Abstract
Water deficit triggers physiological, biochemical, and molecular changes in leaves that could be important for overall plant adaptive response and it can affect tomato yield and quality. To assess the influence of long-term moderate drought on leaves, four tomato accessions from MAGIC TOM populations were selected on the basis of their differences in fruit size and were grown in a glasshouse under control and water deficit conditions. Drought affected stomatal conductance more in large fruit genotypes compared to cherry genotypes and this could be related to higher abscisic acid (ABA) leaf content. Compared to large fruits, cherry tomato genotypes coped better with water stress by reducing leaf area and maintaining photochemical efficiency as important adaptive responses. Accumulation of soluble sugars in the cherry genotypes and organic acid in the leaves of the larger fruit genotypes indicated their role in the osmoregulation and the continuum of source/sink gradient under stress conditions. Long-term moderate drought induced upregulation of NCED gene in all four genotypes that was associated with ABA production. The increase in the expression of ZEP gene was found only in the LA1420 cherry genotype and indicated its possible role in the protection against photooxidative stress induced by prolonged water stress. In addition, upregulation of the APX genes, higher accumulation of vitamin C and total antioxidant capacity in cherry genotype leaves highlighted their greater adaptive response against long-term drought stress compared to larger fruit genotypes that could also reflect at fruit level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Climate change is one of the most serious problems agriculture is facing today. Water scarcity and the occurrence of drought as one of the consequences of climate change may have significant impact on crop production, thus affecting crop growth and yield (Iqbal et al. 2020). To overcome the negative effects of drought, plants have developed different strategies, including drought-avoiding mechanisms (reduction of water losses and increased water uptake) and tolerance via osmotic adjustment and the antioxidant defense systems (Fang and Xiong 2015). Therefore, understanding different plant crop responses and mechanisms is essential to the selection and use of drought-tolerant genotypes.
It is well known that drought has an impact on many physiological processes. Stomatal closure caused by water deficiency has an impact on leaf growth and net photosynthesis, assimilate production and its translocation (Chaves et al. 2009) but can also adversely affect photosynthetic electron transport, and thus increase the risk of photooxidative stress (Miller et al. 2010). The decrease in the maximal photochemical efficiency of photosystem II can affect the CO2 assimilation and the production of NADPH, which are necessary for maintaining the metabolic processes of photosynthesis and plant productivity (Sivakumar et al. 2017). Drought-exposed plants have developed osmotic adjustment as a tolerance mechanism and based on the accumulation of osmolytes they can contribute to maintaining leaf water status under conditions of limited water supply. Accumulation of soluble sugars and organic acids as components of primary metabolism in the leaves has an important role in assimilate transport and the maintenance of source-sink relations (Lemoine et al. 2013). Literature data indicated an important regulatory role of hormones on primary metabolic network during tomato fruit development (Bastías et al. 2014; Li et al. 2018).
Plant growth and stomata conductance response are under the control of hydraulic signals (changes in leaf water status) and chemical signals that are induced in the root under drought conditions and then transferred to the shoot via xylem. One of the most important chemical signals is plant stress hormone abscisic acid (ABA), which is crucial for plant adaptive response to abiotic stress (Vishwakarma et al. 2017). ABA concentration in different tissues and organs is the balance between biosynthesis, catabolic processes, and transport from root to shoot. The NCED gene encodes 9-cis-epoxycarotenoid dioxygenase which is the key regulatory enzyme and corresponds to the rate-limiting step of ABA biosynthesis (Iuchi et al. 2001). Another gene involved in ABA biosynthetic pathway, ZEP, encodes zeaxanthin epoxidase that catalyzes the conversion of zeaxanthin to violaxanthin (Xiong and Zhu 2003). This enzyme also has an important role in the xanthophyll cycle that serves to protect the photosynthetic apparatus from photo-damage under abiotic stress.
Different abiotic stresses such as drought and high temperature stresses induce in the plants the production of reactive oxygen species (ROS) and generate secondary, oxidative stress. Plants respond to oxidative stress by activation of antioxidative protective systems including different non-enzymatic antioxidants and regulation of antioxidant genes and enzymes. Ascorbic acid (vitamin C) is one of the non-enzymatic antioxidants that have a significant potential for scavenging ROS, but also can modulate several physiological functions in plants under stress conditions (Akram et al. 2017). Maintaining the efficiency of photosynthesis and the optimal supply of soluble carbohydrates as precursors are important for biosynthesis and regulation of ascorbic acid levels in plants (Ntagkas et al. 2018). Vitamin C acts as a specific electron donor for ascorbate peroxidase (APX), which plays a key role in the ascorbate- glutathione cycle, one of the major detoxifying systems in plant cells under abiotic stress. Drought stress induces the activation of different antioxidative enzymes in tomato leaves (Zhou et al. 2019), as well as changes in isozyme patterns including the APX enzyme responsible for the sensitivity and tolerance of different tomato varieties against drought (Çelik et al. 2017). Among different APXs, cytosolic APX is one of the most responsive enzymes to abiotic and biotic stresses and its transcription correlates with the intensity and duration of stress (Pandey et al. 2017).
Tomato (Solanum lycopersicum L.) is one of the most widely grown vegetables in the world, with special importance due to its large consumption and high health and nutritional values. For its optimal production, the water supply and optimal temperature are essential, since most of the commercial tomato cultivars are drought and high temperature sensitive, especially at reproductive stage. Furthermore, the interaction of high temperature, water deficit and high irradiance under greenhouse conditions during summer months could generate ROS in tomato plants (Rosales et al. 2011) triggering new oxidative stress.
The study of prolonged drought on the vegetative growth of different tomato varieties (Conti et al. 2019) indicated characteristic genotype-dependent responses to stress. Research with Mediterranean tomato landraces showed that the varieties did not differ in physiological reactions under mild drought conditions, but they had very distinctive response at the biochemical and molecular level under severe stress (Giorio et al. 2018).
Although drought usually has a negative effect on tomato fruit growth and yield, some studies indicated that the positive reactions of plants to stress depended on the genotype and stage of plant development when drought was applied. Experiments with eight tomato accessions corresponding to the parental genotypes of the MAGIC TOM population showed that episodes of water deficiency during the crop cycle of tomato may negatively affect plant and fruit growth, but may improve fruit quality (Ripoll et al. 2016a, b).These data reflect the complexity of underlying mechanisms for specific tomato response at the leaf and fruit level to water stress.
Our previous study showed that long-term moderate water deficiency caused significant differences in the fruit metabolic response in a genotype-dependent manner between the four cherry and large fruit genotypes (Petrović et al. 2019). The aim of presented study was to examine the reactions of leaves of the same tomato genotypes exposed to the same drought stress by monitoring the changes at the physiological, biochemical and molecular levels. This could show whether drought-induced genotypic differences at the leaf level are also reflected at the fruit level, particularly the changes in their primary metabolites and antioxidative components. This is especially important because these components or traits contribute to the quality of the fruit and its nutritional and health value, but they are also important for improving the adaptive response of plants to drought stress.
Materials and methods
Plant material and experimental conditions
Four tomato genotypes (Solanum lycopersicum L.) were chosen among eight parents of the tomato MAGIC population to represent the largest allelic variability among many tomato accessions (Ranc 2010). Two investigated genotypes were large fruit tomatoes (Levovil and LA0147), while the two others were cocktail and cherry tomatoes (Plovdiv and LA1420). The investigations were done from March to July 2014 in a glasshouse in INRA (Avignon, France). Plants were grown in 4 l pots filled with the mixture (pH = 6): 10% white peat, 30% fibrous peat and 60% black peat, with clay. The average daily temperature in the glasshouse was between 24 and 28 °C, and during the night 17–21 °C. The daily humidity was in the range of 51–56% and 69–73% at night. The daily photosynthetically active radiation (PAR) was in the range from 5 to 11 mol m−2 day−1.
Water deficit treatment
Plants were divided into two groups. Control plants were irrigated to 70% of maximum water retention capacity in order to maintain optimal soil humidity. The second group of plants was exposed to drought when the 2nd flower truss reached the phase of anthesis and maintained 25% of maximum water retention capacity of the compost. The plants in both treatments were irrigated until the end of the experiment when the fruits in the 2nd flower truss reached the red-ripe stage. Automated irrigation system was used to maintain the soil humidity and it was controlled by WCM Grodan Control sensor (Grodan Group, Netherlands). Plants were fertilized on daily basis by Liquoplant Rose (Plantin—Courthézon, France).
Stomata conductance and water potential measurement
Stomata conductance was measured on fully developed leaves at the red-ripe stage of the fruits in the 2nd flower truss by porometer (AP4 Leaf Porometer, Delta-T Device, Ltd, UK). At the end of the experiment, young fully developed leaves were collected from 6 plants and leaf length was measured, and leaf area estimated by a LI-3100 areameter (LI-COR, USA). Fresh and dry mass of the leaves (drying at 70 °C) were also measured and specific leaf area (SLA) and leaf dry matter content were calculated. Water potential was measured by using the protocol of Scholander et al. (1965).
Chlorophyll fluorescence measurement
Chlorophyll fluorescence was measured on fully developed leaves at the end of the experiment using a fluorimeter (HANDY-Pocket PEA, Hansatech, King’s Lynn, UK). Leaves were previously dark adapted with leaf clips (15–20 min.) to allow for all reaction centers of PSII to be open and capable for photochemistry reaction. The leaves were exposed to saturating light intensity > 3000 µmol m−2 s−1 PAR for 1 s and the maximum photochemical efficiency of light harvesting of PSII (FV/FM) was calculated.
Soluble sugars and organic acids analysis
Extraction and analysis of sugars and acids from tomato leaves collected at the end of the experiment were conducted by using the protocol of Gomez et al. (2002). Sugar concentration (glucose, fructose and sucrose) was determined with HPLC (Waters, USA) using a 210 nm UV detector. HPLC system was equipped with sugar precolumn (Waters, ref. WAT015209) and Sugar-Pac I column (300 × 6.5 mm) (Waters, ref. WAT088141). Flow rate of the mobile phase (Na2Ca-EDTA—50 mg l−1) was set at 0.6 ml/min. All results were expressed as g of soluble sugar/100 of lyophilized material. Organic acid (citric and malic) analysis was done with HPLC equipped with 50 × 6 mm precolumn (RSpac KC-G, Shodex) and 300 × 8 mm column (RSpac KC-811, Shodex). Flow of the mobile phase (0.1% H3PO4) was set at 1 ml min−1. All results were expressed as g of organic acid/100 of lyophilized material.
Ascorbic acid analysis
Ascorbic acid was extracted and analyzed following the protocol of Stevens et al. (2006). Leaf powder (500 mg) was mixed with 6% TCA (trichloroacetic acid). After centrifugation the supernatant was used for ascorbic acid analysis. Analysis was performed in 96-micro-well plate by treating 20 μl of each sample with 20 μl of 5 mM DTT. DTT activity was stopped after the incubation (20 min, 37 °C) by adding 0.5% N-ethylmaleimide. In each well a coloring reagent was added based on FeCl3. After 60 min of incubation at 37 °C, absorbance was read at 550 nm (Tecan Microplate Reader, Switzerland). Commercial L-ascorbic acid was used to generate the standard curve (0–0.40 mg of ascorbic acid/mL). All results were expressed as mg of ascorbic acid per 100 g of leaf fresh weight.
Antioxidant capacity analysis
Antioxidant capacity analysis was based on the Trolox equivalent antioxidant capacity assay (protocol described by Re et al. 1999). Leaf samples were prepared by dissolving 1 g of ground sample in 80% ethanol and supernatant was used after centrifugation (room temperature, 9000 rpm). ABTS (2, 2’-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) was dissolved in PBS buffer (pH = 7.4). Manganese oxide was used for oxidation of ABTS. Different concentrations of Trolox (0–100 μmol) were used to build a standard curve. Absorbance of samples and standard solutions was measured at 734 nm (SPECTRO UV–VIS, Lambomed, Inc. USA). The results were expressed as Trolox equivalent antioxidant capacity.
ABA analysis
Leaf ABA content was measured by ELISA immunoassay (Asch 2000). Collected leaves were ground in liquid nitrogen and around 0.35 g of each sample was transferred in 2 ml tubes. In each tube 1.5 ml of miliQ water and 0.01 g of PVP (polyvinylpyrrolidone) were added. Tubes were exposed to high temperature for a few seconds in a water bath, and then placed on a thermo shaker for 24 h at 4 °C. After 24 h, tubes were put to centrifugation (15 min, 4 °C, 13,200 rpm). The supernatant was taken, and appropriate dilutions were made.
The first step of ELISA immunoassay was to coat the microplate with 200 µL ABA conjugate, which is an antigen with protein carrier. This was done the day before ELISA assay and the plate was put at 4 ºC. During the coating process, ABA conjugate bonded to the microwell walls. On the day of ELISA test, not-bonded ABA conjugate was washed out 3 times from microwells by sodium phosphate buffer and the third time the buffer was left in microwells for 20 min at 37 ºC. After that, 100 µL of the sample was added and 100 µL of primary antibody-MAC. Plate was put at 4 ºC for 3 h. Antigen from the sample competed with wall-bonded antigen for a limited amount of a primary antibody (MAC). In the next step, we added 200 µL of secondary antibody carrying an enzyme and left the plate for 1 h at 37 ºC for binding primary antibody to secondary. Substrate (p-nitro-phenyl phosphate) was diluted in NaHCO3, and 200 µl of diluted substrate was put in plates. Enzyme reacted with the substrates and it produced color reaction after a few minutes. The absorbance was measured at 405 nm. Commercial ABA was used for preparation of the standard curve (4000, 2000, 1000, 500, 250 and 125 pg /100 μl). All results were expressed as ng of ABA per g of leaf fresh weight.
Measurement of gene expression in tomato leaves by RT-qPCR
Analysis of the gene expression in the leaves included 4 genes whose ID numbers from the Sol genomics network (https://solgenomics.net/) are also presented in parentheses: NCED (9-cis-epoxycarotenoid dioxygenase—Solyc07g056570), ZEP (zeaxanthin epoxidase—Solyc02g090890), sIAPXcp (thylakoid-bound ascorbate peroxidase 6—Solyc11G018550) and sIAPXcyto (cytosolic ascorbate peroxidase 1—Solyc06G005160). The primers were designed using Primer3 Software. Forward and reverse primers of analyzed genes are presented in the Supplementary file 1.
Sample preparation
Fully developed leaves were collected at the end of the experiment and ground with liquid nitrogen and the leaf powder was kept at -80 °C until the RNA extraction step. RNA extraction was performed using TRIzol REAGENT (1 ml of TRI reagent/100 mg tissue). Separation of the sample was done by adding 200 μl of chloroform per 1 ml of TRI Reagent. After shaking and centrifugation (maximum speed for 15 min at 4 °C), 500 μl of the aqueous phase was transferred into a 2 ml tube. To the aqueous phase was added 500 μl of isopropanol, which caused the RNA precipitation. After centrifugation (maximum speed for 15 min at 4 °C) and supernatant removal, RNA pellet was washed by adding 1 ml of 75% ethanol. The sample was centrifuged at 10,000 rpm for 5 min at 4 °C and ethanol was removed. RNA pellet was air dried for 5 min and dissolved in RNase-free water (50 μl). Quality check of extracted RNA was done by agarose gel electrophoresis, while the quantity of extracted RNA was checked by NanoDrop spectrophotometer. Samples were diluted until the concentration of 200 ng of RNA/1 μl of the sample was obtained. Isolated RNA was exposed to DNase enzyme (RNase-Free DNase Qiagen kit, ref: 79,254), in order to remove DNA present in the sample and avoid contamination. To the aqueous phase was added 500 μl of isopropanol, which caused the RNA precipitation. After shaking and centrifugation (maximum speed for 15 min at 4 °C), 500 μl of the aqueous phase was transferred into 2 ml tube.
Two-stepRT-qPCR
The reverse transcription was done for all samples in order to get the cDNA. All samples of cDNA were diluted 1/15 (5 μl of cDNA and 70 μl of ultra-pure water) and 2 μl of diluted cDNA was mixed with 18 μl of reagent mixture (6.2 μl of ultra-pure water, 10 μl of Briliant II Sybr Green Master Mix—Agilent Technologies Stratagene, 0.3 μl of Rox 1/500 and 1.5 μl of primer). Real time PCR was performed (1 cycle – 95 °C for 10 min and 40 cycles – 95 °C for 30 s, 55 °C for 40 s and 72 °C for 30 s). Normalization of data was done using the reference housekeeping genes as internal control (actin depolymerizing factor 6 F-Primer: GCTCTTCCTGAGAATGACTG R-Primer: CCTGAACCTGTCCTTAGATG). This housekeeping gene was chosen after testing since it was showing the lowest variation and the highest stability of expression in 4 lines analyzed in this study. Results are presented as a log2 fold change of up or down regulation of gene expression compared to the control sample (fully irrigated plants).
Statistical analysis
Statistical analyses were performed by using SigmaPlot 12.5 Software. Descriptive statistics and a test for normal distribution was done for each set of data. The difference in physiological and biochemical parameters among two treatments of the same genotype was tested by using the t-test. The data were expressed as mean ± SE, with levels of significance marked with asterisks (p ≤ 0.001−***, p < 0.01−**, p < 0.05−*).
Results
Effect of drought on physiological parameters
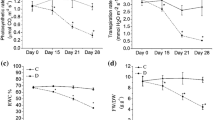
Measurements of stomata conductance as a parameter of the water regime revealed genotypic differences among cultivars in control conditions with the highest value in the genotype with larger fruits, Levovil, and the lowest in cherry genotype Plovdiv. Long-term moderate drought induced significant reduction of stomata conductance in all genotypes (Fig. 1a), but the effect was more expressed in larger fruit genotypes, Levovil and LA0147 (ca. 65%) compared to cherry tomatoes, Plovdiv and LA1420 (ca. 44%). Analysis of ABA leaf content under control conditions indicated that genotypes LA1420 and LA0147 had higher ABA concentrations in leaves than Plovdiv and Levovil (Fig. 1b). Drought-induced accumulation of ABA in tomato leaves was recorded in all genotypes, but higher in larger fruit tomatoes (Levovil + 48%, LA0147 + 37%) compared to cherry genotypes (Plovdiv + 12%, LA1420 + 17%). However, the changes in water potential values indicated the absence of significant differences between investigated genotypes. Under control conditions, the average water potential of all analyzed genotypes was between − 0.20 and − 0.24 MPa, while drought stress induced a decrease and the values of leaf water potential ranged from − 0.96 to − 1.08 MPa.
One of the most sensitive processes under drought conditions is leaf growth. Long-term moderate drought induced the reduction of the leaf length in all genotypes, but more in the cherry tomato (ca. 18%) than in larger fruit genotypes (ca.12%). Similar trend was observed with specific leaf area (SLA) as a one of the leaf traits related to drought tolerance, with a pronounced difference between cherry genotypes (ca. 34%) and larger fruit genotypes (ca. 12%). On the other hand, specific genotype-related differences in dry matter content were not noticed under stress conditions, but the decrease of SLA was followed by a 15–22% increase of dry matter content in all analyzed genotypes (Table 1).
Chlorophyll fluorescence parameters are frequently used in monitoring the drought effects on the photosynthesis, and one of them is the maximum photochemical efficiency of photosystem II. In our study, long-term drought stress significantly decreased the maximal photochemical efficiency of photosystem II (FV/FM) in all investigated genotypes (Table 1), but the effect was more pronounced in genotypes with larger fruits (ca. 22%) than in cherry genotypes (ca. 12%).
Effect of drought on biochemical parameters
Soluble sugars and organic acids are major components of tomato fruit quality, but they also have an important role in osmotic adjustment that contribute to the plant’s adaptive response to drought stress. Our results revealed genotype-specific reactions in soluble sugar content in control conditions, since the leaves of cherry tomato accumulated more glucose and fructose than the leaves of genotypes with larger fruits (Fig. 2a, b). Long-term water deficit significantly increased glucose content in all investigated genotypes, but more in cherry tomatoes (Plovdiv + 67% and LA1420 + 161%). Cherry genotypes also had more fructose under both treatments, control and drought (ca. 30% and 24%, respectively) in comparison to the genotypes with large fruits. Variation in the sucrose content under control conditions was also genotype specific with the highest value in the leaves of LA1420 cherry genotype. Drought induced sucrose accumulation was found in the leaves of all genotypes (Fig. 2c), but statistically significant only in cherry genotype Plovdiv (for 48%).
The analysis of organic acid content in control conditions indicated characteristic genotypic differences where LA1420 and LA0147 accumulated more citric acid than Plovdiv and Levovil. Under stress conditions the accumulation of citric acids was noticed in the leaves of all genotypes (Fig. 3a), but statistically significant difference was only in the genotypes with the larger fruits (Levovil + 90%, LA0147 + 26%). Similar trend was recorded for malic acid content (Fig. 3b), especially for Levovil (+ 164%).
Water deficit also triggered plant's antioxidant system. Ascorbic acid is one of its most important components and prolonged moderate drought stress has caused the accumulation in the leaves of all genotypes (Fig. 4a), but statistically significant only for the cherry tomatoes (Plovdiv + 11% and LA1420 + 63%). Total antioxidant capacity was also increased under drought conditions (Fig. 4b), especially in the leaves of both cherry genotypes (ca. 64%) compared to larger fruit genotypes (ca. 30%).
Two-way ANOVA analysis revealed the effect of genotype, treatment, and genotype-treatment interaction on investigated biochemical parameters (Table 2). The most significant effects related to glucose content as well as ABA and antioxidant parameters, were observed at all levels.
Drought effect on gene expression
One of the well-known responses to drought is the induction of the biosynthesis and accumulation of ABA, which regulates different physiological processes and adaptive reactions in plants. Therefore, our study also included molecular analysis of some of the important genes in ABA biosynthesis. Long term drought stress induced the expression of the NCED gene in the leaves of all investigated genotypes (Fig. 5), but more in the leaves of genotypes with larger fruits (log2 fold change of 0.40 for Levovil and 0.34 for LA0147) in comparison to cherry tomato genotypes(0.24 for Plovdiv and 0.27 for LA1420).The upregulation of ZEP gene in our study was observed only in the genotype LA1420 (log2 fold change 0.15), while it was down-regulated in the other genotypes (Fig. 5).
The moderate long-term drought has also induced upregulation of genes responsible for antioxidative defense (Fig. 5). The upregulation of the cytoplasmic APX gene was more expressed in the leaves of cherry genotypes (Plovdiv + 0.31 and LA1420 + 0.36) than in larger fruit tomatoes (Levovil + 0.17 and LA0147 + 0.18). The expression of chloroplastic IAPXcp was also upregulated in the leaves of cherry tomatoes (Plovdiv + 0.19 and LA1420 + 0.27), while the opposite trend was noticed in larger fruit tomatoes (− 0.17 for Levovil and—0.12 for LA0147).
Discussion
Under limited water supply, the plant water status changed and consequently led to stomata closure and reduced photosynthesis. Long-term moderate drought induced a significant reduction of stomata conductance in all investigated genotypes, but more pronounced in the leaves of the large fruited genotypes Levovil and LA0147 compared to cherry tomatoes, Plovdiv and LA1420 (Fig. 1a). These results indicated that the genotypes with larger fruits were more sensitive to prolonged water deficit than cherry genotypes. On the other hand, the measurements of leaf water potential values, as an indicator of the water status, did not reveal any statistically significant differences between genotypes in both, control and drought conditions. Investigations of Ripoll et al. (2016b) with the same cherry genotypes (Plovdiv and LA1420) showed that moderate drought applied at different stages of fruit development impacted stomata conductance more than leaf water potential and plant water status. Our results are in agreement with these conclusions.
Increase of ABA concentration in leaves and roots under drought conditions is recorded in different crops, and in tomato as well (Manzi et al. 2015; Moles et al. 2018). Our results point out that long-term moderate stress induced the accumulation of ABA in the leaves of all genotypes, but more in tomatoes with larger fruits compared to cherry genotypes (Fig. 1b). Literature data also showed that ABA accumulation in xylem sap and leaves is correlated with stomata closure in many plant species, including the tomato (Thompson et al. 2007). The reduction of stomata conductance in all investigated genotypes in our study could be related to the ABA concentrations in the leaves, which is significantly higher in the genotypes with larger fruits (Levovil and LA0147). The lack of genotype-specific differences regarding leaf water potential, as well as characteristic genotypic differences in stomata conductance and ABA content, indicate the presence of chemical signals in the response of tomato plants to drought. Literature data also demonstrated a primary role of leaf ABA in stomatal control under mild and moderate drought stress in different tomato genotypes (Giorio et al. 2018; Yan et al. 2017).
Inhibition of leaf growth under limited water supply has resulted in the changes in leaf area or dry mass, but also could be a consequence of different sensitivity of the leaf expansion process and photosynthesis to drought. Our results showed that under long-term moderate drought the reduction of the leaf length and specific leaf area (SLA) was more expressed in cherry genotypes compared with larger fruit genotypes (Table 1). This agrees with literature where moderate and severe drought stress induced the reduction of leaf growth parameters and SLA in different tomato genotypes (Calcagno et al. 2011; Rigano et al. 2016) that could be followed by increased leaf dry matter content. An increase in leaf dry matter content under drought conditions may be a consequence of reduced water content of the leaf tissue due to dehydration, or as a result of osmotic adjustment and accumulation of osmotic active compounds in the leaves. Changes of dry matter content in leaves in our study were less expressed in comparison to Ripoll et al. (2016a) experiment where the same genotypes were exposed to a gradual increase in the intensity of drought stress during fruit growth and development. These results indicated that the changes in leaf dry matter content can be a consequence of plant acclimation to stress during recovery periods between successive episodes of water deficit.
Drought stress also affects the photosynthetic apparatus, and induces changes in photosynthetic pigment content and functionality of photosystems. Reduction of FV/FM was recorded in different tomato genotypes under drought conditions (Mishra et al. 2012; Nankishore and Farrell 2016), but this effect depend on the genotype, plant development stage and especially the intensity of stress, due to higher photoinhibition and production of reactive oxygen species (Yuan et al. 2016). Less expressed reduction of Fv/Fm in cherry genotypes in our study indicated that PSII efficiently manages the excess of energy, but it also implies increased activation of antioxidant systems in the leaves of cherry genotypes, as a protective mechanism against excessive production of reactive oxygen species that could occur under long-term drought. This is consistent with the study of Ripoll et al. (2016a) where the same cherry genotypes had a less sensitive photosynthetic apparatus and photosystem II compared to large fruit genotypes under repeated episodes of drought stress with recovery periods. Activation of photoprotective mechanisms under drought conditions provide the efficiency of primary photochemical processes and also the continuity of metabolic reactions of photosynthesis and assimilate production necessary for the maintenance of “source-sink” relation (Osorio et al. 2014).
One of drought induced mechanisms is the accumulation of various soluble compounds in order to maintain a favorable plant water status. Predominant soluble sugars in tomato leaves in our study were hexoses, which accumulated 9-10 times more than sucrose in both the treatments. Specific genotypic differences were noticed in total sugar content (glucose + fructose + sucrose), since cherry genotypes showed higher sugar accumulation in the leaves under control conditions and drought conditions as well. Among cherry genotypes, LA1420 had the highest increase of total sugars under drought conditions, primarily related to higher accumulation of glucose (Fig. 2). Accumulation of hexoses in the leaves of those genotypes under successive periods of water deficit and rehydration have been reported by Ripoll et al. (2016a), but less expressed changes in glucose content in comparison to ours is probably the result of plant acclimation to gradual increase of drought stress intensity. The difference in sucrose and hexose levels depends also on the relative activities of the enzyme sucrose synthase and invertase which play an important role in sugar metabolism in the tomato fruit and are involved in the crop response to environmental factors (Beckles et al. 2012). Drought-related changes in carbohydrate composition may be the result of changes in demand between source and sink organs (Albacete et al. 2014), but also genotype specific, since severe drought induces more sucrose in the leaves of the drought tolerant tomatoes, while less resistant genotypes have reduced sucrose content due to inhibition of the net photosynthesis (Zhou et al. 2017).Organic acids, similarly to sugars, also play an important role in the process of osmotic adjustment, which results in the maintenance of photosynthesis and plant growth in water deficit conditions (Cattivelli et al. 2008). Analysis of total organic acid content indicated that the leaves of the large fruited tomatoes had higher accumulation citric and malic acid under drought conditions compared to cherry tomatoes.
The accumulation of both organic acids and sugars can indicate the presence of osmoregulation in order to maintain metabolic activity in the leaves as a source organ. The study with different tomato mutants including ABA deficient notabilis, indicated that ABA had a significant role in the regulation of primary metabolites at leaf and fruit levels (Li et al. 2018). These results may indirectly point out that drought—induced ABA accumulation in our experiment had a different effect on increasing the osmotic components in the leaves, namely the primary soluble sugars in the leaves of cherry genotypes and the organic acid content in large fruited genotypes. Our previous data indicated that long-term water deficit can influence fruit quality in large tomato fruit genotypes by accumulation of sucrose as well as the increase of citric acid (Petrović et al. 2019).Although our study on the leaf level did not include measurements of osmotic potential and turgor, the accumulation of both organic acids and sugars indicates the presence of an osmoregulation process. Therefore, it could be presumed that higher accumulation of organic acids in the leaves of large tomato fruit genotypes (Fig. 3) is reflected source-sink interactions and they take part in osmotic adjustment necessary for continuing their fruit growth in water shortage conditions as was demonstrated also by Ripoll et al. (2016b).
Water stress may influence the secondary metabolism through two interactive mechanisms: the changes of the transport of primary metabolites as a major source in the biosynthesis of ascorbic acid and carotenoids and oxidative stress which could affect the biosynthetic pathways of antioxidant compounds. Both mechanisms are closely related since the accumulation of carbohydrates may enhance photooxidative stress in photosynthetic organs (Fanciullino et al. 2014). As a result of photochemical changes and production of ROS under prolonged drought conditions plants respond by activation of antioxidant defense systems. Ascorbic acid (vitamin C) is a major antioxidant playing a vital role in the mitigation of ROS and protecting against various environmental abiotic stresses (Smirnof 2018). Vitamin C is also an important cofactor for numerous enzymes involved in plant metabolism, such as violaxanthin de-epoxidase which participates in the xanthophyll cycle and protects photosystem II from photoinhibition (Paciolla et al. 2019). Higher accumulation of vitamin C found in the leaves of cherry genotypes compared to genotypes with larger tomato fruit could be indirectly related with a better photoprotective response of these genotypes under long-term drought.
Drought stress induces an increase in the total antioxidant capacity as well as the activity of antioxidant enzymes in tomato plants (Murshed et al. 2013; Zgallaï et al. 2006; Yuan et al. 2016). The comparison of drought tolerant and susceptible tomato genotypes has shown that highly tolerant genotypes have a stronger antioxidant system, especially under severe drought stress (Rigano et al. 2016). Literature data also showed that high antioxidant activity in cherry tomato varieties was induced by oxidative stress generated by moderate water deficit (Sánchez-Rodríguez et al. 2010). Some studies indicated that ascorbic acid content could be correlated with drought resistance in tomato plants (Garchery et al. 2013). We previously reported that long-term water stress significantly increased ascorbic acid content and total antioxidant capacity in the fruits of all genotypes, especially cherry genotypes Plovdiv and LA1420 (Petrović et al. 2019). It can be assumed that the increase in drought-induced hexoses together with less pronounced fluorescence changes in cherry genotype leaves contribute to the continuous supply of substrate for biosynthesis of the secondary metabolite as vitamin C and their greater accumulation in the leaves and fruits compared to large fruited genotypes. Moreover,greater accumulation of both antioxidant components in the leaves of cherry genotypes in our study (Fig. 4), as well as in the fruits, indicate that these genotypes have a better adaptive antioxidant response to drought than genotypes with large tomato fruit.
Long-term drought stress affected the expression of the genes in ABA biosynthetic pathway. Drought-induced expression of the NCED gene in the leaves of all investigated genotypes is in line with higher leaf ABA content and indicates that the ABA accumulation is the result of increased ABA synthesis. Similar results are obtained in different NCED-over expressing plants. Up-regulated expression of NCED gene in Arabidopsis by drought induces ABA accumulation, decreases transpiration and improves drought tolerance (Iuchi et al. 2001). The upregulation of NCED that resulted in higher ABA content was also recorded in tomato under short and long-term drought conditions (Iovieno et al. 2016; Giorio et al. 2018; Landi et al. 2017). Down-regulation of ZEP gene in the most investigated genotypes in our experiment was not followed by the same pattern for ABA accumulation. Schwarz et al. (2014) showed that drought reduced ZEP protein accumulation in leaves, but this reduction does not affect the accumulation of ABA which are similar in both tissues (roots and leaves). They also confirmed that the accumulation of ZEP protein under drought conditions is tissue-specific,and implies different functions of ZEP protein in ABA biosynthesis and the xanthophylls cycle.
Investigation of over-expression of ZEP genes in Arabidopsis confirms increased drought tolerance (Park et al. 2008). Also, transgenic Arabidopsis plants with overexpression of ZEP had a lower reduction of Fv/Fm and higher activity of antioxidant enzymes, which contributes to the fact that these genotypes are more tolerant to drought (Lou et al. 2017). Taking into account that ZEP products are important for plant acclimation under abiotic stress, upregulation of ZEP gene in cherry tomato LA1420 (Fig. 5) could be related to increased photooxidative protection of this genotype under prolonged drought conditions. Under moderate drought conditions LA1420 had the highest content of phytoene and lycopene in mature fruit (Petrović et al. 2019), which indirectly implied that this genotype also had the highest amount of ZEP substrate—zeaxanthin. Our results also showed that this genotype had the highest level of lutein content, the most important compound for protection of photosynthetic apparatus from severe oxidative stress, so increased synthesis and metabolic conversion of lutein to xanthophyll could be a protection mechanism against ROS forms induced by drought stress (Huang et al. 2010).
Plants exposed to prolonged drought stress are subjected to oxidative stress due to altered photosynthetic processes and accumulation of reactive oxygen species. Effective removal of ROS could be done by different enzymatic antioxidant systems including ascorbate peroxidases (Sofo et al. 2015). Literature data reported the increase of APX expression in tomato plants under drought stress (Iovieno et al. 2016; Landi et al. 2017). APX isoforms have different expression patterns in the same plant.A cytoAPX1- deficient Aradbidopsis mutant was more stress-sensitive under drought and heat stress, while cpAPX deficient mutants did not express drought sensitivity in comparison to non-mutant plants (Caverzan et al. 2012). Although we did not measure the activity of enzymes related to ROS-detoxification mechanisms, an increase in non-enzymatic antioxidant components such as vitamin C and elevated expression of APX genes in the leaves indicated the presence of oxidative stress. Upregulation of both APX genes, cytoplasmic APX and chloroplastic IAPXcp, more expressed in cherry than in large fruited tomatoes in our study (Fig. 5) may be related to the higher drought tolerance of cherry genotypes and better capacity for recovery from oxidative stress induced by prolonged drought stress.
Conclusion
Our study pointed out the characteristic physiological, biochemical and molecular differences in drought induced responses at leaf level between cherry and large fruited tomato genotypes. Prolonged drought stress affected more stomatal conductance in the leaves of large tomato fruit genotypes compared to cherry genotypes due to higher ABA leaf content. Cherry genotypes are more able to withstand water stress due to the production of smaller leaves and more efficient photochemistry of photosystem II, which allows the maintenance of photosynthesis compared to larger fruit genotypes. Biochemical analyses indicated that the osmoregulation was predominantly dependent on the accumulation of sugars in the leaves of the cherry genotypes, while in the larger fruit genotypes it was connected to an increase in the organic acid content. Drought induced accumulation of hexoses and less expressed stomata reduction in cherry genotypes indicate a better capacity to maintain carbon fixation and photosynthesis under the stress conditions, necessary for the “source-sink” gradient, as well as for the production of secondary metabolites. In contrast, lower stomatal conductance, in addition to the changes of fluorescence in the leaves of large fruit genotypes, implies that the effect of drought on photosynthesis could be higher in these genotypes compared to cherry tomatoes. Long-term moderate drought upregulated NCED expression in all of the genotypes is correlated with ABA production. Upregulation of the APX genes in the leaves of cherry genotypes, together with higher vitamin C content and total antioxidant capacity, confirmed their greater adaptive response to drought-induced oxidative stress compared to the response of genotypes with large tomato fruit. The increase in ZEP expression found only in the LA1420 cherry genotype highlighted its role in the protection against photooxidative stress caused by prolonged drought. Further metabolic and molecular studies would help to better understand the regulatory mechanisms underlying the drought response at the leaf level in relation to the differences between cherry genotypes and large fruited tomatoes.
References
Akram NA, Shafiq F, Ashraf M (2017) Ascorbic acid-A potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front Plant Sci 8:613. https://doi.org/10.3389/fpls.2017.00613
Albacete AA, Martínez-Andújar C, Pérez-Alfocea F (2014) Hormonal and metabolic regulation of source-sink relations under salinity and drought: from plant survival to crop yield stability. Biotechnol Adv 32(1):12–30. https://doi.org/10.1016/j.biotechadv.2013.10.005
Asch F (2000) Determination of abscisic acid by indirect enzyme linked immuno sorbent assay (ELISA); Technical report. Taastrup, Denmark, Laboratory for Agrohydrology and Bioclimatology, Department of Agricultural Sciences, The Royal Veterinary and Agricultural University.
Bastías A, Yañez M, Osorio S, Arbona V, Gómez-Cadenas A, Fernie AR, Casaretto JA (2014) The transcription factor AREB1 regulates primary metabolic pathways in tomato fruits. J Exp Bot 65(9):2351–2363. https://doi.org/10.1093/jxb/eru114
Beckles DM, Hong N, Stamova L, Luengwilai K (2012) Biochemical factors contributing to tomato fruit sugar content: a review. Fruits 67(1):49–64. https://doi.org/10.1051/fruits/2011066
Calcagno AM, Rivas M, Castrillo M (2011) Structural, physiological and metabolic integrated responses of two tomato (Solanum lycopersicum L.) cultivars during leaf rehydration. Aust J Crop Sci 5(6):695–701
Cattivelli L, Rizza F, Badeck FW, Mazzucotelli E, Mastrangelo AM, Francia E, Marc C, Tondelli A, Stanca AM (2008) Drought tolerance improvement in crop plants: an integrated view from breeding to genomics. Field Crop Res 105:1–14. https://doi.org/10.1016/j.fcr.2007.07.004
Caverzan A, Passaia G, Rosa SB, Ribeiro CW, Lazzarotto F, Margis-Pinheiro M (2012) Plant responses to stresses, role of ascorbate peroxidase in the antioxidant protection. Genetics Mol Biol 35(4):1011–1019. https://doi.org/10.1590/S1415-47572012000600016
Çelik Ö, Ayan A, Atak Ç (2017) Enzymatic and non-enzymatic comparison of two different industrial tomato (Solanum lycopersicum) varieties against drought stress. Bot Stud 58:32. https://doi.org/10.1186/s40529-017-0186-6
Chaves MM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress, regulation mechanisms from whole plant to cell. Ann Bot 103:551–560. https://doi.org/10.1093/aob/mcn125
Conti V, Mareri L, Faleri C, Nepi M, Romi M, Cai G, Cantini C (2019) Drought Stress Affects the Response of Italian Local Tomato (Solanum lycopersicum L) varieties in a Genotype-Dependent Manner. Plants 8(9):336. https://doi.org/10.3390/plants8090336
Fanciullino AL, Bidel LPR, Urban L (2014) Carotenoid responses to environmental stimuli: integrating redox and carbon controls into a fruit model. Plant Cell Environ 37:273–289. https://doi.org/10.1111/pce.12153
Fang Y, Xiong L (2015) General mechanisms of drought response and their application in drought resistance improvement in plants. Cell Mol Life Sci 72:673–689. https://doi.org/10.1007/s00018-014-1767-0
Garchery C, Gest N, Do PT, Alhagdow M, Baldet P, Menard G, Rothan C, Massot C, Gautier H, Aarrouf J, Fernie AR, Stevens R (2013) A diminution in ascorbate oxidase activity affects carbonal location and improves yield in tomato under water deficit. Plant Cell Environ 36:159–175. https://doi.org/10.1111/j.1365-3040.2012.02564.x
Giorio P, Guida G, Mistretta C, Sellami MH, Oliva M, Punzo P, Iovieno P, Arena C, De Maio A, Grillo S, Albrizio R (2018) Physiological, biochemical and molecular responses to water stress and rehydration in Mediterranean adapted tomato landraces. Plant Biol 20(6):995–1004. https://doi.org/10.1111/plb.12891
Gomez L, Rubio E, Auge M (2002) A new procedure for extraction and measurement of soluble sugars in ligneous plant. J Sci Food Agric 82:360–369. https://doi.org/10.1002/jsfa.1046
Huang HJ, Zhang Q, Feng JH, Peng CL (2010) Does lutein play a key role in protection of photosynthetic apparatus in Arabidopsis under severe oxidative stress? Pak J Bot 42(4):2765–2774
Iovieno P, Punzo P, Guida G, Mistretta C, Van Oosten JM, Nurcato R, Bostan H, Colantuono C, Costa A, Bagnaresi P, Chiusano ML, Albrizio R, Giorio P, Batelli G, Grillo S (2016) Transcriptomic changes drive physiological responsesto progressive drought stress and rehydration in tomato. Front Plant Sci 31:7e371. https://doi.org/10.3389/fpls.2016.00371
Iqbal MS, Singh AK, Ansari MI (2020) Effect of Drought Stress on Crop Production. In: Rakshit A, Singh H, Singh A, Singh U, Fraceto L (eds) New Frontiers in Stress Management for Durable Agriculture. Springer, Singapore, pp 35–47
Iuchi S, Kobayashi M, Taji T, Naramoto M, Seki M, Kato T, Tabata S, Kakubari Y, Yamaguchi-Shinozaki K, Shinozaki K (2001) Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J 27:325–333. https://doi.org/10.1046/j.1365-313x.2001.01096.x
Landi S, De Lillo A, Nurcato R, Grillo S, Esposito S (2017) In-field study on traditional Italian tomato landraces, The constitutive activation of the ROS scavenging machinery reduces effects of drought stress. Plant Physiol Biochem 118:150–160. https://doi.org/10.1016/j.plaphy.2017.06.011
Lemoine R, Camera SL, Atanassova R, Dédaldéchamp F, Allario T, Pourtau N, Bonnemain JL, Laloi M, Coutos-Thevenot P, Maurousset L, Faucher M, Girousse C, Lemonnier P, Parilla J, Durand M (2013) Source-to-sink transport of sugar and regulation by environmental factors. Front Plant Sci 4:272. https://doi.org/10.3389/fpls.2013.00272
Li Y, Lu Y, Li L, Chu Z, Zhang H, Li H, Fernie AR, Ouyang B (2018) Impairment of hormone pathways results in a general disturbance of fruit primary metabolism in tomato. Food Chem 274:170–179. https://doi.org/10.1016/j.foodchem.2018.08.026
Lou Y, Sun H, Li L, Zhao H, Gao Z (2017) Characterization and Primary Functional Analysis of a Bamboo ZEP Gene from Phyllostachys edulis. DNA Cell Biol 36(9):1–12. https://doi.org/10.1089/dna.2017.3705
Manzi M, Lado J, Rodrigo MJ, Zacarías L, Arbona V, Gómez-Cadenas A (2015) Root ABA accumulation in long-termwater-stressed plants is sustained byhormone transport from aerial organs. Plant Cell Physiol 56(12):2457–2466. https://doi.org/10.1093/pcp/pcv161
Miller G, Suziki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen specieshomeostasis and signaling during drought and salinity stresses. Plant Cell Environ 33:453e467. https://doi.org/10.1111/j.1365-3040.2009.02041.x
Mishra KB, Iannacone R, Petrozza A, Mishra A, Armetano N, La Vecchia G, Trtilek M, Cellini F, Nedbal L (2012) Engineered drought tolerance in tomato plants is reflected in chlorophyll fluorescence emission. Plant Sci 182:79–86. https://doi.org/10.1016/j.plantsci.2011.03.022
Moles TM, Mariotti L, De Pedro LF, Guglielminetti L, Picciarelli P, Scartazza A (2018) Drought induced changes of leaf-to-root relationships in two tomato genotypes. Plant Physiol Biochem 128:24–31. https://doi.org/10.1016/j.plaphy.2018.05.008
Murshed R, Lopez-Lauri F, Sallanon H (2013) Effect of water stress on antioxidant systems and oxidative parameters in fruits of tomato (Solanum lycopersicon L, cv. Micro-tom). Physiol Mol Biol Plants 19(3):363–378. https://doi.org/10.1007/s12298-013-0173-7
Nankishore A, Farrell AD (2016) The response of contrasting tomato genotypes to combined heat and drought stress. J Plant Physiol 202:75–82. https://doi.org/10.1016/j.jplph.2016.07.006
Ntagkas N, Woltering EJ, Marcelis LFM (2018) Light regulates ascorbate in plants: an integrated view on physiology and biochemistry. Environ Exp Bot 147:271–280. https://doi.org/10.1016/j.envexpbot.2017.10.009
Osorio S, Ruan YL, Fernie R (2014) An update on source-to-sink carbon partitioning in tomato. Front Plant Sci 5:516. https://doi.org/10.3389/fpls.2014.00516
Paciolla C, Fortunato S, Dipierro N, Paradiso A, De Leonardis S, Mastropasqua L, de Pinto MC (2019) Vitamin C in plants: from functions to biofortification. Antioxidants 8(11):519. https://doi.org/10.3390/antiox8110519
Pandey S, Fartyal D, Agarwal A, Shukla T, James D, Kaul T, Negi YK, Arora S, Reddy MK (2017) Abiotic stress tolerance in plants, myriad roles of ascorbate peroxidase. Front Plant Sci 8:581. https://doi.org/10.3389/fpls.2017.00581
Park HY, Seok HY, Park BK, Kim SH, Goh CH, Lee BH (2008) Overexpression of Arabidopsis ZEP enhances tolerance to osmotic stress. Biochem Biophys Res Commun 375:80–85. https://doi.org/10.1016/j.bbrc.2008.07.128
Petrović I, Savić S, Jovanović Z, Stikić R, Brunel B, Serino S, Bertin N (2019) Fruit quality under water deficit - differences between cherry and large fruited tomato genotypes. Zemdirbyste 106(2):123–128. https://doi.org/10.13080/z-a.2019.106.016
Ranc N (2010)Analyse du PolymorphismeMoléculaire de Gènes de Composantes de la Qualité des Fruits dans les RessourcesGénétiquesSauvages et Cultivées de Tomate; Recherche D’AssociationsGènes/QTL. PhD thesis, Académie de Montpellier, Ecole Nationale Supérieure Agronomique de Montpellier, Montpellier.
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol Med 26:1231–1237. https://doi.org/10.1016/S0891-5849(98)00315-3
Rigano MM, Arena C, Di Matteo A, Sellitto S, Frusciante L, Barone A (2016) Eco- physiological response to water stress of drought-tolerant and drought-sensitive tomato genotypes. Plant Biosyst 150(4):682–691. https://doi.org/10.1080/11263504.2014.989286
Ripoll J, Urban L, Bertin N (2016a) The potential of the MAGIC TOM parental accessions to explore the genetic variability of tomato acclimation to repeated cycles of water deficit and recovery. Front Plant Sci 6:3–15. https://doi.org/10.3389/fpls.2015.01172
Ripoll J, Urban L, Brunel B, Bertin N (2016b) Water deficit effects on tomato quality depend on fruit development stage and genotype. J Plant Physiol 190:26–35. https://doi.org/10.1016/j.jplph.2015.10.006
Rosales MA, Cervilla LM, Sánchez-Rodríguez E, Rubio-Wilhelmi MM, Blasco B, Rios JJ, Soriano T, Castilla N, Romero L, Ruiz JM (2011) The effect of environmental conditions on nutritional quality of cherry tomato fruits, evaluation of two experimental Mediterranean greenhouses. J Sci Food Agric 91:152–162. https://doi.org/10.1002/jsfa.4166
Sánchez-Rodríguez E, Rubio-Wilhelmi M, Cervilla LM, Blasco B, Rios JJ, Rosales MA, Romero L, Ruiz JM (2010) Genotypic differences in some physiological parameters symptomatic for oxidative stress under moderate drought in tomato plants. Plant Sci 178:30–40. https://doi.org/10.1016/j.plantsci.2009.10.001
Scholander PF, Bradstreet ED, Hemmingsen EA, Hammel HT (1965) Sap pressure in vascular plants, negative hydrostatic pressure can be measured in plants. Science 148:339–346. https://doi.org/10.1126/science.148.3668.339
Schwarz N, Armbruster U, Iven T, Brückle L, Melzer M, Feussner I, Jahns P (2014) Tissue-specific accumulation and regulation of zeaxanthin epoxidase in Arabidopsis reflect the multiple functions of the enzyme in plastids. Plant Cell Physiol 56(2):346–357. https://doi.org/10.1093/pcp/pcu167
Sivakumar R, Nandhitha GK, Nithila S (2017) Impact of drought on chlorophyll, soluble protein, abscisic acid, yield and quality characters of contrasting genotypes of tomato (Solanum lycopersicum). British Journal of Applied Science and Technology 21(5):1–10. https://doi.org/10.9734/BJAST/2017/34347
Smirnof N (2018) Ascorbic acid metabolism and functions: A comparison of plants and mammals. Free Radic Biol Med 122:116–129. https://doi.org/10.1016/j.freeradbiomed.2018.03.033
Sofo A, Scopa A, Nuzzaci M, Vitti A (2015) Ascobate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. Int J Mol Sci 16(6):13561–13578. https://doi.org/10.3390/ijms160613561
Stevens R, Buret M, Garchery C, Carretero Y, Causse M (2006) Technique for rapid, small-scale analysis of vitamin C levels in fruit and application to a tomato mutant collection. J Agric Food Chem 54:6159–6165. https://doi.org/10.1021/jf061241e
Thompson AJ, Andrews J, Mulholland BJ, McKee JMT, Hilton HW, Horridge JS, Farquhar GD, Smeeton RC, Smillie IRA, Black CR, Taylor IB (2007) Overproduction of abscisic acid in tomato increases transpiration efficiency and root hydraulic conductivity and influences leaf expansion. Plant Physiol 143(4):1905–1917. https://doi.org/10.1104/pp.106.093559
Vishwakarma K, Upadhyay N, Kumar N, Yadav G, Singh J, Mishra RK, Kumar V, Verma R, Upadhyay RG, Pandey M, Sharma S (2017) Abscisic acid signaling and abiotic stress tolerance in plants, a review on current knowledge and future prospects. Front Plant Sci 8:161. https://doi.org/10.3389/fpls.2017.00161
Xiong L, Zhu JK (2003) Regulation of Abscisic Acid Biosynthesis. Plant Physiol 133:29–36. https://doi.org/10.1104/pp.103.025395
Yan F, Li X, Liu F (2017) ABA signaling and stomatal control in tomato plants exposure to progressive soil drying under ambient and elevated atmospheric CO2 concentration. EnvironExp Bot 139:99–104. https://doi.org/10.1016/j.envexpbot.2017.04.008
Yuan XK, Yang ZQ, LiYX LQ, Han W (2016) Effects of different levels of water stress on leaf photosynthetic characteristics and antioxidant enzyme activities of greenhouse tomato. Photosynthetica 54(1):28–39. https://doi.org/10.1007/s11099-015-0122-5
Zgallaï H, Steppe K, Lemeur R (2006) Effects of different levels of water stress on leaf water potential, stomatal resistance, protein and chlorophyll content and certain antioxidative enzymes in tomato plants. J Integr Plant Biol 48(6):679–685. https://doi.org/10.1111/j.1744-7909.2006.00272.x
Zhou R, Yu X, Ottosen CO, Rosenqvist E, Zhao L, Wang Y, Yu W, Zhao T, Wu Z (2017) Drought stress had a predominant effect over heat stress on three tomato cultivars subjected to combined stress. BMC Plant Biol 17(1):24. https://doi.org/10.1186/s12870-017-0974-x
Zhou R, Kong L, Yu X, Ottosen CO, Zhao T, Jiang F, Wu Z (2019) Oxidative damage and antioxidant mechanism in tomatoes responding to drought and heat stress. Acta Physiol Plant 41:20. https://doi.org/10.1007/s11738-019-2805-1
Funding
This study was supported by the EU Commission (FP7 project AREA) and the Ministry of Education, Science and Technological Development of the Republic of Serbia (Grant No. 451-03-68/2021-14/200116 and Grant No. 451-03-9/2021-14/200216).
Author information
Authors and Affiliations
Contributions
Ivana Petrović: Investigation, Methodology, Formal analysis, Writing—original draft; Justine Gricourt, Slađana Savić: Methodology, Formal analysis, Resources, Zorica Jovanović, Radmila Stikić: Conceptualization, Validation, Supervision; Radmila Stikić, Mathilde Causse: Review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
No ethical issues were violated in this study.
Consent to participate
All authors agree to participate.
Consent for publication
All authors agree on publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Petrović, I., Savić, S., Gricourt, J. et al. Effect of long-term drought on tomato leaves: the impact on metabolic and antioxidative response. Physiol Mol Biol Plants 27, 2805–2817 (2021). https://doi.org/10.1007/s12298-021-01102-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-021-01102-2