Abstract
Chicory (Cichorium intybus L.; Asteraceae) is a small aromatic and medicinal biennial and perennial herb that is distributed in most parts of Europe and Asia including Iran. However, little information is available about seed germination (SG) and seedling growth of this plant in response to abiotic environmental factors. Therefore, this study aimed to investigate the effect of several environmental factors such as temperature (T), water stress (ψ), salinity, pH and burial depth on SG characteristics of chicory. Results indicated that all studied traits including germination percentage (GP), germination rate (GR), germination uniformity (GU), normal seedling percentage (NSP), root length (RL), shoot length (SL) and seedling dry weight (SDW) are significantly influenced by each environmental factor. Estimated cardinal Ts were 3.5, 28.9 and 40.2 °C for the base, optimum and ceiling T, respectively, with a thermal time 330.2 °C h after fitting a beta model in water. The drought tolerance threshold value was − 0.82 MPa for GP and − 0.75 MPa for NSP. The sensitive of each trait to ψ was ranking RL > SL > GR > SDW > NSP > GP. Increasing salinity level from 0 to 250 mM declined GP, GR, NSP, RL, SL and SDW by 75, 83, 88, 85, 80 and 60%, respectively, and also GU decreased seven times compared with the control. The salt tolerance threshold value was 223 and 194 mM for GP and NSP, respectively. Although chicory seeds were able to germinate at all pH levels (84%, ranged from 2 to 10), they could not produce an equivalent normal seedling in the same condition which indicates that seedling growth is more sensitive to pH relative to SG. The best pH for germination and seedling growth was estimated to be ~ 7 for this plant. Seedling emergence increased by 25% as burial depth increased from 0.5 to 2 cm and then sharply decreased by 87% when reached to 4 cm. The best burial depth ranged from 1 to 2 cm (> 88%) for chicory. Consequently, this information could help us to adequately manage the production of this plant under different environmental factors and also to determine its geographic range expansion in the world.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chicory (Cichorium intybus L.; Asteraceae) is a small aromatic and medicinal biennial and perennial herb that is distributed in most parts of Europe, North Africa and Asia including Iran. Different parts (i.e., leaves, stem, root and seed) of the plant contained a number of medicinally important compounds such as sucrose, proteins, caffeic acid, esculin, volatile compounds, flavonoids and vitamins (Nandagopal and Kumari 2007; Street et al. 2013). Chicory is known as an important medicinal plant with great economic potential due to high concentrations of fructooligosaccharide (i.e., inulin in its root which can be used as a replacement material for sugar) and useful effect for maintaining body health against some diseases such as cancer, diabetes, impotence, insomnia and gallstones (Das et al. 2016).
Seed germination (SG) is an important event in the plant’s life history (Bakhshandeh et al. 2013). Following SG, seedling emergence (SE) is the most important phenological event that affects the successful plant establishment particularly in annual plant species (Forcella et al. 2000). Generally, many environmental factors such as temperature (T), water potential (ψ), salinity, pH and burial depth can highly influence SG pattern and seedling growth of many plant species with non-dormant seeds (Baskin and Baskin 2014; Honarmand et al. 2016; Nosratti et al. 2017).
Water deficiency can reduce the final germination percentage (GP), germination rate (GR) and finally seedling growth through its negative effects on the rate of seed imbibition which was well documented in the past (Bradford 2002; Bewley et al. 2013; Atashi et al. 2014; Bakhshandeh et al. 2015; Bakhshandeh and Gholamhossieni 2018; Abdellaoui et al. 2019).
T can influence SG through regulating enzyme activities and or promote/inhibit the synthesis of hormones involved in SG (Baskin and Baskin 2014; Xu et al. 2017). To date, many researchers used several mathematical models such as segmented, dent-like and beta, each with straights and weaknesses, to determine three important Ts for SG in various crops which are defined as cardinal Ts (Bakhshandeh et al. 2013, 2017; Atashi et al. 2015; Javadzadeh et al. 2017; Abdellaoui et al. 2019; Parmoon et al. 2018). These Ts were the base T (Tb), optimum T (To) and ceiling T (Tc) which are important to determine the best planting date for each plant species (Bewley et al. 2013).
The salinity of the soil and/or water is considered as a serious problem for many agricultural lands in the world. Previous researches showed that SG may be influenced by the negative impacts of salt stress, osmotically through decreasing water uptake and ion toxicity through accumulation of salt ions (i.e., Na+ and Cl−), which can affect metabolic cell activities during SG (Parmoon et al. 2018; Parihar et al. 2015). Therefore, investigation of the effect of salt stress on SG of chicory could be useful to determine the best niche for this plant.
Another environmental factor which can influence SG and seedling growth is pH. In fact, soil pH is affected by parent material, precipitation, vegetation and levels of organic matter (Xu et al. 2017). Many species can germinate in a wide range of pH such as muskweed (Myagrum perfoliatum) (Honarmand et al. 2016), Centaurea balsamita (Nosratti et al. 2017) and Cucumis melo L. var. agrestis Naud. (Xu et al. 2017) while for others, it could be a limiting factor (Amini et al. 2015; Javaid et al. 2018). pH can damage several biological systems during SG through reacting with some processes which were involved in SG, damaging organic compounds, organelles and or physical structure of seed (Basto et al. 2013).
However, a successful plant establishment depends on burial depth at which SG occurs (Javaid et al. 2018; Amini et al. 2015). In many plant species, SE declined with the increasing burial depth which could be due to a small amount of seed reserves or lower hydration of seed at greater depths (Dinelli et al. 2013). However, in some plant species such as tobacco (Nicotiana glauca R. Graham) (Florentine et al. 2016) and Cucumis melo var. agrestis Naud (Xu et al. 2017), a maximum SE was observed when the seeds were placed on the soil surface because they needed light for germination (Nosratti et al. 2017).
The available information about the environmental factors’ effect on SG and seedling growth of chicory is scarce yet. Therefore, the objectives of this study were to determine the effect of T, ψ, salinity, pH and burial depth on SG and seedling growth of this plant, and also to calculate the cardinal Ts and the tolerance threshold values for each trait influenced by different environmental factors using mathematical models. However, a better understanding of the seed ecology of chicory could help us to nicely manage the production of this plant under different environmental conditions.
Materials and methods
Seeds of cultivated chicory were provided in Mazandaran province (Sari city, Iran) in September 2017. The seeds were kept in the dark at 5 °C for 3 months. The viability of the seeds under optimal conditions was > 98% before starting the experiment because the seeds had no dormancy. Treatments used in this study were nine constant Ts (5, 10, 15, 20, 25, 30, 35, 38 and 40 °C), four levels of ψ (− 0.3, − 0.6, − 0.9, and − 1.5 MPa), seven levels of salt stress (50, 100, 150, 200, 250, 300 and 350 mM), nine levels of pH (2, 3, 4, 5, 6, 7, 8, 9 and 10) and eight levels of burial depth (0.5, 1, 2, 3, 4, 5, 6 and 7 cm). Distilled water was used as a control in all experiments.
Germination test: 50 seeds were placed on two sheets of filter paper Whatman No. 1 within 8-cm Petri dishes (SUPA Medical Devices, Tehran, Iran) for each replicate of each treatment. The germination tests were carried out with four replicates. 6 ml of distilled water and or test solutions, supplemented with 0.1% Tiram fungicide, was used for each Petri dish to provide adequate moisture for the seeds during the experiment. Petri dishes were randomly placed within an incubator with ± 0.5 °C accuracy (BINDER, Germany) in the dark, except recording times. A thin plastic bag was also used to reduce water evaporation from the Petri dishes. At all experiments, seeds were counted several times daily, depending on the environmental factors (e.g., T, ψ, salinity, and pH) and when the radicle protruded 2 mm from the seed coat it was considered as a germinated seed. The experiments were finished when a new germinated seed was not observed in the three consecutive days.
Seedling growth test: 20 seeds for each replicate were used according to the sandwich method (International Seed Testing Association (ISTA) 2009). Rolled papers were separately treated by each solution of each treatment (e.g., PEG, NaCl, and pH) and then put in a white thin plastic bag (to avoid evaporation from the papers) and randomly incubated in the dark in the incubator at 30 °C (i.e., To) in all experiments. At the end of experiments (seventh day), the normal seedlings were counted. Then, seven of the tallest seedlings were selected from each replicate, and the length of root (RL) and shoot (SL) were measured by a ruler in cm. Seedlings dry weight of (SDW) was also determined by a balance with 0.0001 g precision after oven-drying at 70 °C for 2 days.
The polyethylene glycol (PEG8000, Scharlau, Spain) was used to prepare different levels of ψs according to the formula suggested by Michel and Radecliffe (1995) [ψs = 0.13(PEG)2T − 13.7(PEG)2]. To re-check and modify the ψ values for each T, an osmometer (Model 5100C: Wescor Inc., Logan, UT, USA) was used before starting the experiment. The sodium chloride (NaCl) was used for preparing the salinity levels maintained above. In addition, a citrate–phosphate buffer was used to prepare pH solutions of 2, 3, 4, 5 and 6 [0.1 M citric acid solution + 0.2 M dibasic sodium phosphate solution were combined to make targeted solutions] and by adjusting with 0.1 N HCl solution. In addition, a tricine (N-Tris (hydroxymethyl) methylglycine) solution was used to prepare buffered solutions of 8, 9 and 10 pH by adjusting with 1 N NaOH solution. The pH 7 was prepared using a 2 mM solution of HEPES [N-(2-hydroxymethyl) piperazine-N-2-ethanesulfonic acid)] as in Basto et al. (2013).
To determine the effect of burial depth on SE of chicory, we used three replicates of 50 seeds for each treatment which were planted in soil in 10-cm-diameter plastic pots (black color and with 17 cm height) at the depths of maintained above. The soil type was loam (45, 25 and 30% sand, clay and silt, respectively). Greenhouse T was 28 ± 2 °C during the experiment. The pots were irrigated as required to maintain them at the field capacity during the experiment. SE was counted every 24 h until 14 days. Seeds were considered to be emerged when the two cotyledons could be seen at the soil surface.
The GR for the 50th percentile was estimated by fitting the cumulative SG data (%) vs. time (h) for each replicate and then the time taken to reach 50% of maximum germination (t50) was calculated using the Bakhshandeh et al. (2013) method. Finally, the GR50 was calculated by the formula [GR50 = 1/t50].
Several mathematical models were used to quantify the effect of environmental factors on SG and seedling growth of chicory in the present study. Three T models, segmented, dent-like and beta, were fitted to describe the relationships between GR and T. The models can be written:
Dent-like model (Bakhshandeh et al. 2013):
Segmented model (Bakhshandeh et al. 2013):
Beta model (Yin et al. 1995):
where T, Tb, To1, To2, Tc, and α are the actual T, the base T, the lower optimum T, the upper optimum T (for dent-like model), the ceiling T and the shape parameter (just for the beta model, which determines the curvature of the model), respectively.
We also used a three-parameter segmented model (Eq. 4) for describing the response of SG and seedling growth of chicory to both salt and water stresses:
where G, Gmax, x0 and b are the germination (%) at concentration x, the maximum G (%), the concentration when the linear decrease in G began and the rate of decrease in G per increase in NaCl (mM) and/or per decrease in ψ (MPa), respectively. The model can predict the concentration of NaCl/ψ needed for 50% inhibition of the Gmax (i.e., salinity/drought tolerance threshold) by the formula [x50 = ((Gmax/2)/−b) + x0] and also where SG is stopped by the formula [G0 = (Gmax/−b) + x0]. Based on our knowledge, all parameters estimated by the model (Eq. 4) are easier to understand and also easier to interpret physiologically.
All regression analyses (linear and non-linear) were done using the statistical analysis system (SAS) ver. 9.4 (SAS Institute 2013) to describe the response of chicory germination pattern under different levels of environmental factors maintained above. The graphs were drawn by SigmaPlot 11 software. To compare the means of treatments, an analysis of variance by the GLM procedure was used based on the least significant difference (LSD) test at the 0.05 probability level.
Results and discussion
The results showed that all studied environmental factors (e.g., T, ψ, salinity, pH and burial depth) significantly influenced the pattern of SG and seedling growth of chicory (P ≤ 0.05). Below, the effect of each environmental factor on chicory SG characteristics is presented in more details.
Temperature
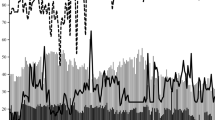
Our results indicated that GP, GR and germination uniformity (GU) are significantly influenced by T (Fig. 1). In fact, there was no significant difference among Ts, ranged from 5 to 38 °C, in terms of GP (> 90%) and then it suddenly decreased by ~ 53% with the addition of T until 40 °C (Fig. 1a). In addition, GR increased from 0.0064 to 0.0761 h− 1 (92%) with the increasing T from 5 to 30 °C and then decreased by 93% when T was equal to 40 °C (Fig. 1c–e). The minimum value of GU was observed at 25, 30 and 35 °C (P > 0.05), then increased at Ts lower than 25 °C and higher than 35 °C (Fig. 1b). Our findings indicated that GR > GU > GP was sensitive to T; therefore, GR as a more sensitive parameter than others was used to estimate the cardinal Ts for this plant. These results are in accordance with the results reported by others (Bradford 2002; Bewley et al. 2013; Bakhshandeh et al. 2015). Three T models (Eqs. 1–3) were fitted to the data GR vs. T (Fig. 1c–e). Results showed that all models could describe well this relationship and the beta model (Eq. 3) as the best had lower RMSE (4.04 h−1) and higher R2 (0.99) values than others (Table 1; Fig. 1e). Estimates based on the beta model were 3.50 °C for Tb, 28.9 °C for To, 40.2 °C for Tc and 13 h for fo (the lowest time taken to reach 50th percentile of SG under optimum condition according to the model estimates). Based on the model parameters, the thermal time (°C h) could easily be calculated by the formula [TT = (To − Tb)fo] suggested by Bradford (2002). TT was equal to 330.2 °C h for this plant. The parameter estimates for all models, segmented, dent-like and beta are summarized in Table 1.
The relationship between the final germination percentage (a), germination uniformity (b) and germination rate (for the 50th percentile) of chicory seeds with temperature in the control conditions (ψ = 0 MPa). The actual germination rate data are shown by the symbols and the lines drawn through these points are based upon the parameters presented in Table 1 using (c) segmented, (d) dent-like and (e) beta models. Bars represent standard error values. In the panels a and b, the same letters are not significant at 5% probability level according to the least significant difference (LSD) test
The cardinal Ts obtained in this study were slightly higher than those reported by Balandary et al. (2011) who showed in short chicory the Tb, To and Tc were 2, 25.5 and 40 °C, respectively, after fitting a beta model. Zarghani et al. (2014) also used a segmented model and showed that the Tb and To were 2.9 and 29.9 °C for this plant which was closer to the results found here but the Tc reported by them was higher (46.3 °C) than that obtained in this study (40.2 °C). Moreover, Corbineau and Come (1989) reported To was ranged 25–30 °C for chicory which is in agreement with our results. Many researchers demonstrated that SG affected by T in various crops through impacting enzymes activity, synthesis of hormones, seed dormancy and translocation of reserve storage material during SG particularly under unfavorable Ts (Bradford 2002; Copeland and McDonald 2012; Bewley et al. 2013). However, other researchers well documented that the segmented (Atashi et al. 2014; Bakhshandeh et al. 2017; Derakhshan et al. 2018), dent-like (Bakhshandeh et al. 2013; Parmoon et al. 2018; Mamedi et al. 2017) and quadratic (Mobli et al. 2018; Javadzadeh et al. 2017) models could nicely describe these relationships and estimate cardinal Ts in various crops.
Water potential
Water is the most important factor for SG and its availability can significantly effect on germination time course (Bradford 2002). To date, many researchers used a three-parameter logistic model to quantify the effect of different levels of ψ on SG (Nosratti et al. 2017; Honarmand et al. 2016; Ghaderi-Far et al. 2012). But in this study, we used a segmented model (Eq. 4) for describing the response of both SG and seedling growth of chicory to ψ because the model parameters are easier to interpret physiologically (author’s opinion).
Based on our results, GP was not influenced by ψ, ranged from 0 to − 0.60 MPa (> 91%), then remarkably decreased with the decreasing ψ until − 1.04 MPa (i.e., G0: where the seeds are unable to germinate). The rate of decrease in GP was 211.7% per MPa decrease in ψ (Fig. 2a). There was no germinated seed at − 1.5 MPa. The same pattern was observed in NSP which means that NSP remained constant at a maximum value (88%) at ψs higher than − 0.53 MPa (more positive), then declined linearly with the rate of 196% per MPa decrease in ψ and finally reached zero at − 0.98 MPa (Fig. 2a). The estimated drought tolerance threshold value was − 0.82 MPa for SG and − 0.75 MPa for NSP (Fig. 2a). GR declined linearly with the decreasing ψ from zero to − 0.98 MPa (0.0707 h− 1 per MPa decrease in ψ) which is indicating that GR relative to GP is more sensitive to ψ, like NSP relative to GP (Fig. 2b). GU also non-linearly declined with the decreasing ψ (Fig. 2c). It is expected because when the seeds germinated under stressful conditions such as lower ψ (more negative), the water uptake by the seeds will be declined and so the time to the beginning of germination and/or the time to reach a maximum germination will be delayed as a result of lower GR. Therefore, low GU can be as the main reason for the low seedling establishment and ultimately low productivity in field condition. Generally, Alvarado and Bradford (2002) on potato (Solanum tuberosum L.), Mesgaran et al. (2017) on two weed species such as wild barley (Hordeum spontaneum [C. Koch] Thell.) and little canarygrass (Phalaris minor L), Bakhshandeh et al. (2017) on sesame (Sesamum indicum L.) Bakhshandeh and Gholamhossieni (2018) on soybean (Glycine max (L.) Merrill) and Atashi et al. (2014) on lemon balm (Melissa officinalis L.) observed that SG characteristics (i.e., GP and GR) significantly declined as ψ decreased (more negative) which was in agreement with the results obtained in this study.
Effect of water potential solutions (prepared using PEG8000) on seed germination and seedling growth of chicory incubated at 30 °C (i.e., optimum temperature). Seedling growth parameters were measured after 7 days. Data represent the mean ± standard error of the mean (n = 4). Final seed germination and normal seedling percentage (a); germination rate (b); germination uniformity (c); root length (d); shoot length (e) and seedling dry weight (f). The seeds are unable to germinate at ≤ − 0.9 MPa in d, e and f, and < − 1.1 MPa in a, b and c so that these data were not used for the model fitting. DTT is the drought tolerance threshold value (water potential needed for 50% inhibition of the trait). In panel a, the solid and broken lines are indicating the final seed germination and normal seedling percentage, respectively, which were fitted by the Eq. 4
The results of seedling growth test showed that the maximum RL was 1.23 cm, remained constant at > − 0.30 MPa (P > 0.05) and then decreased linearly to zero at − 0.64 MPa with the rate of 3.91 cm per MPa decrease in ψ (Fig. 2d). In addition, with the decreasing ψ, SL decreased rapidly (2.95 cm per MPa) from 2.56 cm in water (ψ = 0 MPa) to 0 at − 0.87 MPa which is indicating that RL than SL was more sensitive to ψ in chicory (Fig. 2e). SDW remained constant at its maximum value until − 0.33 MPa (0.642 mg plant− 1) and then decreased linearly to zero at − 0.91 MPa (Fig. 2f). Therefore, the drought tolerance threshold values for RL, SL and SDW were − 0.47, − 0.43 and − 0.62 MPa, respectively (Fig. 2d–f).
In accordance with the results obtained in this study, other researchers showed that RL, SL and SDW significantly declined as ψ decreased such as fennel (Foeniculum vulgare L) and ajowan (Trachyspermum ammi) (Fakheri et al. 2017) and pea (Pisum sativum L.) (Okçu et al. 2005). Kayacetin et al. (2018) in wild mustard (Sinapis arvensis L.) also indicated that SDW, RL and SL significantly decreased (~ 86, 70 and 90%, respectively) when ψ was equal to − 1 MPa relative to the control. In canola, SL decreased from 5.09 cm in water to 0.28 cm at − 0.9 MPa (Channaoui et al. 2017). In some plant species, however, the first change under mild water stress condition is an increase in RL and SDW as a result of higher water uptake by seeds (Fakheri et al. 2017). For example, in canola Channaoui et al. (2017) showed that RL enhanced by 34% until − 0.50 MPa and then decreased by 48% until − 0.9 MPa when compared with the control. In general, decreasing SG and seedling growth with the decreasing ψ can be due to a reduction in water imbibition by seeds during germination which had a negative impact in metabolic processes such as reducing hydrolysis enzymes and utilization of the seed reserve (Toosi et al. 2014). Finally, we conclude that chicory is relatively tolerant to drought stress during germination and so it can be planted in some parts of dryland fields around the world.
Salt stress
Salinity is a serious problem for the growth of many plant species in the world particularly at an early stage of growth (i.e., SG). Like water stress, we used a segmented model (Eq. 4) to describe the response of both SG and seedling growth of chicory to salt stress. Results indicated that the maximum GP and NSP (> 93% for both) observed at NaCl concentration lower than 150 and 100 mM, respectively. On the other hand, differences were not statistically significant (Fig. 3a). However, GP and NSP decreased linearly with the rate of 0.56 and 0.49% per mM increase in salt stress until 317 and 290 mM, respectively, which is defined as G0 (where the seeds are unable to germinate). In addition, the salt tolerance threshold values were 233 and 195 mM for this plant which is indicating that NSP is more sensitive to salt stress than GP (~ 16%).
Effect of salt stress solutions (prepared using NaCl) on seed germination and seedling growth of chicory incubated at 30 °C (i.e., optimum temperature). Seedling growth parameters were measured after 7 days. Data represent the mean ± standard error of the mean (n = 4). Final seed germination and normal seedling percentage (a); germination rate (b); germination uniformity (c); root length (d); shoot length (e) and seedling dry weight (f). The data related to 350 mM in all panels (a–f) were not used for the model fitting. STT is the salt tolerance threshold value (NaCl concentration needed for 50% inhibition of the trait). In panel a, the solid and broken lines are indicating the final seed germination and normal seedling percentage, respectively, which were fitted by the Eq. 4
The maximum GR was 0.0769 h− 1 which was obtained in water and then linearly declined (− 0.0002 h− 1 per mM) with the increasing salt stress to reached zero at 325 mM (i.e., GR0) (Fig. 3b). GU declined non-linearly with the addition of salt stress. In fact, the amount of GU was ~ 14 h at NaCl concentration lower than 100 mM (P < 0.05) then declined eight times relative to the control when NaCl concentration was equal to 300 mM (127 h) (Fig. 3c). This indicates that the seeds were germinated more uniformly in water and/or mild salt stress condition and higher salt stress lead to a significant reduction in GU as a result of lower GR. Our findings are in accordance with the results reported by Bakhshandeh et al. (2017) in sesame. The highest value of RL and SL was 1.31 and 2.76 cm, respectively, which were not significantly affected by salt stress until 100 mM (P < 0.05). After that, both of them decreased linearly with the rate of 0.0068 and 0.0144 cm, respectively, as salt stress increased until 293 mM (Fig. 3d, e). Similar result was observed in SDW which means that SDW remained constant at a maximum value (0.68 mg plant−1) at salt stress lower than 155 mM, then declined linearly (0.0049 mg plant−1 per mM) to reached zero at 293 mM (Fig. 3f). In general, the estimated salt tolerance threshold value was 197 mM for RL, 196 mM for SL and 224 mM for SDW. Moreover, the sensitive of each trait to salt stress can be ranked GR > SL = RL = NSP > SDW > GP.
These findings agree with the results of many published studies that showed salt stress can decline all traits related to SG and seedling growth in various crops. For example, Seghatoleslami (2010) reported an increase in salt stress from 0 to 8 dS m−2 (approximately lower than 100 mM) which had no significant effect on SG and seedling growth of chicory. In other study, Parmoon et al. (2018) showed that salt stress significantly influenced GP, GR and cardinal Ts in both Milk thistle (Silybum marianum) and Calendula (Calendula officinalis). Ghaderi-Far et al. (2012) investigated the effect of salt stress on SG of six medicinal plants and then reported that GP, GR, seedling length and SDW of all plants remarkably declined as salt stress increased. Jamil et al. (2005) also showed that salt stress decreased RL by 58, 82 and 57% and SL by 75, 66 and 78% in canola (Brassica napus), cabbage (Brassica oleracea capitata) and cauliflower (Brassica oleracea botrytis L.), respectively, at 14.1 dS m−2 when compared with the control which is indicating that SL was more sensitive than RL in these species.
However, salt stress can influence SG and seedling growth by either osmotically through decreasing water uptake which is often observed in halophyte crops or ionically through the accumulation of sodium and chloride which resulted in an imbalance nutrient uptake and toxicity effect on metabolic processes (i.e., DNA repair and protein synthesis) which is often observed in non-halophyte crops (Bewley et al. 2013; Seal et al. 2018). Moreover, salt stress can possibly reduce the activity of enzymes involved in SG stage and thus terminates normal seedling growth and development (Neumann 1997; Parihar et al. 2015; Parmoon et al. 2018). However, we conclude that chicory is relatively tolerant to salt stress during germination and so it can be planted in some parts of saline environments in the world.
pH
The response of SG and seedling growth of chicory to different levels of pH is shown in Fig. 4. The maximum GP of chicory (> 84%) was observed at all levels of pH and differences were not statistically significant (Fig. 4a). In opposite, the maximum NSP of chicory (> 93%) was observed at a range of pH 6–8 (P > 0.05) and then significantly decreased at 8 < pH < 6 (Fig. 4c). The seeds were unable to produce a normal seedling at pH 2. This shows the ability of chicory seeds to germinate (radicle length > 2 mm) under a wide range of pH conditions while they could not produce equivalent normal seedling at the same pH. The maximum NSP only obtained at pH of 6–8 (Fig. 4c). Therefore, we can conclude that the SG test is not an accurate test to describe the response of plant species to different levels of pH as documented in the past while the seedling growth test can be introduced as an alternative way. Because the seedling growth is more sensitive to pH solutions than SG particularly in chicory, a quadratic model was used to quantify the relationship between GR, NSP, RL, SL, and SDW with pH solutions (Fig. 4b–f). According to the model parameters, the maximum value of GR (0.172 h−1), NSP (90%), RL (0.836 cm), SL (2.24 cm) and SDW (0.576 mg plant−1) was observed at pH of 7.14, 7.03, 7.35, 7.22 and 8.24 (i.e., optimum pH), respectively (Fig. 4b–f). However, the amount of GR, NSP, RL, and SL declined gradually at pH < 7.18 < pH (averaged all traits) and also the same pattern was observed in SDW (pH < 8.24 < pH). The sensitivity of each trait to acidic solutions is ranked as SL > RL > NSP > SDW > GR > GP. Similar result was obtained for the alkaline solutions which is ranked SL > RL > NSP > GR > SDW > GP (Fig. 4).
Effect of pH solutions on seed germination and seedling growth of chicory incubated at 30 °C (i.e., optimum temperature). Seedling growth parameters were measured after 7 days. Data represent the mean ± standard error of the mean (n = 4). Final seed germination (a); germination rate (b); final normal seedling percentage (c); root length (d); shoot length (e) and seedling dry weight (f). The lines represent the quadratic model fitted to the experimental data. The seeds are unable to produce normal seedling at pH 2. In all panels, the same letters are not significant at 5% probability level according to the least significant difference (LSD) test
To date, the effect of hydrogen ion concentration on SG of various crops was investigated such as muskweed (Honarmand et al. 2016) and white horehound (Marrubium vulgare L.) (Javaid et al. 2018), whereas no investigations were done on the effect of pH on seedling growth. In general, in many plant species, the maximum SG occurred at pH 7 (Honarmand et al. 2016; Amini et al. 2015; Javaid et al. 2018) which is in accordance with our findings in this study. However, a range of pH values lower than 5 and higher than 8 may significantly reduce SG and seedling growth in many plant species such as white horehound (Javaid et al. 2018) while other reports showed that SG was not affected by pH in acidic or alkaline conditions (ranged from 4 to 10) such as muskweed (Honarmand et al. 2016), Centaurea balsamita (Nosratti et al. 2017) and Cucumis melo L. var. agrestis Naud. (Xu et al. 2017). pH can decrease SG and seedling growth through its negative effect on several biological systems during SG such as reacting chemically with some processes during SG, damaging organic compounds, organelles and or physical structure of seed (Basto et al. 2013).
Based on the results presented in this study, chicory seeds could produce normal seedling well at a range of pH 6–8 without any significant decrease. Moreover, more acidic pH greatly decreased the seedling growth of this plant than alkaline pH which resulted in better growth of chicory at alkaline soil conditions. In Iran, the soil pH range is wide, but in general, most parts of Iran had an alkaline soil (Honarmand et al. 2016).
Burial depth
Based on our findings, SE of chicory increased by 25% when seeds were placed at the burial depth of 2 cm relative to 0.5 cm (near to soil surface) and then declined gradually (~ 87%) with the increasing burial depth (Fig. 5a). At the burial depth deeper than ~ 4 cm no seedlings emerged. The best burial depth was 1.56 cm according to the quadratic model parameters for this plant. In addition, the amount of decrease in SE rate was 0.0024 h−1 per cm increase in burial depth (Fig. 5b).
Seedling emergence of chicory in response to burial depth when grown under greenhouse conditions at 28 ± 2 °C (i.e., optimum temperature) for two weeks. Seedling emergence (a) and emergence rate (b). The lines represent the quadratic (a) and linear (b) models fitted to the experimental data. The seeds are unable to emerge at burial depth deeper than 5 cm and so these data were not used for the model fitting
In general, seed burial depth is another factor affecting SG because of its dependency on the soil moisture, soil T, light, physical properties of the soil and/or the physical limitations of the seedling in this condition (Chachalis and Reddy 2000; Xu et al. 2017). Our results are in agreement with the study demonstrating that African mustard seeds were able to emerge in soil surface (29%), but lower than those placed at 1 and 2 cm (decreased by 51 and 44%, respectively), and then completely stopped at 5 cm (Chauhan et al. 2006). Xu et al. (2017) indicated that an increase in burial depth leads to delay in the SE of field muskmelon which is in accordance with our results. In addition, some researchers reported that light may be a requisite for SE such as tobacco (Florentine et al. 2016), but we found that chicory seeds are able to germinate in the dark and light was not a prerequisite for this plant.
Consequently, the main reason for decreasing SE at deeper burial depth (> 2 cm) can probably be due to insufficient seed reserves of chicory seeds (as small-seed species), because seeds usually required higher reserves to reach the soil surface (Baskin and Baskin 2014). Another reason may be due to the oxygen deficiency and/or the invasion of soil pathogens to the seedlings that are growing under inappropriate conditions as a result of the increasing burial depth (Koger et al. 2004).
Conclusion
Having a successful plant establishment requires a good knowledge about the response of each plant species to biotic and abiotic (e.g., T, ψ, salinity, pH and burial depth) environmental factors. Our results indicated that SG and seedling growth of chicory were significantly influenced by the environmental factors. The cardinal Ts were 3.5 °C for Tb, 28.9 °C for To and 40.2 °C for Tc estimated by the beta model. The salt and drought tolerance threshold values were 233 mM and − 0.82 MPa for GP and 195 mM and − 0.75 MPa for NSP, respectively, which is indicating that NSP is more sensitive than GP under both salt and drought stress conditions. Moreover, the best SG and seedling growth were observed at pH of 7 and burial depth of 1.56 cm. Consequently, this information could help us to nicely manage the production of this plant under different environmental factors and also to determine its geographic range expansion in the world. In addition, the models developed in this study and their parameters could be used in chicory SG modeling.
Author contribution statement
EB and FV arranged the experiments. EB conducted the modeling and interpreted the data. EB, FV and HP co-wrote all drafts of the paper and also approved the final draft for submission and take full public responsibility for the content.
Abbreviations
- GP:
-
Final germination percentage
- GR:
-
Germination rate
- GU:
-
Germination uniformity
- mM:
-
Millimolar
- MPa:
-
Megapascal
- NSP:
-
Normal seedling percentage
- PEG:
-
Polyethylene glycol
- RL:
-
Root length
- SDW:
-
Seedling dry weight
- SE:
-
Seedling emergence
- SG:
-
Seed germination
- SL:
-
Shoot length
- T :
-
Temperature
- T b :
-
Minimum temperature (base temperature)
- T c :
-
Maximum temperature (ceiling temperature)
- T o :
-
Optimum temperature
- TT:
-
Thermal time
- ψ :
-
Water potential
References
Abdellaoui R, Boughalleb F, Zayoud D, Neffati M, Bakhshandeh E (2019) Quantification of Retama raetam seed germination response to temperature and water potential using hydrothermal time concept. Environ Exp Bot 157:211–216
Alvarado V, Bradford K (2002) A hydrothermal time model explains the cardinal temperatures for seed germination. Plant Cell Environ 25:1061–1069
Amini V, Zaefarian F, Rezvani M (2015) Interspecific variations in seed germination and seedling emergence of three Setaria species. Braz J Bot 38:539–545
Atashi S, Bakhshandeh E, Zeinali Z, Yassari E, Teixeira da Silva JA (2014) Modeling seed germination in Melisa officinalis L. in response to temperature and water potential. Acta Physiol Plant 36:605–611
Atashi S, Bakhshandeh E, Mehdipour M, Jamali M, Teixeira da Silva JA (2015) Application of a hydrothermal time seed germination model using the Weibull distribution to describe base water potential in zucchini (Cucurbita pepo L.). J Plant Growth Regul 34:150–157
Bakhshandeh E, Gholamhossieni M (2018) Quantification of soybean seed germination response to seed deterioration under PEG-induced water stress using hydrotime concept. Acta Physiol Plant 40:126
Bakhshandeh E, Atashi S, Hafez-Nia M, Pirdashti H (2013) Quantification of the response of germination rate to temperature in sesame (Sesamum indicum). Seed Sci Technol 41:469–473
Bakhshandeh E, Atashi S, Hafeznia M, Pirdashti H, Teixeira da Silva JA (2015) Hydrothermal time analysis of watermelon (Citrullus vulgaris cv.‘Crimson sweet’) seed germination. Acta Physiol Plant 37:1738
Bakhshandeh E, Jamali M, Afshoon E, Gholamhossieni M (2017) Using hydrothermal time concept to describe sesame (Sesamum indicum L.) seed germination response to temperature and water potential. Acta Physiol Plant 39:250
Balandary A, Rezvani Moghaddam P, Nasiri Mahalati M (2011) Determination of seed germination cardinal temperatures in short chicory (Cichorium pumilum Jacq.). Paper presented at the Second National Seed Technology Conference, Mashhad
Baskin CC, Baskin JM (2014) Seeds: ecology, biogeography, and, evolution of dormancy and germination. Academic Press, San Diego, p 1600
Basto S, Dorca-Fornell C, Thompson K, Rees M (2013) Effect of pH buffer solutions on seed germination of Hypericum pulchrum, Campanula rotundifolia and Scabiosa columbaria. Seed Sci Technol 41:298–302
Bewley JD, Bradford K, Hilhorst H, Nonogaki H (2013) seeds: physiology of development, germination and dormancy, 3rd edn. Springer, New York
Bradford KJ (2002) Applications of hydrothermal time to quantifying and modeling seed germination and dormancy. Weed Sci 50:248–260
Chachalis D, Reddy KN (2000) Factors affecting Campsis radicans seed germination and seedling emergence. Weed Sci 48:212–216
Channaoui S, El Kahkahi R, Charafi J, Mazouz H, El Fechtali M, Nabloussi A (2017) Germination and seedling growth of a set of rapeseed (Brassica napus) varieties under drought stress conditions. Int J Agric Environ Biotechnol 2:487–494
Chauhan BS, Gill G, Preston C (2006) African mustard (Brassica tournefortii) germination in southern Australia. Weed Sci 54:891–897
Copeland LO, McDonald MF (2012) Principles of seed science and technology, 4th edn. Springer, New York, p 467
Corbineau F, Come D (1989) Germinability and quality of Cichorium intybus L. seeds. In: VI symposium on the timing of field production of vegetables, vol. 267, pp. 183–190. https://doi.org/10.17660/ActaHortic.1990.267.23
Das S, Vasudeva N, Sharma S (2016) Cichorium intybus: a concise report on its ethnomedicinal, botanical, and phytopharmacological aspects. Drug Dev Ther 7:1–12
Derakhshan A, Bakhshandeh A, Siadat SA-A, Moradi-Telavat M-R, Andarzian SB (2018) Quantifying the germination response of spring canola (Brassica napus L.) to temperature. Ind Crops Prod 122:195–201
Dinelli G, Marotti I, Catizone P, Bosi S, Tanveer A, Abbas R, Pavlovic D (2013) Germination ecology of Ambrosia artemisiifolia L. and Ambrosia trifida L. biotypes suspected of glyphosate resistance. Open Life Sci 8:286–296
Fakheri BA, Mousavi Nick SM, Mohammadpour Vashvaei R (2017) Effect of drought stress induced by polyethylene glycol on germination and morphological properties of fennel and ajowan. J Crop Sci Res Arid Regions 1:35–50
Florentine A, Javaid M, Fernando N, Chauhan BS, Dowling K (2016) Influence of selected environmental factors on seed germination and seedling survival of the arid zone invasive species tobacco bush (Nicotiana glauca R. Graham). The Rangeland J 38:417–425, WesTbrooke M
Forcella F, Arnold RLB, Sanchez R, Ghersa CM (2000) Modeling seedling emergence. Field Crops Res 67:123–139
Ghaderi-Far F, Akbarpour W, Khavari F, Ehteshamnia A (2012) Determination of salinity tolerance threshold in six medicinal plants. J Plant Product 18:15–24
Honarmand SJ, Nosratti I, Nazari K, Heidari H (2016) Factors affecting the seed germination and seedling emergence of muskweed (Myagrum perfoliatum). Weed Biol Manag 16:186–193
International Seed Testing Association (ISTA) (2009) International rules for seed testing. Seed Sci Technol:Zurich, Switzerland
Jamil M, Lee CC, Rehman SU, Lee DB, Ashraf M, Rha ES (2005) Salinity (NaCl) tolerance of Brassica species at germination and early seedling growth. Electron J Environ Agric Food Chem 4:970–976
Javadzadeh M, Rezvani Moghaddam P, Banayan-Aval M, Asili J (2017) Cardinal temperatures for germination of Roselle (Hibiscus sabdariffa). Iran J Seed Res 3:129–141
Javaid MM, Florentine SK, Ali HH, Chauhan BS (2018) Environmental factors affecting the germination and emergence of white horehound (Marrubium vulgare L.): a weed of arid-zone areas. Rangeland J 40:47–54
Kayacetin F, Efeoglu B, Alizadeh B (2018) Effect of NaCl and PEG-induced osmotic stress on germination and seedling growth properties in wild mustard (Sinapis arvensis L.). Anadolu 28:62–68
Koger CH, Reddy KN, Poston DH (2004) Factors affecting seed germination, seedling emergence, and survival of texasweed (Caperonia palustris). Weed Sci 52:989–995
Mamedi A, Tavakkol Afshari R, Oveisi M (2017) Cardinal temperatures for seed germination of three Quinoa (Chenopodium quinoa Willd.) cultivars. Iran J Field Crops Sci 48:89–100
Mesgaran MB, Onofri A, Mashhadi HR, Cousens RD (2017) Water availability shifts the optimal temperatures for seed germination: a modelling approach. Ecol Model 351:87–95
Michel BE, Radcliffe D (1995) A computer program relating solute potential to solution composition for five solutes. Agron J 87:126–130
Mobli A, Ghanbari A, Rastgoo M (2018) Determination of cardinal temperatures of flax-leaf alyssum (Alyssum linifolium Steph. ex. Willd.) response to salinity, pH and drought stress. Weed Sci 66:470–476
Nandagopal S, Kumari BR (2007) Phytochemical and antibacterial studies of Chicory (Cichorium intybus L.)—a multipurpose medicinal plant. Adv Biol Res 1:17–21
Neumann P (1997) Salinity resistance and plant growth revisited. Plant Cell Environ 20:1193–1198
Nosratti I, Soltanabadi S, Honarmand SJ, Chauhan BS (2017) Environmental factors affect seed germination and seedling emergence of invasive Centaurea balsamita. Crop Pasture Sci 68:583–589
Okçu G, Kaya MD, Atak M (2005) Effects of salt and drought stresses on germination and seedling growth of pea (Pisum sativum L.). Turk J Agric For 29:237–242
Parihar P, Singh S, Singh R, Singh VP, Prasad SM (2015) Effect of salinity stress on plants and its tolerance strategies: a review. Environ Sci Pollut Res 22:4056–4075
Parmoon G, Moosavi SA, Siadat SA (2018) How salinity stress influences the thermal time requirements of seed germination in Silybum marianum and Calendula officinalis. Acta Physiol Plant 40:175
SAS Institute Inc (2013) SAS/STAT user’s guide. SAS Institute Inc., Cary
Seal CE, Barwell LJ, Flowers TJ, Wade EM, Pritchard HW (2018) Seed germination niche of the halophyte Suaeda maritima to combined salinity and temperature is characterised by a halothermal time model. Environ Exp Bot 155:177–184
Seghatoleslami MJ (2010) Effect of salinity on germination of three medicinal species of Satureja (Satureja hortensis L.), Chicory (Cichorium intybus L.) and Artichoke (Cynara scolymus L.). Iran J Field Crops Res 8:818–823
Street RA, Sidana J, Prinsloo G (2013) Cichorium intybus: traditional uses, phytochemistry, pharmacology, and toxicology. Evid Based Complement Altern Med 2013
Toosi AF, Bakar BB, Azizi M (2014) Effect of drought stress by using PEG 6000 on germination and early seedling growth of Brassica juncea var. Ensabi Scientific Papers Series A Agron 57:360–363
Xu H, Su W, Zhang D, Sun L, Wang H, Xue F, Zhai S, Zou Z, Wu R (2017) Influence of environmental factors on Cucumis melo L. var. agrestis Naud. seed germination and seedling emergence. PloS One 12:e0178638
Yin X, Kropff MJ, McLaren G, Visperas RM (1995) A nonlinear model for crop development as a function of temperature. Agric For Meteorol 77:1–16
Zarghani H, Mijani S, Nasrabadi SE (2014) Temperature effects on the seed germination of some perennial and annual species of Asteraceae family. Plant Breeding Seed Sci 69:3–14
Acknowledgements
This research is supported by the Genetics and Agricultural Biotechnology Institute of Tabarestan (GABIT) and Sari Agricultural Sciences and Natural Resources University (SANRU) research grant. The authors gratefully acknowledge use of the services and facilities of the GABIT during this research and also Dr. Raoudha Abdellaoui for scientific and linguistic revisions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Weidner.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vahabinia, F., Pirdashti, H. & Bakhshandeh, E. Environmental factors’ effect on seed germination and seedling growth of chicory (Cichorium intybus L.) as an important medicinal plant. Acta Physiol Plant 41, 27 (2019). https://doi.org/10.1007/s11738-019-2820-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-019-2820-2