Abstract
We quantified the seed germination responses of chicory (Cichorium intybus L.; Asteraceae) to heat, water and salt stresses using hydrothermal time (HTT) and halothermal time (HaloTT) models. We extended the HaloTT model based on NaCl concentration to include supra-optimal temperatures. The HTT and HaloTT models enabled quantitative comparison of the effects of reduced water potentials and increased salinity on germination. Below 30 °C, the median threshold water potential permitting germination estimated by the HTT model (− 0.83 MPa) was higher than that estimated by the HaloTT model (− 1.30 MPa, after converting the median threshold NaCl concentration to osmotic potential). Ion uptake into seeds imbibed in salt solutions accounted for an average osmotic adjustment of 36% in the presence of salt stress compared to seeds imbibed in polyethylene glycol solutions. These thresholds became more positive above 30 °C with a common median ceiling temperature of 43 °C. The HaloTT models developed here accurately described germination responses to NaCl concentrations across all temperatures and incorporated both water potential and osmotic adjustment effects. With respect to seed germination, chicory could be considered as a moderately water stress tolerant species and highly tolerant to salt stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Seed germination (SG) is a complex physiological process that is affected by abiotic stresses, including temperature (T), water stress, and salinity, and by other physical and biological factors (Bradford 2002; Bakhshandeh et al. 2020; Vahabinia et al. 2019). T is a critical factor affecting both the germination percentage (GP) and the germination rate (GR, or 1/tg) (Ghaderi-Far et al. 2010; Bewley et al. 2013). During the germination phase of the plant life cycle, there exists a T below which germination is prevented, called the base temperature (Tb), an optimum temperature (To, single and/or range of Ts) at which germination is most rapid, and a T above which germination does not occur, called the ceiling temperature (Tc) (Bakhshandeh et al. 2013). These so-called cardinal Ts vary depending on species and environmental conditions under which they were produced (Hatfield and Prueger 2015). GR usually increases linearly with T between Tb and To, and declines linearly and/or curvilinearly at Ts > To (Bradford 2002; Rowse and Finch-Savage 2003; Mesgaran et al. 2017; Abdellaoui et al. 2019). Water availability is an important factor affecting SG and failure or success of plant establishment. Commonly, GP and GR increase with water availability and decrease at more negative water potential (ψ) (Mesgaran et al. 2013; Atashi et al. 2015). Salinity may limit SG through both osmotic and ion-specific effects (Zhang et al. 2010; Llanes et al. 2016). Dissolved salts decrease the ψ of saline solutions, with a 1 M solution of NaCl having a ψ of approximately − 4.4 MPa at 25 °C (Nobel 1983). In addition, accumulations of inorganic ions (e.g., sodium [Na+] and chloride [Cl−]) and compatible organic solutes (e.g., amino acids, proline, etc.) result in osmotic adjustment, or a reduction of ψ inside the seed cells, that can enable SG to occur at lower water potentials (Parihar et al. 2015; Seal et al. 2018). These factors, T, ψ and salinity, influence SG alone and/or in combination, and are relevant to crop production and to ecological distributions of species (Baskin and Baskin 2014).
The hydrotime model developed by Gummerson (1986) and Bradford (1990) has been used to quantify the influence of ψ on germination at a given T. According to the model, the time to germination is inversely proportional to the difference between the ψ of the seed environment and the physiological ψ threshold for radicle emergence (base ψ or ψb), which varies among seeds in the population. The ψb values for various germination percentiles (ψb(g)) usually exhibit a normal distribution within seed populations (Dahal and Bradford 1994; Bradford and Still 2004; Bakhshandeh and Gholamhossieni 2018), although other distributions, such as Gumbel, Weibull, Log-Normal, Logistic, Log-logistic, Inverse Normal and Gamma, have been applied to specific seed populations or mixtures of populations (Mesgaran et al. 2013; Atashi et al. 2015; Moltchanova et al. 2020).
Gummerson (1986) also proposed a hydrothermal time (HTT) model of SG stating that at Ts ≤ To (i.e., between Tb and To) and at any constant ψ, the time to germination (tg) of any germination percentile of a seed population is a function of the degree to which T and ψ exceed their respective base values, Tb and ψb, at which germination is inhibited (Gummerson 1986; Dahal and Bradford 1994; Bradford 1995). In this model, Gummerson (1986) assumed that θHT and Tb are constant and equal among seeds and only ψb(g) varies in the seed population. However, this model does not predict the inhibition of SG (both GR and GP) at Ts > To (i.e., between To and Tc). Consequently, Alvarado and Bradford (2002) suggested another model in which the relationship between GR vs. T will be negatively linear at Ts > To with a common To for all germination percentiles but differing Tc values in the population. This decline in GR and GP was due to a linear increase in ψb(g) as T increased above To, and a constant value (i.e., kT) was defined as the increase in ψb(50) per degree as T increased above To. This modeling approach successfully predicted SG in many crops, such as potato (Solanum tuberosum L.) (Alvarado and Bradford 2002), watermelon (Citrullus vulgaris) (Bakhshandeh et al. 2015), sesame (Sesamum indicum L.) (Bakhshandeh et al. 2017) and eruca (Eruca sativa) (Bakhshandeh et al. 2019).
However, for some other crops such as wild barley (Hordeum spontaneum Thell.) and little canarygrass (Phalaris minor L.) (Mesgaran et al. 2017) and Retama reatam (Abdellaoui et al. 2019), the relationship between GR and T did not transition sharply from increasing to decreasing at To. Rowse and Finch-Savage (2003) showed also that a T lower than To should be defined (Td) at which ψb(50) starts to change, i.e., [ψ − ψb(50) + kT (T − Td)], resulting in a curvilinear peak in GR around and above To (e.g., Bakhshandeh and Gholamhossieni 2019).
Seal et al. (2018) used the same methodology to quantify the SG characteristics of the halophyte Suaeda maritima under different NaCl concentrations at sub-optimal Ts. They developed a halothermal time (HaloTT) model that utilized log NaCl to replace water potential in the HTT model. That is, the germination behavior at sub-optimal Ts was governed by the salt concentration threshold distribution (NaClb(g)) relative to the NaCl of the environment. In the current work, we employ the HaloTT model and extend it to describe SG in response to T across all Ts at any constant NaCl concentration. To our knowledge, no HaloTT model to quantify SG responses at supra-optimal Ts has been proposed as yet, thus, our study is the first report in this case.
Chicory (Cichorium intybus L.; Asteraceae) is a perennial herb, native to Europe, Africa, America and many parts of Asia, including Iran (Jouad et al. 2001; Benítez et al. 2010; Guarrera and Savo 2016). The medicinal effects of chicory are well documented (Abbas et al. 2015; Sampaio et al. 2009; Yoo et al. 2011). Furthermore, this plant copes with different soil types and abiotic and biotic stresses and can be used as food and feed. Based on its broad tolerance to environmental conditions, we have investigated the SG responses of chicory to combinations of T, ψ and salinity using the models described above. The aims of this study were: (1) to study the effects of heat, water and salt stresses on SG characteristics of chicory; (2) to estimate the cardinal Ts for germination using the HTT and HaloTT models; (3) to measure the uptake of salt ions into chicory seeds in response to salinity; and (4) to calculate the amount of osmotic adjustment attributable to NaCl uptake into the seeds when imbibed in salt solutions.
Materials and methods
Seed collection and treatments
Chicory seeds were provided from Mazandaran province, Iran, in 2017. They were maintained in the dark at 5 °C for 3 months. The seed viability [assessed according to ISTA (2018)] was > 98% under optimal conditions at the beginning of the experiments. The treatments were seven constant Ts (10, 15, 20, 25, 30, 35 and 40 °C), four levels of ψ (− 0.3, − 0.6, − 0.9 and − 1.5 MPa) and four levels of salinity (0.075, 0.150, 0.225 and 0.300 M). For the control we used distilled water in all experiments. Polyethylene glycol (PEG8000, Scharlau, Spain) was applied to make solutions having different ψs (and corrected for Ts) based on Michel and Radcliffe (1995) method. An osmometer (Model 5520: Wescor Inc., USA) was used initially and also every two days during the experiments to re-check the ψs for each T. Sodium chloride (NaCl) was applied to prepare different levels of salt stress that are mentioned above. The NaCl concentration (M) at each T was converted to water potential (ψ, MPa) according to the Van’t Hoff relation (Van’t Hoff 1887), and confirmed using the osmometer as well.
Seed germination tests
Fifty seeds for each of four replicates per treatment were placed on two sheets of Whatman No. 1 filter paper within 8 cm Petri dishes with six mL of the test solutions, supported by 0.1% Thiram. An incubator with ± 0.5 °C precision was used and the Petri dishes were randomly placed within in the dark, except during recording times. The Petri dishes were put into a plastic bag to avoid water evaporation. Seeds were counted several times daily, depending on the T, ψ and salt stress in all experiments. The seeds at least 2 mm long of radicle were considered as germinated seeds. At each counting time, germinated seeds were removed to avoid an error. The experiments were ended when no additional seeds germinated for three consecutive days.
Hydrotime and halotime models
A repeated probit regression analysis as followed by Bradford (1990) were used to analyses and determine the model parameters. GR50 (h−1) was calculated by interpolation by curves fit to the time course data according to:
where GR50 is the GR for the 50th percentile of germination (h−1) and t50 is the time to reach 50% germination.
To quantify the germination time courses data in the osmotic solutions at 10, 15, 20, 25, 30, 35 and 40 °C, the hydrotime model was used. This model is expressed as (Gummerson 1986):
where θH is the hydrotime constant (MPa h or MPa d); ψ is the actual water potential of the medium (MPa); ψb(g) is the base value of ψ inhibiting radicle emergence of percentage g (MPa); and tg is the actual time to germination of percentage g. Thus, the ψb(50) is the base water potential for the median or 50th percentile SG fraction.
A similar method was used to determine the parameters of halotime model by substituting the NaCl for ψ, the NaClb(g) for ψb(g) and the θHalo for θH. For each salt treatment at 10, 15, 20, 25, 30, 35 and 40 °C, the halotime model can be written as (Seal et al. 2018):
where θHalo is the halotime constant (M h or M d); NaClb(g) is the base value of NaCl inhibiting radicle emergence of percentage g (M); NaCl is concentration of the imbibition medium (M); and tg is the actual time to germination percentage g (h or d). Thus, the NaClb(50) is the median base NaCl concentration for the 50th percentile SG fraction. We note that the original equation in Seal et al. (2018) would result in negative values for θHalo since the term used there (NaCl − NaClb(g)) [or NaClmax g as defined in Seal et al. (2018)] will be a negative number, as the magnitude of NaCl is smaller than NaClb(50) at any NaCl permitting germination. We have reversed the order of NaClb(g) and NaCl in Eq. 3 to give positive values of θHaloTT.
Hydrothermal time and halothermal time models
At Ts ≤ To, the HTT model is expressed as (Gummerson 1986):
or
At Ts > To, the model should be modified to (Alvarado and Bradford 2002):
or
where Td represents the T at which GR begins to be affected by warm Ts, which can be lower than To (Rowse and Finch-Savage 2003).
For the HaloTT model, the model proposed by Seal et al. (2018) (with correction as above) at Ts ≤ To was used:
or
where θHaloTT is the HaloTT constant (M °C h or M °C d) and σNaClb is the standard deviation of the NaClb(g) distribution.
To our knowledge, no HaloTT model to quantify SG responses at Ts > To has been proposed as yet. Thus, we developed the model below as the first report:
or
Thus, SG response can be quantified at all Ts and ψs by the HTT models (Eqs. 4–7) and at all Ts and NaCls using the HaloTT models (Eqs. 8–11).
Seed moisture contents
Three replicates of 1 g chicory seeds were sown on two sheets of Whatman No. 1 filter paper in 8 cm Petri dishes containing 6 mL of water, 0.075, 0.150, 0.225 and 0.300 M NaCl at 10, 15, 20, 25, 30, 35 and 40 °C. Immediately prior to the initiation of germination (estimated from the cumulative GP vs. time curve, Fig. 2), seeds were rinsed several times with distilled water and blotted with filter paper to remove any solution from the testa. The seeds weighted, then dried in an oven at 103 °C for 17 h (ISTA 2018) and seed moisture content (SMC) was expressed on a dry weight basis.
Seed Na+ and Cl− concentrations and estimated osmolality
A flame photometric method as described by Estefan et al. (2013) was used to measure Na+ concentration in the chicory seeds obtained from the experiment described above (seed moisture contents section). Dried chicory seeds (103 °C for 17 h) were powdered by a mill and 0.5 g for each replicate was oxidized at 550 °C for 5 h and 2 N hydrochloric acid was used to digest the dry residue. Na+ was analyzed using a flame photometric instrument (model PFP7, Jenway, UK) and standard curves were constructed using 1000 ppm Na flame photometry standard solution (Product No: 025021, Jenway, UK) for quantification. To determine chloride (Cl−) concentration in the seeds, 0.2 g of dry powdered seeds for each replicate was placed in 20 mL of distilled water at > 90 °C for 1 h, then the amount of Cl− was measured by AgNO3 (Mohrs’s titration) method (Estefan et al. 2013). The summed concentrations of Na+ and Cl− (mmol kg DW−1) were divided by the seed water content (kg H2O kg DW−1) at the time of sampling to estimate the total osmolality (mmol/kg H2O) contributed by these ions, making the simplifying assumption that all of the water in the seed was symplastic. We then used the Van’t Hoff relation to convert the osmolality of salt ions in the seeds to ψ (MPa).
Statistical analyses
All statistical analyses were performed using the Statistical Analysis System ver. 9.4 (SAS Institute 2015), Excel and Sigma Plot ver. 11 software (Systat Software Inc., San Jose CA, USA, www.systatsoftware.com). A two-way ANOVA with the least significant difference test (LSD) at 0.05 probability level was used to analyze the effects of T, ψ, salinity and their interactions on SG, seed Na+ and Cl− concentrations and SMC.
Results
Effects of water potential, temperature and salinity on GP and GR
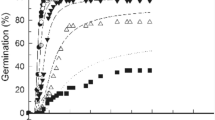
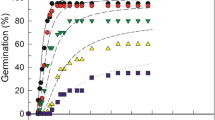
Water potential, NaCl and their interactions significantly affected GP and GR50 (p > 0.001) (Table 1). Supra-optimal T reduced GP (in water) while ψ and/or NaCl reduced GP at both lower and higher T. Both GP and GR50 decreased with decreasing ψ (Fig. 1) and with increasing NaCl in the medium at each tested T (Fig. 2). The relationship between GR50 and T was linear at T < 30 °C (sub-optimal Ts) under both conditions and shared a common Tb (Fig. 3). In general, GP and GR50 were more sensitive to changes in ψ and/or NaCl than to changes in T (Figs. 1, 2).
Hydrotime constant (θH, a), base water potential (ψb(50), closed circles and solid line) and base NaClb(50) after converting to MPa by van’t Hoff equation (open circles and dashed line; b), halotime constant (θHalo, c) and base NaCl concentration (NaClb(50), d) as a function of temperature (T) for chicory seeds. In b and d, the predicted lines are for the 50th percentile intercepts ψb(50) = 0 MPa and NaClb(50) = 0 MPa at Tc (43 °C), respectively. Inset a1: the inverse of θH vs. T, which will be linear below To (and extrapolates to Tb at the intercept on the x axis) and then is constant at T > To, as this maximized the fitting of the model. Inset c1: the inverse of θHalo vs. T, which continues increasing linearly up to 40 °C
Hydrotime and halotime models
The hydrotime model successfully described germination data across all ψs at each T, with R2 > 0.88 (Table 2; Fig. 1). The θH values declined with increasing T from 62.9 MPa h at 10 °C to 9.0 MPa h at 30 °C and was fixed to a constant value of 9.0 MPa h at Ts > 30 °C (i.e., Ts > To) because this optimized the model (Table 2; Fig. 3a). The inverse of θH values (1/θH) was linear below To (and extrapolated to Tb = 4.8 °C, on the x axis) (Fig. 3a). The ψb(50) remained approximately constant at sub-optimal Ts (i.e., ≤ 30 °C), ranging from − 0.813 to − 0.839 MPa, then increased linearly to − 0.573 and − 0.210 MPa at 35 and 40 °C, respectively, and intersected 0 MPa at 43 °C (i.e., Tc(50)) (Table 2; Fig. 3b) The ψb(50) values increased with increasing T above To with a kT (slope) value of 0.066 MPa °C−1 (Table 2; Fig. 3b).
We used the same method to estimate the parameters of the halotime model at each T, with R2 ranging from 0.82 to 0.97 (Table 2; Fig. 2). The θHalo values non-linearly decreased with increasing T from 14.1 M h at 10 °C to 2.1 M h at 40 °C (Table 2; Fig. 3c). The inverse of θHalo values (1/θHalo) were linear with T up to 40 °C and intercepted the x axis at Tb = 4.8 °C (Fig. 3c). The NaClb(50) for chicory seeds remained approximately constant at sub-optimal Ts (i.e., < 30 °C), ranging from 0.294 to 0.326 M, then decreased linearly (kT = 0.024 M °C−1) with increasing T above To until it intersected 0 M at 43 °C (i.e., Tc(50)) (Table 2; Fig. 3d). That is, the NaCl required to prevent germination decreased by 0.024 M per degree above To (Table 2; Fig. 3d).
The median GR50 (1/t50) across Ts at different ψs increased linearly at Ts ≤ Td, the T at which ψb(50) begins to change (which is equal to To in this case), and then decreased non-linearly at Ts above To until Tc (Fig. 4a). The maximum GR50 (0.0973 h−1) was observed at To when ψ = 0 MPa and then decreased linearly as ψ increased, reaching zero at − 0.83 MPa with a rate of decrease of 0.111 h−1 per MPa with decreasing ψ (Fig. 4b). The common intercept at Tb = 4.8 °C (Table 3; Fig. 4a), indicates that base temperature was not affected by ψ. In contrast, the linear decrease in Tc(50) values predicted by the HTT model as T increases above To (i.e., 30 °C) is illustrated in Fig. 4c.
The effect of temperature (T) and water potential (MPa) (a) and T and NaCl concentration (M) (d) on the median germination rates (= 1/t50) of chicory seeds. The symbols represent the actual data and the lines drawn through these points are based upon the parameters of the hydrothermal time and halothermal time models (Table 3). The effects of ψ and of NaCl on GR50 at To (b, e, respectively) intersect the calculated ψb(50) and NaClb(50) values at GR50 = 0 or Tc = Td = To and the linear relationships were highly significant (P < 0.0001). The Tc(50) values predicted by the hydrothermal and halothermal models at each ψ or NaCl also are shown (c, f, respectively), and therefore fall exactly on the modeled lines
The relationship of GR50 with T at different NaCl concentrations showed a similar pattern as was observed for ψ (Fig. 4d). GR50 reached its maximum (0.0979 h−1) at To when NaCl = 0 M and then decreased linearly as NaCl concentration increased, reaching zero at 0.304 M (− 1.37 MPa, after converting to ψ) with a rate of decrease of 0.32 h−1 per M of increase in NaCl (Fig. 4e). The linear decrease in Tc(50) values predicted by the HaloTT model relative to the control (NaCl = 0 M) as NaCl increased between 43 and 30 °C is also shown (Fig. 4f).
When the initial salinity levels tested were converted into ψ values (using the Van’t Hoff equation), the calculated ψb(50) values were much lower (more negative) in comparison with PEG data (Table 2; Fig. 3b). For example, at 40 °C, the estimated ψb(50) was − 0.21 MPa in PEG but was − 0.45 in NaCl. This could be due to Na+ and Cl− ions entering into seed cells, lowering their osmotic potential and increasing embryonic turgor, enabling the seeds to germinate at lower ψs. The relative osmotic adjustment of the chicory seeds imbibed in NaCl was calculated as [(1 − (estimated ψb(50) using hydrotime model/estimated ψb(50) using the halotime model after converting NaCl to ψ)) × 100]. This Osmotic Adjustment Value was relatively constant (32 to 40%) at T < To, then increased above To as the value of ψb(50) became less negative (Tables 2, 3).
The σψb and σNaClb values varied little across Ts (Table 2), indicating that to the variation of ψ and NaCl thresholds among individual seeds did not change across the range of temperatures tested.
Hydrothermal time model, halothermal time model and cardinal temperatures
To quantify the response of chicory SG to across all Ts under different ψ and NaCl, HTT (Eqs. 4, 7) and HaloTT (Eqs. 8, 11) models were applied to the respective data. The HTT model described the SG data of chicory well, with R2 of 0.86 (Table 3; Fig. 5a). The estimated parameters were 220 MPa °C h for θHTT, − 0.83 MPa for ψb(50), 0.211 MPa for σψb and 0.066 MPa °C−1 for kT (Table 3).
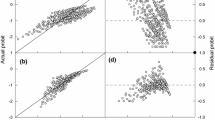
Linear regression of probit (g) plotted against base water potentials (ψb(g), MPa) and adjusted base water potentials (ψb(g) + kT(T − Td)) (a) and linear regression of probit (g) plotted against base NaCl concentrations (NaClb(g), M) and adjusted base NaCl concentrations (−NaClb(g) + kT(T − Td) (b). Symbols are the observed values of probit germination fraction at each T versus the calculated base values for different germination fractions (g). The highly significant linear relationships with probit (g) indicate that the base thresholds are normally distributed in the seed population and that the hydrothermal and halothermal models account for 83–86% of the variance in the germination behavior across all conditions
The HaloTT model fit the data for chicory SG in NaCl with a high R2 value (0.83) (Table 3; Fig. 5b) and an estimated θHaloTT value of 74.9 M °C h (Table 3). The osmotic adjustment value was 36% when the ψb(50) value estimated by the HTT (− 0.83 MPa) was compared to the ψb(50) value estimated by the HaloTT (− 1.30 MPa) (Table 3).
To determine the cardinal Ts for chicory, GR50 vs. T was plotted in both conditions. The relationships between GR50 and Ts ≤ To were linear at all ψs and all NaCls and were limited to a single Tb of 4.8 °C (Fig. 4a, d). GR50 reached its maximum at To (30 °C) in both conditions (water and/or salt stresses) and also was not influenced by ψ and NaCl. Similarly, Tc(50) was 43 °C based on both the HTT and HaloTT models (Fig. 3b, d).
Seed moisture content and osmotic adjustment to salt
SMC was measured just prior to radicle emergence at different Ts and NaCls (Fig. 6a). At 10 °C, SMC was unchanged under salt stress relative to control (NaCl = 0 M). However, SMC decreased with increasing T from 15 to 30 °C under salt and control treatments, but with less decrease for salt treatments. SMC tended to increase with NaCl at each T, but decreased as T increased to To then increased again. The reason for the decrease in SMC with increasing T is unclear, but may be due to more rapid GR50 around To and less time to achieve maximum moisture content prior to radicle emergence. At Ts ≥ 35 °C, the SMC increased and tended to increase with increasing NaCl concentrations relative to control (Fig. 6a).
Seed moisture content (a), concentrations of Na+ (b), Cl− (c) and calculated osmotic potential due to NaCl (d) into the chicory seeds. Lower case letters show significant differences among NaCl treatments within a temperature and upper case letters show significant differences among temperatures, using the least significant difference test (LSD) at 0.05 probability level
At all Ts, Na+ concentration in the seed tissues increased significantly with increasing NaCl (p < 0.05) when compared to the control, attaining 132 mmol kg−1 DW at 0.300 M NaCl (Fig. 6b). Cl− content of chicory seeds also increased remarkably as salinity levels increased (p < 0.05) at all Ts (Fig. 6c). Seed Cl− content reached its highest levels at supra-optimal Ts (> 30 °C) (Fig. 6c).
As the median base water potentials preventing germination were − 0.5 MPa lower in NaCl in comparison with PEG (Table 2; Fig. 3b), we estimated the osmotic adjustment in the seeds that could be attributed to additional Na+ and Cl− ion uptake. Using the ion contents and water contents on a dry weight basis, we calculated the total NaCl concentrations in the seeds for each treatment and converted these into osmotic potential values. These values were approximately − 0.5 MPa in water and became more negative as NaCl concentration increased, reaching approximately − 1.3 MPa across Ts at 0.300 M (Fig. 6d). Thus, sufficient Na+ and Cl− ions were taken up by the seeds to account for the observed ability of seeds to germinate to lower osmotic potential in salt as compared to PEG, which does not enter the cells. Osmotic adjustment due to NaCl uptake is also consistent with the increase in SMC with increasing NaCl concentration (Fig. 6a), while SMC decreases with increasing PEG concentration (Bradford 1986). There is uncertainty as to the locations of the ions and the water inside of the seed tissues (i.e., apoplastic or symplastic), and only symplastic ions would presumably contribute to increasing turgor and therefore to germination capacity. Nonetheless, the data indicate that salt ion uptake into the seeds was at least sufficient to account for the differences between ψb(50) and NaClb(50) (converted to osmotic potential) (Table 3; Fig. 3b).
Discussion
In general, the temperature responses of seeds can be defined by their cardinal Ts (i.e., Tb, To and Tc) (Bewley et al. 2013). The relationship between GR50 and T identified values of 4.8, 30 and 43 °C for Tb, To and Tc, respectively (Table 3). Similar Tb values have been reported for witloof chicory (Cichorium intybus; 5.3 °C) (Bierhuizen and Wagenvoort 1974), chicory (Cichorium intybus cv. Grasslands Puna; 3.7 °C) (Moot et al. 2000) and rapeseed (0–5 °C, depending on cultivars) (Farzaneh et al. 2014). The maximum GR50 was observed at 30 °C (To) in the control treatment (0 MPa and 0 M), similar to previous reports for chicory (29.9 °C, Zarghani et al. 2014; 25 to 30 °C, Corbineau and Come 1989), although Balandary et al. (2011) reported a value of 25.5 °C after fitting an empirical beta model. Our findings showed also that Tb and To were relatively unaffected by PEG and NaCl stresses, which corroborate results for To of Silybum marianum and Calendula officinalis (Parmoon et al. 2018) and flax-leaf alyssum (Alyssum linifolium Steph. ex. Willd.) (Mobli et al. 2018). The median Tc value estimated in this study (43 °C based on both hydrotime and halotime models) was approximately equal to the values reported for chicory by Zarghani et al. (2014) (46.3 °C) and Vahabinia et al. (2019) (40.2 °C) based on different models. At T > To, the value of Tc varies among seeds in the population (Alvarado and Bradford 2002), and also depends upon the stress level (Atashi et al. 2014; Bakhshandeh et al. 2017; Parmoon et al. 2018; Abdellaoui et al. 2019). As stress intensity increases, Tc approaches nearer to To, as illustrated here for chicory (Fig. 4), because of the threshold sensitivities to ψ or NaCl increase as the T increases above To (Fig. 3c, d).
The HTT model (Gummerson 1986), generalized as population-based threshold models, describes well the observed GRs and GPs response to increasing stress, aging, hormones and other factors affecting seed germination (Bello and Bradford 2016). GR50 slows as the stress intensity increases due to reduced ψ or increased NaCl concentrations, and GP also declines as the stress exceeds the threshold that prevents germination for a given fraction of the seed population. The HTT and HaloTT models applied here described these patterns well for chicory seeds (Figs. 1, 2, 5). The time constants in these models (e.g., θH or θHalo) provided useful overall values for germination kinetics for estimating the cardinal Ts under both water and salt stress (Fig. 3). Following the work of Seal et al. (2018) on the halophyte Suaeda maritima, we used the NaCl concentrations to fit halotime and HaloTT models to our germination data for chicory seeds. In the suboptimal T range, NaClb(50) was not affected by NaCl concentration (Fig. 3d), which is in contrast to the results reported by Seal et al. (2018), who found that NaClb(50) values significantly decreased with increasing T (between 5 and 25 °C). In contrast, threshold values in the halophyte Chloris virgata and the glycophyte Digitaria sanguinalis tended to increase with T before declining (Zhang et al. 2012). Thus, these responses may vary among species and the environments to which they are adapted (Huarte 2006). In addition, we extended the HaloTT model for quantifying SG responses at Ts > To. The NaClb(50) values for chicory decreased linearly above To, consistent with seed germination responses to ψ at high temperature (Alvarado and Bradford 2002). Thus, germination patterns across the entire range of temperatures from Tb to Tc could be described by the HaloTT model on the basis of NaCl concentration (Figs. 4a, d; 5b).
However, SG characteristics of chicory were more influenced by ψ in comparison with NaCl at equivalent osmotic potentials (Table 2). This agrees with the findings for other species, such as soybean (Khajeh-Hosseini et al. 2003), alfalfa (Tilaki et al. 2009), barley (Zhang et al. 2010), sunflower (Luan et al. 2014) and rapeseed (Bakhshandeh and Jamali 2020). This is likely due to the uptake of salt ions by the chicory seed, enabling osmotic adjustment to maintain a ψ gradient allowing water uptake during imbibition and germination (Heshmat et al. 2011; Seal et al. 2018). SMC, Na+ and Cl− concentrations increased in chicory seeds with increasing salinity (Fig. 6), consistent with results with barley (Zhang et al. 2010), several halophytic species (Khan et al. 1985; Seal et al. 2018; Song et al. 2005) and rapeseed (Bakhshandeh and Jamali 2020). In our case, the measured uptake of Na+ and Cl− ions during imbibition in salt solutions was sufficient to account for the lower apparent ψb(50) values when NaCl was converted to osmotic potentials. As the halotime model described germination behavior well up to the highest NaCl tested (0.300 M), chicory SG is controlled primarily by the osmotic rather than toxic effects of salt, as also reported for barley (Zhang et al. 2010), Acacia harpophylla (Arnold et al. 2014) and Atriplex halimus (Shaygan et al. 2017).
In conclusion, the models used in this work accurately and successfully describe chicory SG across all Ts at a range of ψs and NaCls and provide reliable tools for assessing germination under these conditions. We developed a HaloTT model for quantifying the SG responses (both GR and GP) in the supra-optimal T range, which has not been reported previously. This model was also able to predict germination responses of salt tolerant (rapeseed), moderately salt-sensitive (cucumber), and salt-sensitive (green bean) species (Bakhshandeh et al. unpublished data). Thus, the hydrotime, halotime, HTT and HaloTT models enable prediction of SG behavior across entire the range of temperature, water and salt conditions.
Of course, conditions can vary in the field and performance may not exactly match model predictions. However, we note that Liu et al. (2020) used the HTT model to characterize germination behavior of 13 native desert annual species. They found that relative germination sensitivities to T and ψ determined from laboratory tests were highly correlated with average seedling emergence data in desert conditions over 25 years of field observations in Arizona. Both HTT and HaloTT models use parameters based on mechanistic assumptions about the underlying physiology of SG to describe patterns of germination timing. Therefore, we believe that while field conditions can vary and influence germination at a particular time, the HaloTT model developed here can quantify and predict the relative germination performance that can be expected in field conditions.
Author contribution statement
EB and KB designed the experiments. EB and FV performed the experiments. EB and KB conducted the modeling and interpreted the data. EB, KB, HP, FV and RA co-wrote all drafts of the paper and also approved the final draft for submission.
Abbreviations
- Cl– :
-
Chloride ion
- GP:
-
Germination percentage
- GR:
-
Germination rate, or 1/tg
- HaloTT:
-
Halothermal time model
- HTT:
-
Hydrothermal time model
- k T :
-
The slope of the relationship between ψb(50) and/or NaClb(50) and T above To
- M:
-
Molar
- MPa:
-
Megapascal
- Na+ :
-
Sodium ion
- NaCl:
-
NaCl concentration (M)
- NaClb(50):
-
The base NaCl of the 50th percentile
- NaClb :
-
Median base NaCl concentration
- OAV:
-
Osmotic adjustment value
- SG:
-
Seed germination
- SMC:
-
Seed moisture content
- T :
-
Temperature
- T b :
-
Minimum temperature (base temperature)
- T c :
-
Maximum temperature (ceiling temperature)
- T d :
-
The temperature at which ψb(50) and/or NaClb(50) trend begins to change
- t g :
-
The time required for fraction or percentage (g) of seeds to germinate
- T o :
-
Optimum temperature
- θ H :
-
Hydrotime constant
- θ Halo :
-
Halotime constant
- σ NaClb :
-
Standard deviation of NaClb within the seed population
- σψ b :
-
Standard deviation of ψb within the seed population
- ψ :
-
Water potential
- ψb(50):
-
The base ψ of the 50th percentile
- ψ b :
-
Base water potential
References
Abbas ZK, Saggu S, Sakeran MI, Zidan N, Rehman H, Ansari AA (2015) Phytochemical, antioxidant and mineral composition of hydroalcoholic extract of chicory (Cichorium intybus L.) leaves. Saudi J Biol. Sci. 22:322–326
Abdellaoui R, Boughalleb F, Zayoud D, Neffati M, Bakhshandeh E (2019) Quantification of Retama raetam seed germination response to temperature and water potential using hydrothermal time concept. Environ Exp Bot 157:211–216
Alvarado V, Bradford K (2002) A hydrothermal time model explains the cardinal temperatures for seed germination. Plant Cell Environ 25:1061–1069
Arnold S, Kailichova Y, Baumgartl T (2014) Germination of Acacia harpophylla (Brigalow) seeds in relation to soil water potential: implications for rehabilitation of a threatened ecosystem. PeerJ 2:e268
Atashi S, Bakhshandeh E, Zeinali Z, Yassari E, Teixeira da Silva JA (2014) Modeling seed germination in Melisa officinalis L. in response to temperature and water potential. Acta Physiol Plant 36:605–611
Atashi S, Bakhshandeh E, Mehdipour M, Jamali M, Teixeira da Silva JA (2015) Application of a hydrothermal time seed germination model using the Weibull distribution to describe base water potential in zucchini (Cucurbita pepo L.). J Plant Growth Regul 34:150–157
Bakhshandeh E, Gholamhossieni M (2018) Quantification of soybean seed germination response to seed deterioration under PEG-induced water stress using hydrotime concept. Acta Physiol Plant 40:126
Bakhshandeh E, Gholamhossieni M (2019) Modelling the effects of water stress and temperature on seed germination of radish and cantaloupe. J Plant Growth Regul. https://doi.org/10.1007/s00344-00019-09942-00349
Bakhshandeh E, Jamali M (2020) Population-based threshold models: a reliable tool for describing aged seeds response of rapeseed under salinity and water stress. Environ Exp Bot. https://doi.org/10.1016/j.envexpbot.2020.104077
Bakhshandeh E, Atashi S, Hafez-Nia M, Pirdashti H (2013) Quantification of the response of germination rate to temperature in sesame (Sesamum indicum). Seed Sci Technol 41:469–473
Bakhshandeh E, Atashi S, Hafeznia M, Pirdashti H, Teixeira da Silva JA (2015) Hydrothermal time analysis of watermelon (Citrullus vulgaris cv ‘Crimson sweet’) seed germination. Acta Physiol Plant 37:1738
Bakhshandeh E, Jamali M, Afshoon E, Gholamhossieni M (2017) Using hydrothermal time concept to describe sesame (Sesamum indicum L.) seed germination response to temperature and water potential. Acta Physiol Plant 39:250
Bakhshandeh E, Pirdashti H, Vahabinia F, Gholamhossieni M (2019) Quantification of the effect of environmental factors on seed germination and seedling growth of Eruca (Eruca sativa) using mathematical models. J Plant Growth Regul. https://doi.org/10.1007/s00344-00019-09974-00341
Bakhshandeh E, Gholamhosseini M, Yaghoubian Y, Pirdashti H (2020) Plant growth promoting microorganisms can improve germination, seedling growth and potassium uptake of soybean under drought and salt stress. Plant Growth Regul 90:123–136
Balandary A, Rezvani Moghaddam P, Nasiri Mahalati M (2011) Determination of seed germination cardinal temperatures in short chicory (Cichorium pumilum Jacq.). Second National Seed Technology Conference, Mashhad, pp 1818–1822
Baskin CC, Baskin JM (2014) Seeds: ecology, biogeography, and evolution of dormancy and germination. Academic, San Diego, p 1600
Bello P, Bradford KJ (2016) Single-seed oxygen consumption measurements and population-based threshold models link respiration and germination rates under diverse conditions. Seed Sci Res 26:199–221
Benítez G, González-Tejero M, Molero-Mesa J (2010) Pharmaceutical ethnobotany in the western part of Granada province (southern Spain): ethnopharmacological synthesis. J Ethnopharmacol 129:87–105
Bewley JD, Bradford K, Hilhorst H, Nonogaki H (2013) Seeds: physiology of development, germination and dormancy, 3rd edn. Springer, New York
Bierhuizen J, Wagenvoort W (1974) Some aspects of seed germination in vegetables. 1. The determination and application of heat sums and minimum temperature for germination. Sci Hortic 2:213–219
Bradford KJ (1986) Manipulation of seed water relations via osmotic priming to improve germination under stress conditions. HortScience 21:1105–1112
Bradford KJ (1990) A water relations analysis of seed germination rates. Plant Physiol 94:840–849
Bradford KJ (1995) Water relations in seed germination. Seed Dev Germ 1:351–396
Bradford KJ (2002) Applications of hydrothermal time to quantifying and modeling seed germination and dormancy. Weed Sci 50:248–260
Bradford KJ, Still DW (2004) Applications of hydrotime analysis in seed testing. Seed Technol 26:75–85
Corbineau F, Come D (1989) Germinability and quality of Cichorium intybus L. seeds. VI Symp Timing Field Prod Veg 267:183–190
Dahal P, Bradford KJ (1994) Hydrothermal time analysis of tomato seed germination at suboptimal temperature and reduced water potential. Seed Sci Res 4:71–80
Estefan G, Sommer R, Ryan J (2013) Methods of soil, plant, and water analysis. A manual for the West Asia and North Africa region, International Center for Agricultural Research in the Dry Areas (ICARDA), p 244
Farzaneh S, Soltani E, Zeinali E, Ghaderi-Far F (2014) Screening oilseed rape germination for thermotolerance using a laboratory-based method. Seed Technol 15–27
Ghaderi-Far F, Bakhshandeh E, Ghadirian R (2010) Evaluating seed quality in sesame (Sesamum indicum L.) by the accelerated ageing test. Seed Technol 32:69–72
Guarrera P, Savo V (2016) Wild food plants used in traditional vegetable mixtures in Italy. J Ethnopharmacol 185:202–234
Gummerson R (1986) The effect of constant temperatures and osmotic potentials on the germination of sugar beet. J Exp Bot 37:729–741
Hatfield JL, Prueger JH (2015) Temperature extremes: effect on plant growth and development. Weather Clim Extrem 10:4–10
Heshmat O, Saeed H, Fardin K (2011) The improvement of seed germination traits in canola (Brassica napus L.) as affected by saline and drought stress. J Agric Sci Technol 7:611–622
Huarte R (2006) Hydrotime analysis of the effect of fluctuating temperatures on seed germination in several non-cultivated species. Seed Sci Technol 34:533–547
International Seed Testing Association (ISTA) (2018) International rules for seed testing. Seed Science and Technology, Zurich
Jouad H, Haloui M, Rhiouani H, El Hilaly J, Eddouks M (2001) Ethnobotanical survey of medicinal plants used for the treatment of diabetes, cardiac and renal diseases in the North centre region of Morocco (Fez–Boulemane). J Ethnopharmacol 77:175–182
Kamkar B, Al-Alahmadi MJ, Mahdavi-Damghani A, Villalobos FJ (2012) Quantification of the cardinal temperatures and thermal time requirement of opium poppy (Papaver somniferum L.) seeds to germinate using non-linear regression models. Ind Crops Prod 35:192–198
Khajeh-Hosseini M, Powell A, Bingham I (2003) The interaction between salinity stress and seed vigour during germination of soyabean seeds. Seed Sci Technol 31:715–725
Khan M, Weber D, Hess W (1985) Elemental distribution in seeds of the halophytes Salicornia pacifica var. utahensis and Atriplex canescens. Am J Bot 72:1672–1675
Liu S, Bradford KJ, Huang Z, Venable DL (2020) Hydrothermal sensitivities of seed populations underlie fluctuations of dormancy states in an annual plant community. Ecology 101(3):e02958
Llanes A, Andrade A, Masciarelli O, Alemano S, Luna V (2016) Drought and salinity alter endogenous hormonal profiles at the seed germination phase. Seed Sci Res 26:1–13
Luan Z, Xiao M, Zhou D, Zhang H, Tian Y, Wu Y, Guan B, Song Y (2014) Effects of salinity, temperature, and polyethylene glycol on the seed germination of sunflower (Helianthus annuus L.). Sci World J 2014:170418. https://doi.org/10.1155/2014/170418
Mesgaran M, Mashhadi H, Alizadeh H, Hunt J, Young K, Cousens R (2013) Importance of distribution function selection for hydrothermal time models of seed germination. Weed Res 53:89–101
Mesgaran MB, Onofri A, Mashhadi HR, Cousens RD (2017) Water availability shifts the optimal temperatures for seed germination: a modelling approach. Ecol Model 351:87–95
Michel BE, Radcliffe D (1995) A computer program relating solute potential to solution composition for five solutes. Agron J 87:126–130
Mobli A, Ghanbari A, Rastgoo M (2018) Determination of cardinal temperatures of Flax-leaf Alyssum (Alyssum linifolium) in response to salinity, pH, and drought stress. Weed Sci 66:470–476
Moltchanova E, Sharifiamina S, Moot DJ, Shayanfar A, Bloomberg M (2020) Comparison of three different statistical approaches (non-linear least-squares regression, survival analysis and Bayesian inference) in their usefulness for estimating hydrothermal time models of seed germination. Seed Sci Res. https://doi.org/10.1017/S0960258520000082
Moot D, Scott W, Roy A, Nicholls A (2000) Base temperature and thermal time requirements for germination and emergence of temperate pasture species. New Zealand J Agric Res 43:15–25
Nobel PS (1983) Biophysical plant physiology and ecology. W. H. Freeman and Co., San Francisco
Parihar P, Singh S, Singh R, Singh VP, Prasad SM (2015) Effect of salinity stress on plants and its tolerance strategies: a review. Environ Sci Pollut Res 22:4056–4075
Parmoon G, Moosavi SA, Siadat SA (2018) How salinity stress influences the thermal time requirements of seed germination in Silybum marianum and Calendula officinalis. Acta Physiol Plant 40:175
Rowse H, Finch-Savage W (2003) Hydrothermal threshold models can describe the germination response of carrot (Daucus carota) and onion (Allium cepa) seed populations across both sub-and supra-optimal temperatures. New Phytol 158:101–108
Sampaio FC, Maria do Socorro VP, Dias CS, Costa VCO, Conde NC, Buzalaf MA (2009) In vitro antimicrobial activity of Caesalpinia ferrea Martius fruits against oral pathogens. J Ethnopharmacol 124:289–294
SAS Institute Inc (2015) SAS/STAT user’s guide. SAS Institute Inc., Cary
Seal CE, Barwell LJ, Flowers TJ, Wade EM, Pritchard HW (2018) Seed germination niche of the halophyte Suaeda maritima to combined salinity and temperature is characterised by a halothermal time model. Environ Exp Bot 155:177–184
Shaygan M, Baumgartl T, Arnold S (2017) Germination of Atriplex halimus seeds under salinity and water stress. Ecol Eng 102:636–640
Song J, Feng G, Tian C, Zhang F (2005) Strategies for adaptation of Suaeda physophora, Haloxylon ammodendron and Haloxylon persicum to a saline environment during seed-germination stage. Ann Bot 96:399–405
Tilaki GAD, Behtari B, Behtari B (2009) Effect of salt and water stress on the germination of alfalfa (Medicago sativa L.) seed. Пoвoлжcкий экoлoгичecкий жypнaл, 158–164
Vahabinia F, Pirdashti H, Bakhshandeh E (2019) Environmental factors’ effect on seed germination and seedling growth of chicory (Cichorium intybus L.) as an important medicinal plant. Acta Physiol Plant 41:27
Van’t Hoff JH (1887) The role of osmotic pressure in the analogy between solutions and gases. Zeitschrift fur physikalische Chemie 1:481–508
Wang R, Bai Y, Tanino K (2005) Germination of winterfat (Eurotia lanata (Pursh) Moq.) seeds at reduced water potentials: testing assumptions of hydrothermal time model. Environ Exp Bot 53:49–63
Yoo S, Murata R, Duarte S (2011) Antimicrobial traits of tea-and cranberry-derived polyphenols against Streptococcus mutans. Caries Res 45:327–335
Zarghani H, Mijani S, Nasrabadi SE (2014) Temperature effects on the seed germination of some perennial and annual species of Asteraceae family. Plant Breed Seed Sci 69:3–14
Zhang H, Irving LJ, McGill C, Matthew C, Zhou D, Kemp P (2010) The effects of salinity and osmotic stress on barley germination rate: sodium as an osmotic regulator. Ann Bot 106:1027–1035
Zhang H, Irving L, Tian Y, Zhou D (2012) Influence of salinity and temperature on seed germination rate and the hydrotime model parameters for the halophyte, Chloris virgata, and the glycophyte, Digitaria sanguinalis. South Afr J Bot 78:203–210
Acknowledgements
This work is financially supported by the Genetics and Agricultural Biotechnology Institute of Tabarestan (GABIT) and Sari Agricultural Sciences and Natural Resources University (SANRU) research Grant (D.2169.97.31). We also gratefully acknowledge use of the services and facilities of the GABIT during this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Communicated by S. Renault.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bakhshandeh, E., Bradford, K.J., Pirdashti, H. et al. A new halothermal time model describes seed germination responses to salinity across both sub- and supra-optimal temperatures. Acta Physiol Plant 42, 137 (2020). https://doi.org/10.1007/s11738-020-03126-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-020-03126-9