Abstract
Seed germination (SG) is crucial for the survival of plant species; however, it is often subjected to unpredictable environmental conditions such as temperature (T) and salinity. Therefore, the objectives of this work were to concurrently investigate salinity and T effects on SG behavior of green bean using a halothermal time (HaloTT) model, to compute the cardinal Ts, and to explore the effect of salinity on seeds’ salt ion assimilation (i.e., Na+ and Cl−). Our findings revealed that the effect of salt stress on SG was quantified well by the HaloTT model (R2 > 0.82). The estimated cardinal Ts at 0 mM were 8.15, 30.0, and 39 °C for the base (Tb), optimum (To), and ceiling (Tc) temperatures, respectively. The median threshold NaCl concentration (NaClb(50)) was 305 mM at To, then declined linearly at Ts ≥ To, reaching 0 mM at Tc. Decreasing germination capability at Ts above To was due to the shifts in NaClb(50), which appears as a thermoinhibition phenomenon. Na+ and Cl− assimilation enhanced significantly with T and seed ψ decreased compared to control. In conclusion, green bean SG was initially controlled by salt osmotic effect (≤ 150 mM), when exceeding this concentration, its effect became both osmotic and toxic.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Green bean (Phaseolus vulgaris L.) is one of the most important fresh vegetable crops cultivated in many countries. The world production of this plant is estimated at 27 million tons in 2019, with an annual rising of 2.8% over the period from 2013 to 2019 (FAO 2019). It is a very widespread crop of most temperate, moist-to-dry tropical and subtropical climates. Green bean is mainly produced in China, India, Indonesia, Turkey, and Thailand (FAO 2019). The global green bean market was $31.1B in 2019, 4.7% higher than the previous year.
Seed germination (SG) is one of the key phases for the survival of plant species; however, it only occurs if external conditions (e.g., humidity, temperature, light, etc.) and internal factors (e.g., maturity, viability, dormancy, etc.) are favorable (Bewley et al. 2013). Temperature (T) is one of the major environmental factors that control SG (Abdellaoui et al. 2017; Bakhshandeh et al. 2021). Each species is characterized by cardinal Ts, varying from one species to another depending on the environment to which it is adapted (Bakhshandeh et al. 2021; Bakhshandeh and Gholamhossieni 2019; Hardegree 2006). An optimum temperature (To, single and/or range of Ts) at which seeds germinate quickly and vigorously, a base temperature (Tb), and ceiling temperature (Tc) upon which, germination is inhibited (Bewley et al. 2013).
Numerous studies showed that germination rate (GR), that is, the number of days/hours required to reach a specific percentile of germination capacity are seriously affected by T (Bakhshandeh and Eslami 2013; Bakhshandeh et al. 2013, 2021; Derakhshan et al. 2018; Abdellaoui et al. 2019). It increases linearly at Tb ≤ Ts ≤ To, while at Ts ≥ To, it decreases linearly and/or curvilinearly (Alvarado and Bradford 2002; Bakhshandeh and Gholamhossieni 2019; Elahifard et al. 2021).
Several unpredictable environmental factors such as T, water stress, and salinity, etc. on one hand and seed hydration, storage conditions, seed dormancy, etc. on the other hand often aggravate SG (Bewley et al. 2013; Baskin and Baskin 2014; Bakhshandeh and Gholamhossieni 2019; Bakhshandeh and Jamali 2020; Elahifard et al. 2021).
Changes in climate patterns are significantly influencing SG. Based on climate simulation models, an increase in the average global T, altered weather patterns with shifts in rainfall frequency and intensity, and an increase in saline land area are expected (Corwin, 2021). Soil salinization is a global problem that has influenced 833 million ha of agricultural land in over the world (FAO 2021). Also, it was estimated that fifty percent of the irrigated land could be salinized by the year 2050 (Talat 2020; Singh 2021). Indeed, soil salinization is increasing at the rate of 1–2 million ha year–1 globally, influencing a remarkable portion of crop production and making land unsuitable for cultivation (Devkota et al. 2022). To meet global food security, the agricultural land (i.e., irrigated area) needs to be increased from current 202–242 million ha in 2030 (FAO News 2021). In addition, the demand for irrigation is greater in arid- and semi-arid regions, where more than 90% of agriculture depends on irrigation. However, the increase of salt concentration in the medium, delayed SG, and increased latency. This is explained by the time needed for seeds to develop mechanisms to regulate their internal osmotic pressure during saline conditions (Hajlaoui et al. 2007). In general sodium chloride (NaCl) in soil or irrigation water decreases GR and reduces germination capacity (Llanes et al. 2016). This effect depends on the species, the severity, and the duration of the salt stress (Ben Naceur et al. 2001; Tobe et al. 2001). Therefore, monitoring the effects of salinity due to climate change is crucial to study the extent of the problem, to recognize trends, and to formulate some management strategies, particularly during the early stage of plant growth (i.e., SG).
Seed germination decrease is due to either an increase in the external osmotic pressure (i.e., in soil solution), which slows down seed imbibition and limits the absorption of water necessary to trigger the metabolic processes involved in germination and/or excess accumulation of Na+ and Cl– in the embryo, which can lead to impaired metabolic processes of germination and in extreme cases, death of the embryo. However, to some extent, the accumulation of compatible solutes (i.e., soluble carbohydrates, proline, betaine, organic acids, etc.) and the assimilation of inorganic ions (i.e., Na+ and Cl–) are necessary or even mandatory to maintain seed hydration following osmotic adjustment leading to an increase in the negativity of the osmotic potential of seeds (Bakhshandeh et al. 2020; Zhang et al. 2010). Nevertheless, the combined effect of salinity and T is more pronounced for Ts > or < To.
El-Bastawisy et al. (2018) showed that Vicia faba SG was markedly affected, especially germination speed, at 200 mM NaCl. Cokkizgin (2012) reported that the coefficient of the velocity of germination, germination index, and seed vigor index, in Phaseolus vulgaris L., decreased with increasing NaCl concentration while mean germination time increased. They also revealed that the germination percentage (GP) of this plant decreased up to 60% for seeds germinating in Petri dishes at − 1.5 MPa NaCl. Contrarily, Bayuelo‐Jiménez et al. (2002) pointed out that increasing salinity (180 mM NaCl) decreased germinability by 50% in species of the genus Phaseolus.
Population-based threshold models are frequently used to depict seed response to the environmental conditions during germination and emergence stages (Bello and Bradford 2016; Abdellaoui et al. 2019; Bakhshandeh et al. 2020; Liu et al. 2020b). One type of these models are halotime (HT) and halothermal time (HTT) models which were successfully used for concurrently describing the effect of NaCl and T and, their interactions on SG of diverse plants such as Suaeda maritima (a model halophyte plant) (Seal et al. 2018), rapeseed (salt-tolerant) (Bakhshandeh and Jamali 2020), chicory (moderately salt-tolerant) (Vahabinia et al. 2019), and cucumber (moderately salt-sensitive) (Bakhshandeh et al. 2021). Indeed, knowing this information at the species level could increase our ability to predict species distribution shifts under climate change. The parameters of these models are strongly flexible, interpreting simply and physiologically, and also describing how environmental factors participate to regulate SG within a seed population (Bradford 2018). Also, a high correlation between the germination results obtained in laboratory tests and the emergence results collected from field conditions was reported by Liu et al. (2020a), who studied the SG of 13 desert species in Arizona over 25 years.
Therefore, our objectives in the present study were (1) to concurrently investigate salinity and T effects on SG of green bean using the HaloTT model, (2) to compute the cardinal Ts for this plant, (3) to explore the effect of salinity on salt ions accumulation (i.e., Na+ and Cl−) into the seeds of green bean.
Materials and methods
Seed source
Seeds of green bean (Phaseolus vulgaris L.; Fabaceae, var. Sunray) were provided in Mazandaran province, Iran, in 2018. It was produced by PROSEED Company (Netherlands). At the beginning of the experiments, the seeds had six months old and their viability was > 90% (assessed by ISTA method). It was also kept at 5 °C before use.
Treatments
In the present work, the effect of five constant Ts (i.e., 15, 20, 25, 30, and 35 °C) and five salinity levels (0, 75, 150, 225, and 300 mM) were concurrently investigated. This means that, at each T, all five salinity levels were studied. Distilled water was used at 0 mM treatment (as control) in all Ts. For preparing the above-mentioned salt concentrations, NaCl was used.
Seed germination test protocol
Twenty-five seeds per Petri dish (9 cm diameter) were placed between germination filter paper (two layers below and one layer on top of the seeds) with three replicates. Then, 13 mL of the test solutions, supplemented with 0.1% Thiram fungicide, was added per Petri dish. The dishes were put into a thin white plastic bag for minimizing water evaporation losses and then randomly placed within an incubator with ± 0.5 °C precision. The dishes were checked several times daily, depending on the T and salinity levels and the germinated seeds were immediately counted. In each recording time, the seed at least 0.2 cm radicle length was considered as germinated seed. The experiments were finished when no more germination occurred in each Petri dish for three continuous days.
Halotime and halothermal time models
To quantify salt stress effect on SG of green bean, a halotime model proposed by Bakhshandeh et al. (2020) was fitted in each T. This model can be written as:
A HaloTT model, first developed by Bakhshandeh et al. (2020), was also fitted to the experimental data obtained at all Ts and salinity levels. The model can be expressed as
According to the results reported by Bakhshandeh and Gholamhossieni (2019), the Td value may be equal and/or lower than To, depending on plant species. All the parameters of these models are defined in detail in Bakhshandeh and Jamali (2020).
Seed moisture content, Na + , and Cl– concentration
To determine the Na+ and Cl− concentration (mmol kg DW−1) into the green bean seeds, at all tested Ts and salinity, the method reported by Bakhshandeh et al. (2020) was used. Briefly, 15 g of the seeds was grown under the same conditions that are described fully in “Seed germination test protocol”. Then, the seeds were harvested from each Petri dish immediately before germination (this time was accurately estimated from Fig. 1) and washed carefully with distilled water for removing any tested solution from the testa. For the seed moisture content (SMC, %) measurement, the seeds were immediately ground and dried in an oven at 130 °C for 1 h (ISTA 2009), then determined as a dry weight basis. For the Na+ and Cl− contents measurement, a flame photometric and the Mohrs’s titration method were used, respectively (Estefan et al. 2013). To calculate the total osmolality (mmol/kg H2O) of the seeds, made by the salt ions (i.e., Na+ and Cl−), we assumed that all of the water into the seed will be symplastic. To convert the total osmolality of the seeds to ψ (MPa) the method suggested by Van’t Hoff (1887) was used:
where R is the gas constant (i.e. 0.0083 L/atm/mol/K), T is the absolute temperature in degrees in Kelvin (273.15 °C), C is the solute concentration in mol L−1, and i is the dissociation constant of the salt (i.e. NaCl = 1.8). The unit for ψs is MPa.
Statistical analysis
To determine the model parameters, a repeated probit regression analysis reported by Bradford (1990) was applied. The statistical analysis system ver. 9.4 (SAS Institute Inc 2015) and Excel software ver. 2013 was used for analyzing the data. Besides, the Sigma Plot ver. 11 software was used to draw the figures. Also, analysis of variance of the data and means comparison using the least significant difference test was carried out using SAS software.
Results
Germination characteristics affected by temperature and salt stress
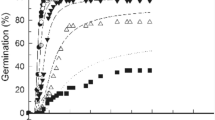
The T, salinity and the combined effect of them [T × salinity] had significantly (P < 0.001) affected GP of green bean seeds (Table 1; Fig. 1a–e). The seeds in distilled water showed a germination > 90% at 25 °C and then declined remarkably with T reaching ~ 70% at 35 °C. The control (0 mM NaCl) and treated (75 mM NaCl) seeds showed no significant difference at 20–30 °C relating to the final GP. However, for the same treatment (75 mM NaCl), a significant decrease was detected at 15 and 35 °C. By increasing salinity (> 75 mM NaCl), GP decreased significantly compared to the control (at all Ts) and was inhibited at 300 mM NaCl at both 15 and 35 °C (Fig. 1a–e).
The same results were observed for GR, meaning that the T, salinity and the combined effect of them [T × salinity] had significantly affected GR (P < 0.001, Table 1). Following the same pattern as GP, the highest GR50 (GR for the 50th percentile) of green bean seeds was found at 25–35 °C (Fig. 1a–e). GR50 also decreased severely with increasing NaCl concentration at all Ts. It should be noted that the salt stress effect was harsher at 20 °C ≥ Ts ≥ 30 °C for both GP and GR parameters (Fig. 1a–e; Table 1).
Halotime and halothermal time models
The halotime model (Eq. 1), fitted to the green bean SG data showed a coefficient of determination (R2) value of > 0.80 (Table 2). The registered θHalo value non-linearly decreased from 27,000 mM h for 15 °C to 7000 mM h for 35 °C (Table 2; Fig. 2a). However, at Ts < 30 °C, the NaClb(50) remained constant (averaged 308.7 mM), then declined linearly (kT = 34.0 mM °C−1) with T at Ts > To, reaching 0 mM at Tc (i.e., 36.0, 39.0 and 42.2 °C for the 16th, 50th and 84th percentiles, respectively) (Table 2; Fig. 3b). For instance, NaClb(50) was 132.8 mM at 35 °C (Table 2). The σNaClb values were similar at all Ts (averaged 107.1 mM), except for 15 °C (150.3 mM), indicating that SG was homogenous at 20 °C ≤ Ts (Table 2). A linear model was used to quantify the inverse of θHalo values (1/θHalo) versus T. The interception value of the linear model broken the x-axis at 8.2 °C (defined as Tb) (Fig. 2b).
a Changes in halotime constant for green bean as a function of temperature. b The relationship between the inverse of halotime constant values with temperature which is linear and the intercept is equal to 8.2 °C (Tb). In each panel, the symbols and the lines are represented the actual and predicted data for the 50th percentile, respectively
a The cardinal temperatures for germination of green bean seeds using the halothermal time model. Based on the models' behavior, the base temperature (Tb) and optimal temperature (To) were constant for all seed fractions in this species while the ceiling temperatures (Tc) varies with the seed fraction, being 36.0, 39.0, and 42.2 °C in water at the 16th, 50th and 84th percentiles, respectively. b The NaClb(g) decreased linearly as T increased at the supra-optimal T range for the seed fractions. In panel b, the lines for different seed fractions (16, 50 and 84%) intercept the NaClb(g) = 0 mM axis at the Tc values for these fractions. The actual data are shown by the symbols and the lines drawn through these points are based upon the parameters presented in Table 3
The HaloTT (Eqs. 2 and 3) model was fitted to investigate green bean SG response under different NaCl concentrations at all ranges of Ts. Results revealed that a high R2 value (R2 = 0.82), which assigns a high correlation between observed and predicted germination data for each set of T (Fig. 4; Table 3).
Linear regression of probit (g) plotted against base NaCl concentrations (NaClb(g), mM) and adjusted base NaCl concentrations (− NaClb(g) + kT(T – Td)). Points are the observed values of probit germination fraction at each set of temperature and the lines represent the predicted values by the models (p < 0.05)
The cardinal Ts of green bean seeds were calculated based on the HaloTT model (Fig. 4). The Tb and To values remained steady in all seed fractions with 8.2 and 30 °C, respectively (Table 3; Fig. 3a) whereas the Tc was 36.0, 39.0, and 42.2 °C at 0 mM NaCl (i.e., control) for the 16th, 50th, and 84th percentiles, respectively (Fig. 4a). Besides, the GR variation under the combined effect of salinity and T indicated a high remarkable relation between GR and NaCl concentrations at all Ts. At Ts ≤ 30 °C, GR showed a linear increase then curvilinearly decreased at Ts ≥ To with increasing T (Fig. 4a).
In Fig. 3b, NaClb(g) was roughly steady at Ts below To and then dropped off linearly at Ts ≥ To, reaching 0 mM at the intersection of the x-axis at the Tc values. At sub-optimal Ts, the NaClb(g) values were 198.3 mM for the 16th, 305.0 mM for the 50th, and 411.7 mM for the 84th percentiles (Fig. 4b). After conversing these values to ψ (MPa) by the Van’t Hoff method, they correspond to − 0.90, − 1.38, and − 1.86 MPa, respectively.
Seed moisture content, salt ions (Na+ and Cl−) content into the seeds
The SMC (determined immediately before germination) showed a significant increase at all studied Ts under salt stress in compassion with the control (Fig. 5a; small letters) and decreased significantly at T < 30 °C then enhanced notably at T > 30 °C (Fig. 5a; capital letters).
a–d Seed moisture content, the concentration of Na+, Cl− and osmotic/solute potential of the seeds (after converting NaCl to ψ based on Van’t Hoff equation) for green bean following imbibition in different concentrations of NaCl at five constant temperatures. In all panels, the values in the y-axis are different. Lower case letters show significant differences among NaCl treatments within a temperature and upper case letters show significant differences among temperatures, using the least significant difference test (LSD) at 0.05 probability level. **Significant at 0.01 probability level
At each studied T, seed Na+ concentration enhanced remarkably (P < 0.05) under salt stress in compassion with the control (Fig. 5c; small letters). For example, at 30 °C the Na+ concentration registered was 57.6 mmol kg−1 DW at 0 mM NaCl (i.e., control seeds) and 181.8 mmol kg−1 DW for treated seeds (300 mM NaCl). However, the combined effect of both stresses (i.e., NaCl and T) gave the most important Na+ content (221.5 mmol kg−1 DW, averaged for all salt stress levels) for treated seeds under 300 mM NaCl at 35 °C. When considering the influence of T under various concentrations of salinity, Na+ content decreased with T at T > 15 °C, remained approximately steady between 20 and 30 °C, then enhanced at the Ts above 30 °C (Fig. 5c; capital letters).
Similarly, seed Cl− content showed the same pattern as Na+ content; it increased remarkably (P < 0.05) with enhancing salt stress levels at all Ts (Fig. 5b; small letters). The treated seeds (300 mM NaCl) concentrated more Cl− content (99.2 mmol kg−1 DW) compared to control (30.6 mmol kg−1 DW) at supra-optimal Ts (i.e., 35 °C). However, the least Cl− content was registered for control seeds (15.7 mmol kg−1 DW) at 30 °C (as To). The lowest seeds Cl− content was observed at 20 ≤ T ≤ 30 °C (averaged for all levels of salt stress), then increased significantly at 15 > T > 30 °C (Fig. 5b; capital letters).
Treated seeds showed a significant decrease (P < 0.05) of their osmotic/solute potential (estimated by the Van’t Hoff method) across all studied Ts in compassion with the control (Fig. 5d; small letters). For example, seed ψ became more negative from − 0.45 MPa (control) to − 1.17 MPa (300 mM NaCl) at 35 °C. Besides, the ψ of green bean seeds decreased significantly (P < 0.05) at Ts above 15 °C and remained unchanged for Ts ≥ 20 °C (Fig. 5d; capital letters).
Accordingly, at lower osmotic potentials, the salt ions (i.e., Na+ and Cl−) could be absorbed by the seeds and so enhancing the turgescence and germination capacity. However, this osmotic adjustment was achieved for treated seeds (75 mM NaCl) at 20 to 30 °C (Figs. 1b–d and 5d). Salt ions uptake is therefore satisfactory at some extent (lower ψs) to enhance SG rather than being toxic.
Discussion
Seed germination is strongly affected by different environmental factors (i.e., T, NaCl, ψ, etc.). Therefore, SG modeling under stressful environmental conditions could help us to better understand germination requirements for finding appropriate geographical areas, where a specific species can grow.
The results showed that the GP and GR were significantly influenced by T (across all studied Ts), which agrees with the findings reported in previous studies (Abdellaoui et al. 2019; Bakhshandeh and Eslami 2013; Bakhshandeh and Gholamhossieni 2019; Derakhshan et al. 2018). In addition, decreasing GR and GP with salt stress was reported, especially when salinity exceeding the threshold above which SG is inhibited (Bakhshandeh et al. 2021; Seal et al. 2018). These results are totally in agreement with those showing that salinity to some extent reduces and/or delays the SG and the loss of seeds before germination (Bakhshandeh et al. 2021; Song et al. 2005). In general, salinity influences SG in several ways: (i) high accumulation of salt ions in the medium causes osmotic and pseudo-drought stress, leading to a decrease in water absorption by the seeds which is necessary for nutrient mobilization during SG (Rajabi Dehnavi et al. 2020), (ii) it can cause changes in enzyme activity by the toxicity effect of ions, leading to a changing in the metabolism of nucleic acid and protein (Gomes-Filho et al. 2008), disturbing the hormonal balance (Ryu and Cho 2015), and reducing the use of seed reserves and (iii) the high levels of salt ions may be toxic to the embryo growth (Kaymakanova 2009). However, different internal factors of a seed, such as coat properties, age, polymorphism, dormancy and, seedling vigor; and external factors, such as T, light, and water, can influence SG under salt stress (Wahid et al. 2016).
The models applied in this study (i.e., halotime and HaloTT) illustrated well green bean SG under salt stress (Tables 2 and 3). Results showed that the NaClb(50) of green bean was 305 mM in line with the results of Cokkizgin (2012), who showed that GP of common bean was reduced to 60% at 20 °C when seeds were treated with 17.7 g/L of NaCl (302.8 mM). The NaClb(50) was unvarying with Ts < To in the studied seed population, similar to the results on chicory (Bakhshandeh et al. 2020) and opposing to the results on Suaeda maritima (Seal et al. 2018). At Ts > To = 30 °C, NaClb(50) values decreased linearly confirming results obtained in chicory (Bakhshandeh et al. 2020) and cucumber (Bakhshandeh et al. 2021). Indeed, the decline in SG capability at Ts > To is due to the shifts in the NaClb(50) value, indicating the seeds needed to absorb much water for germination, which appears as a thermoinhibition phenomenon (Derakhshan et al. 2018). On the other hand, this phenomenon can be defined as an adaptive strategy by the seeds, meaning that SG will not occur when they are exposed under stressful conditions (i.e., high T and salinity), keeping their germination capability to reach an appropriate environmental condition (Hosseini Sanehkoori et al. 2021).
In previous researches on common beans, the Tb was 7–8 °C (White and Montes 1993) and 8.4–9.2 °C (Cardoso and Bianconi 2013) in line with our results (8.2 °C). Raveneau et al. (2011) also reported that Tb of common bean was 5.1–9.6 °C depending on seed lot. Green bean seeds germination necessitates temperate Ts with moderately high Tb (8.2 °C) and an extremely high To (30 °C). Contrarily, Balkaya (2004) showed that To ranged from 23.0 to 24.6 °C depending on green bean varieties. In all studied seed fractions, both Ts (Tb and To) were steady under salinity levels (Fig. 3a), whereas the Tc values were changed (i.e., 36.0, 39.0, and 42.2 °C for the 16th, 50th, and 84th percentiles at 0 mM, respectively) confirmedly to Alvarado and Bradford (2002), and Bakhshandeh et al. (2021) results. In common bean, Kurtar (2010) found the Tc value was 40 and 42 °C, respectively, distinguishable from our results on green bean (39.0 °C). Our findings are also fully agreed with the results obtained from different cultivars of green bean (Carioca, Grauna, and Uirapuru with Tc = 38.5, 38.6, and 38.9 °C, respectively) (Cardoso and Bianconi 2013). The T interval between Tb and Tc varies according to the physiological state of the seeds, is often restricted for dormant seeds, and heightens when dormancy is broken (Bradford and Somasco 1994).
The SMC, Na+ and Cl− contents of green bean seeds increased with increasing salinity levels (Fig. 5), which is in agreement with the results on Atriplex canescens and Salicornia pacifica (Khan et al. 1985), Haloxylon ammodendron and Suaeda physophora (Song et al. 2005), Suaeda maritima (Seal et al. 2018), Cichorium intybus (Bakhshandeh et al. 2020), and Cucumis sativus (Bakhshandeh et al. 2021). In our study, the measured uptake of Na+ and Cl−
ions during imbibition in salt solutions was sufficient to account for the lower apparent ψb(50) values after converting the NaCl concentrations to osmotic potentials, meaning that the salt ions absorption permitted the seeds grown in a salty medium to preserve lower ψb(50) (i.e., − 1.38 MPa; corresponding to elevated NaClb(50) = 305 mM NaCl at To) (Bakhshandeh et al. 2021, 2020). This could be explained by the fact that SG was primarily controlled by salt osmotic effect rather than toxic to some extent (150 mM NaCl for green bean), when exceeding this NaCl concentration, its effect became both osmotic and toxic (Bakhshandeh et al. 2021; Seal et al. 2018; Zhang et al. 2010).
Of course, it is not only a physical–chemical effect, but also depends on the physiology and genetics of the species (Tlahig et al. 2021). However, the water gradient between the seed and the medium is important for the uptake of water in the germinated seed, but also are the cellular strategies that include the detoxification and cell protection mechanisms that allow the seed to germinate until 150 mM NaCl.
Seed germination of green bean, across all studied Ts and salinity levels, was accurately modeled by the HaloTT model. All the evidence showed that green bean (e.g., Sunray variety) could be considered as a moderately salt-tolerant species in the seed germination stage and also could successfully be cultivated in moderate water-deficient regions (Bourgault 2009; Saleh et al. 2018). Also, we believe that the parameters found in this work can be applied as an extrapolative tool in green bean growth simulation models to predict SG behavior of this plant (especially Sunray variety) under different conditions of T and salinity that could exist in the field, although, further study is required under field condition to verify these findings.
Author contribution statement
EB designed and performed the experiments. EB conducted the modeling. EB, RA, FB and MJ interpreted the data. EB, RA, FB and MJ co-wrote all drafts of the paper and also approved the final draft for submission.
Abbreviations
- Cl– :
-
Chloride ion
- GP:
-
Germination percentage
- GR:
-
Germination rate, or 1/tg
- HaloTT:
-
Halothermal time model
- k T :
-
The line slope of the relationship between NaClb(50) and T above Td
- mM:
-
Millimolar
- MPa:
-
Megapascal
- Na+ :
-
Sodium ion
- NaCl:
-
The real tested NaCl concentration (mM)
- NaClb(50):
-
The base NaCl of the 50th percentile
- NaClb :
-
Median base NaCl concentration
- SG:
-
Seed germination
- SMC:
-
Seed moisture content
- T :
-
Temperature
- T b :
-
Minimum temperature (base temperature)
- T c :
-
Maximum temperature (ceiling temperature)
- T d :
-
The temperature at which NaClb(50) trend begins to change
- t g :
-
The time required for fraction or percentage (g) of seeds to germinate
- T o :
-
Optimum temperature
- θ Halo :
-
Halotime constant
- σ NaClb :
-
Standard deviation of NaClb within the seed population
- ψ :
-
Water potential
- ψ b(50):
-
The base ψ of the 50th percentile
References
Abdellaoui R, Boughalleb F, Chebil Z, Mahmoudi M, Belgacem AO (2017) Physiological, anatomical and antioxidant responses to salinity in the Mediterranean pastoral grass plant Stipa lagascae. Crop Pasture Sci 68:872–884
Abdellaoui R, Boughalleb F, Zayoud D, Neffati M, Bakhshandeh E (2019) Quantification of Retama raetam seed germination response to temperature and water potential using hydrothermal time concept. Environ Exp Bot 157:211–216
Alvarado V, Bradford K (2002) A hydrothermal time model explains the cardinal temperatures for seed germination. Plant Cell Environ 25:1061–1069
Bakhshandeh A, Eslami Z (2013) An authenticated image encryption scheme based on chaotic maps and memory cellular automata. Optics Lasers Engin 51:665–673
Bakhshandeh E, Gholamhossieni M (2019) Modelling the effects of water stress and temperature on seed germination of radish and cantaloupe. J Plant Growth Regul. https://doi.org/10.1007/s00344-00019-09942-00349
Bakhshandeh E, Jamali M (2020) Population-based threshold models: a reliable tool for describing aged seeds response of rapeseed under salinity and water stress. Environ Exp Bot 176:104077
Bakhshandeh E, Atashi S, Hafez-Nia M, Pirdashti H (2013) Quantification of the response of germination rate to temperature in sesame (Sesamum indicum). Seed Sci Technol 41:469–473
Bakhshandeh E, Bradford KJ, Pirdashti H, Vahabinia F, Abdellaoui R (2020) A new halothermal time model describes seed germination responses to salinity across both sub-and supra-optimal temperatures. Acta Physiol Plant 42:1–15
Bakhshandeh E, Abdellaoui R, Boughalleb F (2021) Modeling the effects of salt stress and temperature on seed germination of cucumber using halothermal time concept. Theor Exp Plant Physiol 33:79–93
Balkaya A (2004) Modelling the effect of temperature on the germination speed in some legume crops. J Agron 3:179–183
Baskin JM, Baskin CC (2014) What kind of seed dormancy might palms have? Seed Sci Res 24:17–22
Bayuelo-Jiménez JS, Craig R, Lynch JP (2002) Salinity tolerance of Phaseolus species during germination and early seedling growth. Crop Sci 42:1584–1594
Bello P, Bradford KJ (2016) Single-seed oxygen consumption measurements and population-based threshold models link respiration and germination rates under diverse conditions. Seed Sci Res 26:199–221
Ben Naceur M, Rahmoune C, Sdiri H, Meddahi M, Selmi M (2001) Effect of salt stress on germination, growth and grain yield in some North African wheat varieties. Sécheresse 12:167–174
Bewley JD, Bradford KJ, Hilhorst HW, Nonogaki H (2013) Germination. In: Seeds. Springer, pp 133–181
Bourgault M (2009). Legume production in semi-arid areas: comparative study of the physiology of drought tolerance in common bean (Phaseolus vulgaris L.) and mungbean (Vigna radiata (L.) Wilczek). A thesis submitted to McGill University in partial fulfilment of the requirements of the degree of doctor of philosophy
Bradford KJ (1990) A water relations analysis of seed germination rates. Plant Physiol 94:840–849
Bradford KJ (2018) Interpreting biological variation: seeds, populations and sensitivity thresholds. Seed Sci Res 28:158–167
Bradford KJ, Somasco OA (1994) Water relations of lettuce seed thermoinhibition. I. Priming and endosperm effects on base water potential. Seed Sci Res 4:1–10
Cardoso VJM, Bianconi A (2013) Hydrotime model can describe the response of common bean (Phaseolus vulgaris L.) seeds to temperature and reduced water potential. Acta Scientiarum Biol Sci 35:255–261
Cokkizgin A (2012) Salinity stress in common bean (Phaseolus vulgaris L.) seed germination. Not Bot Horti Agrobot Cluj Napoca 40:177–182
Corwin DL (2021) Climate change impacts on soil salinity in agricultural areas. Eur J Soil Sci 72(2):842–862
Derakhshan A, Bakhshandeh A, Siadat SA, Moradi-Telavat MR, Andarzian SB (2018) Quantifying the germination response of spring canola (Brassica napus L.) to temperature. Ind Crops Prod 122:195–201
Devkota KP, Devkota M, Rezaei M, Oosterbaan R (2022) Managing salinity for sustainable agricultural production in salt-affected soils of irrigated drylands. Agric Systems 198:103390
Elahifard E, Derakhshan A, Sardrood BP (2021) Does seed heteromorphism affect the critical temperature thresholds for wild mustard (Sinapis arvensis L.) germination? A modeling approach. Botany 99:507–514
El-Bastawisy ZM, El-Katony TM, Abd El-Fatah SN (2018) Genotypic variability in salt tolerance of Vicia faba during germination and early seedling growth. J King Saud Univ Sci 30:270–277
Estefan G, Sommer R, Ryan J (2013) Methods of soil, plant, and water analysis. A manual for the West Asia and North Africa region 3:65–119
FAO (2019) FAOSTAT/Productionstat/Crops [Online]. Food and Agriculture Organization of the United Nations. http://www.fao.org/faostat/en/#data/QC. Accessed 22 Jan 2019
FAO (2021) The World Map of Salt Affected Soil [WWW Document]. FOOD Agric. Organ, UNITED NATIONS. https://www.fao.org/soils-portal/data-hub/soil-maps-and-databases/global-map-of-salt-affected-soils/en/. Accessed 12 May 2021
FAO News (2021) World agriculture 2030: Main findings [WWW Document]. https://www.fao.org/english/newsroom/news/2002/7833-en.html. Accessed 12 May 2021
Gomes-Filho E, Lima CRFM, Costa JH, da Silva ACM, da Guia Silva Lima M, de Lacerda CF, Prisco JT (2008) Cowpea ribonuclease: properties and effect of NaCl-salinity on its activation during seed germination and seedling establishment. Plant Cell Rep 27:147–157
Hajlaoui H, Denden M, Bouslama M (2007) Etude de la variabilité intraspécifique de tolérance au stress salin du pois chiche (Cicer arietinum L.) au stade germination. Tropicultura 25:168–173
Hardegree SP (2006) Predicting germination response to temperature. I. Cardinal-temperature models and subpopulation-specific regression. Ann Bot 97:1115–1125
Hosseini Sanehkoori F, Pirdashti H, Bakhshandeh E (2021) Quantifying water stress and temperature effects on camelina (Camelina sativa L.) seed germination. Environ Exp Bot 186:104450
International Seed Testing Association (ISTA) (2009) International rules for seed testing. Seed Science and Technology, Zurich
Kaymakanova M (2009) Effect of salinity on germination and seed physiology in bean (Phaseolus Vulgaris L.). Biotechnol Biotechnol Equip 23:326–329. https://doi.org/10.1080/13102818.2009.10818430
Khan M, Weber D, Hess W (1985) Elemental distribution in seeds of the halophytes Salicornia pacifica var. utahensis and Atriplex canescens. Am J Bot 72:1672–1675
Kurtar ES (2010) Modelling the effect of temperature on seed germination in some cucurbits. Afr J Biotechnol 9:1343–1353
Liu Q, Zhang Q, Yan Y, Zhang X, Niu J, Svenning J-C (2020a) Ecological restoration is the dominant driver of the recent reversal of desertification in the Mu Us Desert (China). J Clean Prod 268:122241
Liu S, Bradford KJ, Huang Z, Venable DL (2020b) Hydrothermal sensitivities of seed populations underlie fluctuations of dormancy states in an annual plant community. Ecology 101:e02958
Llanes A, Andrade A, Masciarelli O, Alemano S, Luna V (2016) Drought and salinity alter endogenous hormonal profiles at the seed germination phase. Seed Sci Res 26:1–13
Rajabi Dehnavi A, Zahedi M, Ludwiczak A, Cardenas Perez S, Piernik A (2020) Effect of salinity on seed germination and seedling development of sorghum (Sorghum bicolor (L.) Moench) genotypes. Agronomy 10:859
Raveneau M, Coste F, Moreau-Valancogne P, Lejeune-Henaut I, Durr C (2011) Pea and bean germination and seedling responses to temperature and water potential. Seed Sci Res 21:205–213
Ryu H, Cho YG (2015) Plant hormones in salt stress tolerance. J Plant Biol 58:147–155
Saleh S, Liu G, Liu M, Ji Y, He H, Gruda N (2018) Effect of irrigation on growth, yield, and chemical composition of two green bean cultivars. Horticulturae 4:3
SAS Institute Inc (2015) SAS/STAT user’s guide. SAS Institute Inc, Cary
Seal CE, Barwell LJ, Flowers TJ, Wade EM, Pritchard HW (2018) Seed germination niche of the halophyte Suaeda maritima to combined salinity and temperature is characterised by a halothermal time model. Environ Exp Bot 155:177–184
Singh A (2021) Soil salinization management for sustainable development: a review. J Environ Manage 277:111383
Song J, Feng G, Tian C, Zhang F (2005) Strategies for adaptation of Suaeda physophora, Haloxylon ammodendron and Haloxylon persicum to a saline environment during seed-germination stage. Ann Bot 96:399–405
Talat N (2020) Alleviation of soil salinization and the management of saline soils, climate change, and soil interactions. In: Climate Change and Soil Interactions. Elsevier, pp 305–329
Tlahig S, Bellani L, Karmous I, Barbieri F, Loumerem M, Muccifora S (2021) Response to salinity in legume species: an Insight on the effects of salt stress during seed germination and seedling growth. Chem Biodiver 18:e2000917
Tobe K, Zhang L, Qiu GY, Shimizu H, Omasa K (2001) Characteristics of seed germination in five non-halophytic Chinese desert shrub species. J Arid Environ 47:191–201
Vahabinia F, Pirdashti H, Bakhshandeh E (2019) Environmental factors’ effect on seed germination and seedling growth of chicory (Cichorium intybus L.) as an important medicinal plant. Acta Physiol Plant 41:1–13
Van’t Hoff JH (1887) Die Rolle des osmotischen Druckes in der Analogie zwischen Lösungen und Gasen. Z Phys Chem 1:481–508
Wahid A, Farooq M, Basra SM, Rasul E, Siddique KH (2016) Germination of seeds and propagules under salt stress. In: Pessarakli M (ed) Handbook of plant and crop stress, 3rd edn. CRC Press, Boca Raton, pp 321–337
White JW, Montes C (1993) The influence of temperature on seed germination in cultivars of common bean. J Exp Bot 44:1795–1800
Zhang H, Irving LJ, McGill C, Matthew C, Zhou D, Kemp P (2010) The effects of salinity and osmotic stress on barley germination rate: sodium as an osmotic regulator. Ann Bot 106:1027–1035
Acknowledgements
This work is financially supported by the Genetics and Agricultural Biotechnology Institute of Tabarestan (GABIT) and Sari Agricultural Sciences and Natural Resources University (SANRU) (under grant ID: D.2169.97.31). The data shared with the Institut des Régions Arides (IRA-Medenine, Tunisia) based on the memorandum of understanding between both institutes which was signed in January 2020. The authors would also like to thank Miss Fatemeh Vahabinia for her assistance with the collection of some experimental data during this research.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Communicated by S. Renault.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bakhshandeh, E., Abdellaoui, R., Boughalleb, F. et al. Quantification of green bean germination response to simultaneous salt and temperature stress: a modeling approach. Acta Physiol Plant 44, 134 (2022). https://doi.org/10.1007/s11738-022-03461-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-022-03461-z