase (CRTISO), zeta-carotene isomerase (ZISO) and NCED were up-regulated in treatment with ABA and down-regulated in treatment with NDGA (Fig. S1). On the other hand, the genes LeNCED and zeaxanthin epoxidase (ZEP) showed higher expression in the NDGA treatment, whereas neoxanthin synthase (NSY), 1-deoxy-d-xylulose 5-phosphate synthase (DXS), geranylgeranyl diphosphate synthase (GGPS) and CRTR-B2 had higher expression levels in the control treatment. Both phytoene desaturases (PDS) were inhibited by NDGA, however, their expressions varied between the control and ABA treatments.
The ABA-regulated carotenoid biosynthesis genes were selected for further analysis of their expression patterns under the water deficit conditions established in the present study, through RT-qPCR. Most carotenoid biosynthesis genes were either down-regulated or negligibly affected by water deficit in fruits of ‘Santa Clara’ (Fig. 7 and Table S2). The only exception was ZISO, which was strongly up-regulated. Because these genes belong to the same biosynthetic pathway and to avoid a Type I error with successive comparisons of means, the relationships of the set of genes with different water regimes were evaluated by means of multivariate analysis. The Lambda Wilks test showed no statistical difference (P ≤ 0.05) in the effects of water regimes on the set of expression data of the carotenoid biosynthesis genes. In exploring the expression behavior of these genes, principal component analysis showed the construction of two antagonistic factors. The first factor grouped the genes IPI, PSY, PDS, ZISO, CRTISO, CRTR-B1, CRTR-B2, NCED and LeNCED, which share 68.5% of the total variance, while the second factor grouped the genes DXS and GGPS, which share 22.7% of the total variance (Fig. S2). The genes LeNCED and PSY, followed by the genes CRTR-B2 and CRTR-B1, made the greatest contribution to the variations observed in the expression patterns of the carotenoid biosynthesis genes under different water conditions (Fig. S2).
‘Micro-Tom’ NILs harboring the r, og and B mutant alleles were subjected to the same experimental conditions as ‘Santa Clara’ to test the hypothesis of whether changes in fruit carotenoid metabolites alter the effects of water deficit on the transcription of the pathway genes. In the WT ‘Micro-Tom’, most carotenoid biosynthesis genes showed small changes of expression in fruits in response to water deficit, except for CRTISO and CRTR-B2, which were notably up- and down-regulated, respectively (Fig. 8 and Table S2). On the other hand, different effects of water deficit on the expression of carotenoid biosynthesis genes in fruits were observed depending on the mutant allele. All the carotenoid biosynthesis genes were down-regulated by water deficit in the r and og mutants, whereas the opposite was observed in the B mutant (Fig. 8 and Table S2).
Gene expression of the carotenoid biosynthesis genes in fruits of near-isogenic lines (NILs) in Solanum lycopersicum L. cv. Micro-Tom harboring the mutations yellow flesh (r), Beta-carotene (B) and old-gold crimsom (og), subjected to control (irrigated) and water deficit treatments. Expression levels were normalized to the corresponding expression value of the gene ACT. WT wild-type
Discussion
To assess how moderate water deficit applied at the fruit development stage can compromise the physiology of cultivated tomato, physiological and plant growth parameters, as well as other parameters related to photosynthesis, were monitored. Ψw decreased according to water reduction (Fig. 1a), with minimum mean values of − 0.38 MPa for the control and − 0.53 MPa for the water deficit treatment. Reduction in the water potential values was observed for a commercial tomato line under mild water deficit conditions, even when grafted in a drought-tolerant rootstock (Nilsen et al. 2014). According to Chaves et al. (2003), drought tolerance can be defined as the plant’s capacity to cope with low leaf water potential under water deficit. Our results indicate that the plants were already acclimated to the water deficit at the B stage.
One of the most important physiological responses of plants to water deficit is the inhibition or reduction of photosynthesis, which consequently leads to loss of yield (Perez-Martin et al. 2014). In Santa Clara, the effect of moderate water deficit also reflected in the decrease of A, gs, E and Ci/Ca (Table 1), with a significant relationship with Ψw (Fig. 2). The gs of plants under water deficit conditions showed a smaller variation than that of the control plants, in which the stomata may be contributing to different degrees of opening, revealing the importance of stomatal limitation in water deficit conditions. Photosynthetic efficiency is most affected by water deficit due to the impairment of photosystem II (PSII), one of the most sensitive components (Gururani et al. 2015).
Plants have developed several strategies to avoid damage caused by dehydration (Hsieh et al. 2010). According to our results, the carboxylation efficiency was lower under water deficit conditions since the decrease in stomatal conductance contributes to the reduction of the internal concentration of CO2. This relationship between photosynthetic rate and stomatal conductance confirms the important role of internal CO2 diffusion in promoting photosynthesis (Terashima et al. 2011; Pallozzi et al. 2013). Reduced CO2 flux at the carboxylation sites is one of the main causes for the reduced photosynthetic rates since water controls the stomatal opening (Bosco et al. 2009). Thus, carboxylation efficiency is affected by the availability of CO2 in the leaf mesophyll, as well as by temperature, light amount, and enzymatic activity (Taiz et al. 2017).
Minimizing water loss from stomata is a valuable approach for improving water use efficiency and drought tolerance (Hsieh et al. 2010; Kim et al. 2012). In Santa Clara, the RWC was not significantly different between the two water regimes (Fig. 1b), while the intrinsic and instantaneous water use efficiencies were significantly higher in the water deficit treatment. Yu et al. (2020) performed a meta-analysis to examine the impact of water deficit on water use efficiency and observed that water use efficiency and productivity in tomato plants can be improved under water deficit conditions. The wild tomato species S. pennellii shows greater water use efficiency than cultivated tomato and for this reason used as a source of drought resistance (Borba et al. 2017; Oliveira et al. 2021).
The leaf chloroplast pigments were not significantly different between the water treatments in Santa Clara (Fig. 3). However, together with Ψw, the effect of water deficit was pronounced. Abiotic stresses generally reduce chlorophyll contents in plants, and it may be related to the generation of reactive oxygen species (Silva and Asaeda 2017). A 45.4% decrease in total chlorophyll content was found in tomato seedlings under water deficit compared to the control conditions, a similar pattern also observed with chlorophylls a and b (Raja et al. 2020).
Another symptomatic parameter of stress-induced damage is plant growth, which is linked to energy resources generated by photosynthesis. Stress exposure leads to excessive excitation of the photosynthetic electron transport chain, altering the activity of the photosystem and causing decreased energy synthesis necessary for photosynthesis (Bechtold and Field 2018). Among the plant growth-related data, the variables with significant alterations induced by moderate water deficit were stem diameter, total plant height, number of leaves and fruits, and leaf, stem and total biomass (Table 2). These results are consistent with other studies that report a reduction in these variables, especially in cultivated tomato, which is highly susceptible to drought. Wahb-Allah et al. (2011) showed that plant height was impaired by reduced irrigation, with significant variations observed among the different genotypes analyzed. Regarding stem diameter, Conti et al. (2019) noted a slight drop in different tomato varieties 16 days after withholding irrigation.
The biomass allocation to different plant organs was similar between treatments, but significant differences were observed in leaf and stem biomass allocation (Fig. 4). The root dry mass ratio did not differ between treatments. Liu and Stützel (2004) developed a study similar to ours and observed a gain in the allocation of root biomass in stressed plants, indicating that plants under water deficit invest more energy in root development for better use of available water in the soil.
The relative growth rate had a significant effect between the water regimes, leading to its decrease in plants under water deficit conditions, as also observed by Bian et al. (2019). This can be explained by the reduction of carboxylation efficiency in plants because of partial stomatal closure under water stress conditions. Net assimilation rate and leaf area ratio had no significant changes under water deficit conditions in Santa Clara (Table 2).
Fruit quality is associated with different characteristics, including SSC and pH. In our study, the effect of moderate water deficit was significant in the fruit weight, upper polar diameter and equatorial diameter (Table 3). These were the variables that most contributed as indicators to estimate fruit quality (Fig. 5). Despite the variations observed, pH and SSC did not show a significant difference between the two water regimes, corroborating the results found in previous studies (Di Gioia et al. 2013; Nicoletto et al. 2013; Djidonou et al. 2017; Lang et al. 2020). Although there is no significant variation in these parameters between irrigation treatments, Grieneisen et al. (2018) suggest that pH and SSC measurements continue to be included in evaluations, since these variables can show large differences depending on the stress conditions imposed.
The partial closure of the stomata in response to water deficit in plants causes a reduction in CO2 and an increase in O2, thus causing an imbalance of electrons in the photosystem and the formation of reactive oxygen species (Carvalho et al. 2011). In the present study, the enzymatic activities of SOD, APX, GPX and PAL in fruits did not differ between the two treatments (Fig. 6). In tomato leaf tissue from the work of Raja et al. (2020), SOD activity increased 2.5-fold in plants under water deficit conditions compared to control, while APX activities increased three-fold. These differences in relation to our results are probably due to the different duration of the water deficit period, which was only 10 days in the study by Raja et al. (2020) and 50 days in our study, thus allowing the plants to become acclimatized to the water deficit. Consequently, the fruits that matured in this situation of more prolonged water deficit showed no differences in enzymatic activities between treatments.
In the final development stage of the tomato fruit, the color changes from green to red due to carotenoid accumulation and chlorophyll reduction (Rodrigo and Zacarias 2007). RNA-Seq data showed that the genes of the carotenoid biosynthesis pathway DXS, IPI, GGPS, PSY, PDS, ZDS, CRTISO, ZISO, CRTR-B1, CRTR-B2, ZEP, NSY, NCED and LeNCED were significantly responsive to the effects of ABA and NDGA (Fig. S1). In our study, the effects of moderate water deficit on the expression of these genes in fruits of ‘Santa Clara’ and WT ‘Micro-Tom’ were either negative or negligible, except for the carotene isomerases (Figs. 7 and 8 and Table S2). Two clusters of anticorrelated genes were observed (Fig. S2), indicating that their expression patterns were mutually exclusive. The carotenoid biosynthesis genes that most contributed to the variations in expression profiles under water deficit conditions were LeNCED and PSY, followed by the genes CRTR-B1 and CRTR-B2 (Fig. S2). These results are not quite surprising, as these enzymes catalyze limiting steps in the carotenoid biosynthesis pathway (Galpaz et al. 2006; Li et al. 2009; Zhang et al. 2009). Our study using the r, og and B mutants also confirmed the hypothesis that changes in carotenoid metabolites are able to shift the water deficit effects on the expression of the carotenoid biosynthesis genes in fruits (Fig. 8 and Table S2). These results suggest that there is an interaction between water deficit and carotenoids (r mutant) and that the increase in the concentrations of lycopene (og mutant) and beta-carotene (B mutant) in fruits produce signals that are transduced to either negatively or positively regulate, respectively, the transcription of the carotenoid biosynthesis genes in response to water deficit. The fact that carotenoid biosynthesis genes and enzymes are subjected to feedback and feedforward regulation by carotenoid metabolites supports our observations (Kachanovsky et al. 2012; Enfissi et al. 2017). Therefore, our results suggest the involvement of carotenoid metabolites in regulating feedback and feedforward the water deficit-mediated responses of pathway genes.
Conclusion
Solanum lycopersicum L. cv. Santa Clara showed adaptive responses to moderate water deficit since the photosynthetic efficiency was higher under these conditions, and with the reduction of transpiration rate, the RWC did not differ significantly from plants under control conditions. This adaptive response was also observed with an increase in the intrinsic and instantaneous water use efficiencies, as well as in the control of oxidative stress and changes in the expression profiles of carotenoid biosynthesis genes in fruits. However, these adaptive responses were not sufficient to maintain the relative growth rate of the plant and the fruit weight, diameter and yield under conditions of moderate water deficit.
Author contribution statement
DSRJ, LRC and MGCC conceived and designed the experiments. DSRJ, ACOB, IAB and NSL conducted the experiments. DSRJ, LRC and MGCC analyzed the data and drafted the manuscript. MGCC supported the project. All authors read and approved the final manuscript.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Andersen CL, Jensen JL, Ørntoft TF (2004) Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res 64:5245–5250
Anjum SA, Xie X-Y, Wang L-C et al (2011) Morphological, physiological and biochemical responses of plants to drought stress. African J Agric Res 6:2026–2032. https://doi.org/10.5897/AJAR10.027
Arms EM, Yan Z, St.Clair DA (2017) Differential transcriptional regulation in roots of tomato near-isogenic lines in response to rapid-onset water stress. Front Plant Sci 8:1–14. https://doi.org/10.3389/fpls.2017.00166
Bechtold U, Field B (2018) Molecular mechanisms controlling plant growth during abiotic stress. J Exp Bot 69:2753–2758. https://doi.org/10.1093/jxb/ery157
Bian Z, Zhang X, Wang Y, Lu C (2019) Improving drought tolerance by altering the photosynthetic rate and stomatal aperture via green light in tomato (Solanum lycopersicum L) seedlings under drought conditions. Environ Exp Bot 167:103844. https://doi.org/10.1016/j.envexpbot.2019.103844
Bolger A, Scossa F, Bolger ME et al (2014) The genome of the stress-tolerant wild tomato species Solanum pennellii. Nat Genet 46:1034–1038. https://doi.org/10.1038/ng.3046
Borba MEA, Maciel GM, Fraga Júnior EF et al (2017) Gas exchanges and water use efficiency in the selection of tomato genotypes tolerant to water stress. Genet Mol Res. https://doi.org/10.4238/gmr16029685
Bosco MR et al (2009) Efeito do NaCl sobre o crescimento, fotossíntese e relações hídricas de plantas de berinjela. Rev Ceres 56:296–302
Carvalho RF, Campos ML, Pino LE, Crestana SL, Zsogon A, Lima JE, Benedito VA, Peres LEP (2011) Convergence of developmental mutants into a single tomato model system: “Micro-Tom” as an effective toolkit for plant development research. Plant Methods 7:18. https://doi.org/10.1186/1746-4811-7-18
Chaves MM, Oliveira MM (2004) Mechanisms underlying plant resilience to water deficits: Prospects for water-saving agriculture. J Exp Bot 55:2365–2384. https://doi.org/10.1093/jxb/erh269
Chaves MM, Maroco JP, Pereira JS (2003) Understanding plant responses to drought—from genes to the whole plant. Funct Plant Biol 30:239–264. https://doi.org/10.1071/FP02076
Cidade LC, de Oliveira TM, Mendes AFS et al (2012) Ectopic expression of a fruit phytoene synthase from Citrus paradisi Macf. promotes abiotic stress tolerance in transgenic tobacco. Mol Biol Rep 39:10201–10209. https://doi.org/10.1007/s11033-012-1895-2
Claeys H, Inzé D (2013) The agony of choice: How plants balance growth and survival under water-limiting conditions. Plant Physiol 162:1768–1779. https://doi.org/10.1104/pp.113.220921
Conti V, Mareri L, Faleri C et al (2019) Drought stress affects the response of italian local tomato (Solanum lycopersicum L.) varieties in a genotype-dependent manner. Plants. https://doi.org/10.3390/plants8090336
Dai A (2013) Increasing drought under global warming in observations and models. Nat Clim Change 3:52–58. https://doi.org/10.1038/nclimate1633
de Carvalho MHC (2008) Drought stress and reactive oxygen species: production, scavenging and signaling. Plant Signal Behav 3:156–165. https://doi.org/10.4161/psb.3.3.5536
de Silva HCC, Asaeda T (2017) Effects of heat stress on growth, photosynthetic pigments, oxidative damage and competitive capacity of three submerged macrophytes. J Plant Interact 12:228–236. https://doi.org/10.1080/17429145.2017.1322153
Di Gioia F, Signore A, Serio F, Santamaria P (2013) Grafting improves tomato salinity tolerance through sodium partitioning within the shoot. HortScience 48:855–862. https://doi.org/10.21273/hortsci.48.7.855
Djidonou D, Zhao X, Brecht JK, Cordasco KM (2017) Influence of interspecific hybrid rootstocks on tomato growth, nutrient accumulation, yield, and fruit composition under greenhouse conditions. HortTechnology 27:868–877. https://doi.org/10.21273/HORTTECH03810-17
Enfissi EM, Nogueira M, Bramley PM, Fraser PD (2017) The regulation of carotenoid formation in tomato fruit. Plant J 89:774–788. https://doi.org/10.1111/tpj.13428
Fan Y, Wang Q, Kang L et al (2015) Transcriptome-wide characterization of candidate genes for improving the water use effciency of energy crops grown on semiarid land. J Exp Bot 66:6415–6429. https://doi.org/10.1093/jxb/erv353
Galpaz N, Ronen G, Khalfa Z et al (2006) A chromoplast-specific carotenoid biosynthesis pathway is revealed by cloning of the tomato white-flower locus. Plant Cell 18:1947–1960. https://doi.org/10.1105/tpc.105.039966
Gonçalves LP, Alves TFO, Martins CPS et al (2016) Rootstock-induced physiological and biochemical mechanisms of drought tolerance in sweet orange. Acta Physiol Plant. https://doi.org/10.1007/s11738-016-2198-3
Grieneisen ML, Aegerter BJ, Scott Stoddard C, Zhang M (2018) Yield and fruit quality of grafted tomatoes, and their potential for soil fumigant use reduction. A meta-analysis. Agron Sustain Dev. https://doi.org/10.1007/s13593-018-0507-5
Gururani MA, Venkatesh J, Tran LSP (2015) Regulation of photosynthesis during abiotic stress-induced photoinhibition. Mol Plant 8:1304–1320. https://doi.org/10.1016/j.molp.2015.05.005
Hill CB, Taylor JD, Edwards J et al (2013) Whole-genome mapping of agronomic and metabolic traits to identify novel quantitative trait loci in bread wheat grown in a water-limited environment. Plant Physiol 162:1266–1281. https://doi.org/10.1104/pp.113.217851
Hsieh TH, Li CW, Su RC et al (2010) A tomato bZIP transcription factor, SlAREB, is involved in water deficit and salt stress response. Planta 231:1459–1473. https://doi.org/10.1007/s00425-010-1147-4
Hunt R (1990) Basic growth analysis. Unwin Hyman, London
Iovieno P, Punzo P, Guida G et al (2016) Transcriptomic changes drive physiological responses to progressive drought stress and rehydration in tomato. Front Plant Sci 7:1–14. https://doi.org/10.3389/fpls.2016.00371
Jaleel CA, Riadh K, Gopi R et al (2009) Antioxidant defense responses: physiological plasticity in higher plants under abiotic constraints. Acta Physiol Plant 31:427–436. https://doi.org/10.1007/s11738-009-0275-6
Kachanovsky DE, Filler S, Isaacson T, Hirschberg J (2012) Epistasis in tomato color mutations involves regulation of phytoene synthase 1 expression by cis-carotenoids. Proc Natl Acad Sci USA 109:19021–19026. https://doi.org/10.1073/pnas.1214808109
Kim YM, Han YJ, Hwang OJ et al (2012) Overexpression of arabidopsis translationally controlled tumor protein gene AtTCTP enhances drought tolerance with rapid ABA-induced stomatal closure. Mol Cells 33:617–626. https://doi.org/10.1007/s10059-012-0080-8
Kissoudis C, Sunarti S, Van De Wiel C et al (2016) Responses to combined abiotic and biotic stress in tomato are governed by stress intensity and resistance mechanism. J Exp Bot 67:5119–5132. https://doi.org/10.1093/jxb/erw285
Kusvuran S, Dasgan HY (2017) Drought induced physiological and biochemical responses in Solanum lycopersicum genotypes differing to tolerance. Acta Sci Pol Hortorum Cultus 16:19–27. https://doi.org/10.24326/asphc.2017.6.2
Lang KM, Nair A, Moore KJ (2020) The impact of eight hybrid tomato rootstocks on ‘BHN 589’ scion yield, fruit quality, and plant growth traits in a midwest high tunnel production system. HortScience 55:936–944. https://doi.org/10.21273/hortsci14713-20
Langridge P, Reynolds MP (2015) Genomic tools to assist breeding for drought tolerance. Curr Opin Biotechnol 32:130–135. https://doi.org/10.1016/j.copbio.2014.11.027
Li F, Tzfadia O, Wurtzel ET (2009) The phytoene synthase gene family in the Grasses. Plant Signal Behav 4:208–211. https://doi.org/10.4161/psb.4.3.7798
Liu F, Stützel H (2004) Biomass partitioning, specific leaf area, and water use efficiency of vegetable amaranth (Amaranthus spp.) in response to drought stress. Sci Hortic (Amsterdam) 102:15–27. https://doi.org/10.1016/j.scienta.2003.11.014
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Mareri L, Faleri C, Romi M et al (2016) Heat stress affects the distribution of JIM8-labelled arabinogalactan proteins in pistils of Solanum lycopersicum cv Micro-Tom. Acta Physiol Plant. https://doi.org/10.1007/s11738-016-2203-x
Mickelbart MV, Hasegawa PM, Bailey-Serres J (2015) Genetic mechanisms of abiotic stress tolerance that translate to crop yield stability. Nat Rev Genet 16:237–251. https://doi.org/10.1038/nrg3901
Mou W, Li D, Luo Z et al (2015) Transcriptomic analysis reveals possible influences of ABA on secondary metabolism of pigments, flavonoids and antioxidants in tomato fruit during ripening. PLoS ONE. https://doi.org/10.1371/journal.pone.0129598
Nicoletto C, Tosini F, Sambo P (2013) Effect of grafting on biochemical and nutritional traits of “Cuore di Bue” tomatoes harvested at different ripening stages. Acta Agric Scand Sect B Soil Plant Sci 63:114–122. https://doi.org/10.1080/09064710.2012.729606
Nilsen ET, Freeman J, Grene R, Tokuhisa J (2014) A rootstock provides water conservation for a grafted commercial tomato (Solanum lycopersicum L.) line in response to mild-drought conditions: a focus on vegetative growth and photosynthetic. PLoS ONE 9:1–22. https://doi.org/10.1371/journal.pone.0115380
Oliveira CS, Maciel GM, Fraga Júnior EF et al (2021) Selection of tomato genotypes for drought tolerance and agronomic potential through different selection indexes. Hortic Bras 39:102–111. https://doi.org/10.1590/s0102-0536-20210115
Pallozzi E, Tsonev T, Marino G et al (2013) Isoprenoid emissions, photosynthesis and mesophyll diffusion conductance in response to blue light. Environ Exp Bot 95:50–58. https://doi.org/10.1016/j.envexpbot.2013.06.001
Perez-Martin A, Michelazzo C, Torres-Ruiz JM et al (2014) Regulation of photosynthesis and stomatal and mesophyll conductance under water stress and recovery in olive trees: correlation with gene expression of carbonic anhydrase and aquaporins. J Exp Bot 65:3143–3156. https://doi.org/10.1093/jxb/eru160
Perveen R, Suleria HAR, Anjum FM et al (2015) Tomato (Solanum lycopersicum) carotenoids and lycopenes chemistry; metabolism, absorption, nutrition, and allied health claims—a comprehensive review. Crit Rev Food Sci Nutr 55:919–929. https://doi.org/10.1080/10408398.2012.657809
Pervez MA, Ayub CM, Khan HA et al (2009) Effect of drought stress on growth, yield and seed quality of tomato (Lycopersicon esculentum L.). Pakistan J Agric Sci 46:174–178
Raja V, Qadir SU, Alyemeni MN, Ahmad P (2020) Impact of drought and heat stress individually and in combination on physio-biochemical parameters, antioxidant responses, and gene expression in Solanum lycopersicum. 3 Biotech 10:1–18. https://doi.org/10.1007/s13205-020-02206-4
Ripoll J, Urban L, Staudt M et al (2014) Water shortage and quality of fleshy fruits-making the most of the unavoidable. J Exp Bot 65:4097–4117. https://doi.org/10.1093/jxb/eru197
Ripoll J, Urban L, Brunel B, Bertin N (2016) Water deficit effects on tomato quality depend on fruit developmental stage and genotype. J Plant Physiol 190:26–35. https://doi.org/10.1016/j.jplph.2015.10.006
Rodrigo MJ, Zacarias L (2007) Effect of postharvest ethylene treatment on carotenoid accumulation and the expression of carotenoid biosynthetic genes in the flavedo of orange (Citrus sinensis L. Osbeck) fruit. Postharvest Biol Technol 43:14–22. https://doi.org/10.1016/j.postharvbio.2006.07.008
Sestari I, Zsögön A, Rehder GG, Teixeira LL, Hassimoto NMA, Purgatto E, Benedito VA, Peres LEP (2014) Near-isogenic line enhancing ascorbic acid, anthocyanin and carotenoid content in tomato (Solanum lycopersicum L. cv Micro-Tom) as a tool to produce nutrient-rich fruits. Sci Hortic 175:111–120. https://doi.org/10.1016/j.scienta.2014.06.010
Sharma P, Kothari M, Lakhawat SS (2015) Water requirement on drip irrigated tomatoes grown under shade net house. Eng Technol India 6:12–18. https://doi.org/10.15740/has/eti/6.1/12-18
Siddiqui MH, Al-Khaishany MY, Al-Qutami MA et al (2015) Morphological and physiological characterization of different genotypes of faba bean under heat stress. Saudi J Biol Sci 22:656–663. https://doi.org/10.1016/j.sjbs.2015.06.002
Sivakumar R, Srividhya S (2016) Impact of drought on flowering yield and quality parameters in diverse genotypes of tomato Solanum lycopersicum L. Adv Hortic Sci 30:3–11. https://doi.org/10.13128/ahs-18696
Slovak R, Ogura T, Satbhai SB et al (2016) Genetic control of root growth: from genes to networks. Ann Bot 117:9–24. https://doi.org/10.1093/aob/mcv160
Smart RE, Bingham GE (1974) Rapid estimates of relative water content. Plant Physiol 53:258–260. https://doi.org/10.1104/pp.53.2.258
Sprenger H, Kurowsky C, Horn R et al (2016) The drought response of potato reference cultivars with contrasting tolerance. Plant Cell Environ 39:2370–2389. https://doi.org/10.1111/pce.12780
Taiz L, Zeiger E, Møller IM, Murphy A (2017) Fisiologia e Desenvolvimento Vegetal, 6th edn. Artmed, Porto Alegre
Terashima I, Hanba YT, Tholen D, Niinemets Ü (2011) Leaf functional anatomy in relation to photosynthesis. Plant Physiol 155:108–116. https://doi.org/10.1104/pp.110.165472
Wahb-Allah MA, Alsadon AA, Ibrahim AA (2011) Drought tolerance of several tomato genotypes under greenhouse conditions. World Appl Sci J 15:933–940
Yamaguchi M, Sharp RE (2010) Complexity and coordination of root growth at low water potentials: recent advances from transcriptomic and proteomic analyses. Plant Cell Environ 33:590–603. https://doi.org/10.1111/j.1365-3040.2009.02064.x
Yu L, Gao X, Zhao X (2020) Global synthesis of the impact of droughts on crops’ water-use efficiency (WUE): towards both high WUE and productivity. Agric Syst 177:102723. https://doi.org/10.1016/j.agsy.2019.102723
Zandalinas SI, Mittler R, Balfagón D et al (2018) Plant adaptations to the combination of drought and high temperatures. Physiol Plant 162:2–12. https://doi.org/10.1111/ppl.12540
Zhang M, Yuan B, Leng P (2009) Cloning of 9-cis-epoxycarotenoid dioxygenase (NCED) gene and the role of ABA on fruit ripening. Plant Signal Behav 4:460–463. https://doi.org/10.4161/psb.4.5.8542
Zhou R, Yu X, Kjær KH et al (2015) Screening and validation of tomato genotypes under heat stress using Fv/Fm to reveal the physiological mechanism of heat tolerance. Environ Exp Bot 118:1–11. https://doi.org/10.1016/j.envexpbot.2015.05.006
Zhu M, Monroe JG, Suhail Y et al (2016) Molecular and systems approaches towards drought-tolerant canola crops. New Phytol 210:1169–1189. https://doi.org/10.1111/nph.13866
Acknowledgements
We thank Dr. Lázaro E. P. Peres (ESALQ-USP) for the donation of ‘Micro-Tom’ near-isogenic lines (NILs) seeds.
Funding
This work was supported by a research grant from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; Brasília, DF, Brazil; Process # 304878/2018-9). D.S. Rocha Júnior was a recipient of a Ph.D. scholarship provided by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES; Brasília, DF, Brazil). M.G.C. Costa is a CNPq Research Fellow. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Communicated by M. Prasad.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11738_2023_3549_MOESM1_ESM.docx
Supplementary file1 Table S1. Oligonucleotides used in the RT-qPCR analysis. Table S2. Quantitative real-time PCR (RT-qPCR) expression data for carotenoid biosynthesis genes in fruits of Solanum lycopersicum L. cvs. Santa Clara and Micro-Tom (WT and mutants) subjected to control (irrigated) and water deficit treatments. Expression levels were normalized to the corresponding expression value of the gene ACT and the relative expression was calculated by the method 2−ΔΔCt (Livak and Schmittgen 2001). Values shown are the log2 fold change of gene expression between water deficit and control treatments. Fig. S1. Heatmap of expression of the carotenoid biosynthesis genes in fruits of Solanum lycopersicum L. treated with exogenous ABA or NDGA (Nordihydroguaiaretic acid ABA biosynthesis inhibitor) and control. Data show mean FPKM values of each gene in each RNA-Seq library (Mou et al. 2015). Fig. S2. Principal component analysis performed with Euclidean distance for gene expression data of carotenoid biosynthesis genes in fruits of Solanum lycopersicum L. cv. Santa Clara subjected to different water regimes. (DOCX 156 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rocha Júnior, D.S., Barbosa, A.C.O., Batista, I.A. et al. Impact of moderate water deficit at the fruit development stage of tomato (Solanum lycopersicum L.): effects on plant growth, physiology, fruit yield and quality and expression of carotenoid biosynthesis genes. Acta Physiol Plant 45, 65 (2023). https://doi.org/10.1007/s11738-023-03549-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-023-03549-0