Abstract
High level of salinity present in the soil severely affects plant growth and metabolism, eventually reduces crop productivity. In the present study, we have made an effort to obtain detailed insight on the effect of various levels of salinity on various physiological, biochemical, genetic, and phytochemical parameters of Andrographis paniculata genotype, CIM-Megha, in an attempt towards development of a salt-tolerant variety. The results showed that maximum seed germination efficiency was observed at 100 mM among the various salt concentrations. Moreover, with the increase in salt concentration, the overall growth of the plant was stunted. High salinity had a negative effect on photosynthetic pigments, free cysteine content, non-protein thiol content, and nitrate reductase activity. However, proline accumulation and phenol content were found to increase with the increasing salt concentration. The results from the study demonstrated that activities of CAT and APX antioxidant enzymes increased with the applied salt stress. The accumulation of reactive oxygen species in response to salinity is the most important DNA-damaging factor causing a decrease in the genomic template stability of the plant. Quantification of important bioactive constituents (andrographolide, neo-andrographolide, and 14-DDA) was done through HPLC, and the results showed high variability in constituents. In summary, Andrographis paniculata could be grown at large scale in saline areas having up to 100 mM salt concentration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Andrographis paniculata (Burm. f.) Wallich ex Nees is an annual and herbaceous plant belonging to the family Acanthaceae. The plant is commonly known as Kalmegh, Chiretta or King of Bitters and is extensively used in Ayurvedic, Unani, and Siddha systems of medicine (Raina et al. 2013). The plant is an important constituent of over 26 Ayurvedic formulations mentioned in the Indian pharmacopoeia (Raina et al. 2013). The aerial parts extract comprises of several phytopharmaceutical metabolites such as, labdane diterpenes, phenylpropanoids, flavonoids, and xanthones (Rao et al. 2004). Among the labdane diterpenes, andrographolide, neoandrographolide, and 14-deoxy-11, 12-didehydroandrographolide (14-DDA) are considered to be the main bioactive constituents (Garg et al. 2015), which are responsible for the wide spectrum of pharmacological properties of the plant, such as, anti-malarial (Wiart et al. 2005), anti-inflammatory (Shen et al. 2002), anti-cancer (Kumar et al. 2004), anti-hepatitis (Sharma et al. 1991), anti-diabetic (Yu et al. 2003; Rao 2006), anti-diarrheal (Gupta et al. 1993), antioxidant (Akowuah et al. 2008), remedy for sexual dysfunction (Akbarsha and Murugaian 2000), cytotoxic (Nanduri et al. 2004), cardio-protectant (Tan and Zhang 2004), immunostimulatory (Calabrese et al. 2000; Kumar et al. 2004), anti-microbial (Singh et al. 2003), anti-HIV (Calabrese et al. 2000), anti-oedema, and analgesic (Lin et al. 2009), and renal-protective (Rao 2006). Because of its immense pharmaceutical importance, the plant is included in the Indian Herbal Pharmacopoeia (IDMA 2002), Herbs of Commerce of American Herbal Products Association (AHPA 2004), and in Indian Pharmacopoeia (IPC 2007). A capsule dosage form KalmCold™, prepared from the leaf extracts of A. paniculata by M/s Natural Remedies Pvt. Ltd., Bangalore, India, is popular for its efficacy in the treatment of upper respiratory tract infection (Saxena et al. 2010).
Presently, due to the tremendous demand of medicinal plants by the herbal industry, immediate attention is required for their commercial production. However, several abiotic factors including soil salinity have been known to significantly hinder medicinal plant productivity (Qureshi et al. 2005). Soil salinity is considered to be a major abiotic factor resulting in reduction in plant growth and productivity. Around 20% of the world’s cultivated area and around 33% of the world’s irrigated lands are reported to be acutely affected by water logging and salinity (FAO 2008; Shrivastava and Kumar 2015). Further, salinity in the soil is increasing at the rate of 10% annually depending on the several biotic and abiotic factors (Jamil et al. 2011). India (7 million hectares of land) stands second after China among the Asian countries possessing high saline and alkaline lands (Patel et al. 2011). Thus, improved varieties that are tolerant to salt stress are the ultimate solution for the large-scale commercial cultivation of important medicinal plants like A. paniculata. Detailed studies encompassing morphological, physiological, biochemical, genetic, as well as chemical changes in plants due to various levels of salinity stress is a prerequisite for breeding efforts directed towards development of stress-tolerant varieties. High salinity stress affects various metabolic and physiological processes such as seed germination and seedling vigour, vegetative and reproductive growth, secondary metabolites content, which ultimately define the economic yield of the crop.
Secondary metabolites produced by the plant do not have a direct role in the maintenance of plant life processes but are used to counter various stresses and play a role in plant defense. High salinity conditions in the soil results in the increased production of reactive oxygen species (ROS) leading to oxidative stress and ionic imbalance in the cell. Consequently it causes damage to the lipids, proteins, nucleic acids, and cell membrane integrity (Nabi et al. 2019). Oxidative stress developed in the plants can be mitigated through antioxidative metabolites such as glutathione, ascorbic acid, α-tocopherol and antioxidant enzymes such as superoxide dismutase (SOD), guaiacol peroxidase (GPX), catalase (CAT), ascorbate peroxidase (APX), and glutathione reductase (GR) (Hayat et al. 2012). CAT and APX are involved in ROS scavenging through the removal of free H2O2 (Gharsallah et al. 2016). Seed germination and the vigour of plumule and radicle are vital stages during the plant life cycle. High salinity conditions impede the water absorption to the seeds, causing decreased germination rate and low viability percentage. The decline in photosynthetic efficiency is also responsible for low productivity and has been successfully utilized in evaluating salt tolerance ability of plants (Kumar et al. 2017). Reports from earlier literature stated that carotenoids help in scavenging ROS, and also act as a signaling precursor under abiotic stress thus protecting and stabilizing photochemical processes under stress conditions (Zhang et al. 2014). For maintaining plant cellular homeostasis during salt stress, several organic solutes are synthesized in the plant cytosol, like proline, trehalose, betaine, polyols, etc. (Hasegawa et al. 2000), of which proline accumulation during stress has been extensively studied. Further, it has also been reported that proline accumulation in the plant as a result of the stress signal influences adaptive responses (Hayat et al. 2012). For individual plant species, the relationship between proline accumulation and stress tolerance is unclear (Huang et al. 2013). Cysteine is a thiol-containing biogenic amino acid, which is considered as the sulfur center in the formation of the sulfhydryl group of sulfur-containing amino acids and also involved in the synthesis of methionine (Met), iron–sulfur clusters, and some vitamins through donating its sulfur group. Cysteine also acts as an antioxidant that scavenges ROS, thereby protecting the cell from oxidative damage (Genisel et al. 2015). Non-protein thiols are water-soluble antioxidant molecules having an important role in ROS scavenging. It has also been reported that phenols play an important role in absorbing and neutralizing ROS (Valifard et al. 2018). Reduction of available nitrate in the soil to nitrite during nitrogen assimilation process is carried out by nitrate reductase enzyme. Growth and yield of the plant are often correlated with the nitrogen content present in the plant, which is directly correlated with the nitrate reductase activity (Katiyar and Dubey 1992).
Salt stress not only affects the various physiological and metabolic processes but also greatly affects the genetic material through point mutations and chromosome rearrangements. The accumulations of free radicals are the most important DNA-damaging factors causing a decrease in the genomic template stability of the plant. The changes in the genetic material can be detected through the use of molecular markers (Genisel et al. 2015). Inter-simple sequence repeats (ISSRs) are dominant markers present throughout the genome, and use a single primer for amplification (Kumar et al. 2016). Genomic template stability (GTS) in the salt-treated plants is calculated using the differences in the banding pattern in comparison to the control.
Salt toxicity has been studied in various traditional crops as well as in some medicinal and aromatic plants, and it has been reported that some plants are tolerant to salinity conditions. The present study on A. paniculata responses to different levels of salinity is valuable. Hitherto, several studies have been reported on the effect of salt stress on A. paniculata, viz. macro and micronutrient uptake (Talei et al. 2012), morphological, physiological and biochemical traits in seedlings (Talei et al. 2012, 2013a, b), growth indices (Talei et al. 2013a, b), proteomic analysis of leaf and root (Talei et al. 2014), physiological characteristics (Chen et al. 2014), photosynthetic parameters and andrographolide content (Talei et al. 2015), effect on growth and antioxidant enzymes (Kumar and Srivastava 2018). In the present study, we explore the effect of various concentrations of salts on seed germination efficiency, morphology, several biochemical markers, genomic template stability, and content of main bioactive chemical constituents in the elite variety CIM-Megha (Misra et al. 2005), developed by CSIR-Central Institute of Medicinal and Aromatic Plants, Lucknow, India, in an attempt towards development of a salt-tolerant variety.
Materials and methods
Plant materials and salt treatments
Seeds of A. paniculata variety CIM-Megha were provided by the CSIR-Central Institute of Medicinal and Aromatic Plants, Research Centre, Bengaluru (12° 58′ N latitude; 77° 35′ E longitude). Seeds were sown in flat tray (30 cm length, 20 cm width, and 5 cm height) containing a 1:1 mixture of soil and farmyard manure, and allowed to grow in a glass house for 3 weeks under standard conditions (60–75% relative humidity, 25 ± 2 °C temperature, and 16/8 h photoperiod). Further, the seedlings were transferred in pots (20 cm high, and 20 cm internal diameter) containing a 1:1 mixture of soil and farmyard manure in green house conditions. Until acclimatization, the plants were irrigated with field water twice a week. After 2 weeks of acclimatization, the plants were treated with the salt (non-iodized NaCl) solutions of different concentration—0 mM (control), 50 mM, 100 mM, 150 mM, 200 mM, and 250 mM. The salt treatment was given to the plants weekly for 2 months during which field water was not applied to the plants. The experiment was conducted in triplicates for each treatment. After 2 months of salt treatment, morphological characters [plant height (cm), primary branches, leaf length and width (cm)] were recorded; biochemical assays, genomic template stability, and the quantification of main bioactive constituents were performed. For genomic template stability, leaf samples were stored in − 80 °C until further use.
Seed germination under salinity condition
To determine the seed germination threshold, seeds of CIM-Megha were first surface sterilized using 5% sodium hypochlorite (NaClO) and were then sown in 90 mm diameter petri dishes (each 50 seeds) containing blotting papers for maintaining uniformity in salt solutions. Each petri dish was subjected to 10 mL of different salt concentrations (10 mM, 50 mM, 100 mM, 150 mM, 200 mM, 220 mM, 240 mM, 260 mM, 280 mM, and 300 mM) and the control was provided with distilled water. Parafilm was applied to the petri dishes to prevent any water loss and then incubated in a growth chamber maintaining at 27 °C temperature, 75% relative humidity with 14/10 h photoperiod and 120 µmoles/m2/s light intensity. Germination efficiency, shoot height and tap root length were recorded after ten days of sowing. The experiment was conducted in triplicates for each treatment.
Biochemical assays
Photosynthetic pigment content
Chlorophyll and carotenoid pigments were measured following the method developed by Arnon (1949). Finely cut fresh leaves (10 mg) of treated A. paniculata were ground to a fine pulp with the addition of 0.2 mL of acetone (100%). The slurry was then centrifuged for 5 min at 5000 rpm. The supernatant was transferred to a fresh 2 mL micro-centrifuge tube. The process was repeated till the residue became colorless and a final volume of 1 mL was maintained in the micro-centrifuge tube. The absorbance of the solution was read at 470, 645 and 662 nm against the solvent (acetone) blank. The quantities of chlorophyll a, b, total chlorophyll, and total carotenoids were expressed as mg gFW−1.
Proline content
Proline quantity was estimated as per the protocol developed by Bates et al. (1973). Plant tissue (50 mg) was homogenized in 2.5 mL of 3% aqueous sulphosalicylic acid and the homogenate was centrifuged at 5000 rpm for 10 min. The extract (0.5 mL) was boiled with 0.5 mL acid-ninhydrin and 0.5 mL of acetic acid and incubated for 1 h at 100 °C. Further, the extract was transferred to an ice bath for 20 min. The mixture was then extracted with 1 mL of toluene and mixed vigorously for 15–20 s. The absorbance of the pink coloured phase was recorded at 520 nm. Free proline content was determined against the standard and was expressed in µ mol gFW−1.
Cysteine content
Measurement of cysteine content was done according to the method developed by Gaitonde (1967). The plant tissue (100 mg) was pulverized in 1 mL of 5% cold perchloric acid and the suspension was centrifuged at 5000 rpm for 1 h at 4 °C. The perchloric extract (0.2 mL) was mixed with 0.2 mL of acid-ninhydrin and 0.2 mL of acetic acid and incubated for 10 min at 100 °C. The reaction mixture was rapidly cooled on ice and diluted by adding 1 mL of 95% ethanol. The spectral measurement of the resulting mixture was made at 560 nm. Cysteine content was determined against the standard and was expressed as n mol gFW−1.
Non-protein thiol content
Non-protein thiol content was measured following the method developed by Ellman (1959). The plant tissue (50 mg) was homogenized with 1 mL of 6.67% sulphosalicylic acid and centrifuged at 13,000 g for 10 min. To 250 μL of the supernatant, 50 μL of Ellman’s reagent solution and 2.5 mL of reaction buffer (0.1 M sodium phosphate, pH 8.0; 1 mM EDTA) was added and incubated for 15 min at room temperature. The spectral measurements were recorded at 412 nm. The concentration of sulfhydryls groups was estimated based on the molar extinction coefficient of 2-nitro-5-thiobenzoic acid (TNB; 14,150 M−1 cm−1).
Total phenolic contents
The Folin–Ciocalteu’s phenol reagent method was used for determining the total phenolic content as described earlier by Dai et al. (2006). The plant tissue (50 mg) was pulverized in 1 mL of acidic methanol, filled in micro-centrifuge tubes and incubated at 4 °C under darkness for 24 h. The mixture was centrifuged at 4000 g for 10 min, and the supernatant was collected in a fresh tube. To remove the residual chlorophyll, ether (0.5 mL) was added to the supernatant. To 0.5 mL of the resulting solution an equal volume of Folin–Ciocalteu reagent (1:5 dilutions with de-ionized water) was added. Further, 1 mL of 6% sodium carbonate was added and was incubated in the dark for 1 h. The absorbance at 760 nm was recorded and the linear equation of a standard curve obtained with gallic acid was used to determine the concentration of phenols. The total phenolic content was expressed in ng gFW−1.
Assay of nitrate reductase (EC: 1·6.6·1) activity
The assay for nitrate reductase activity in A. paniculata was performed as per the procedure of Hageman and Hucklesby (1971) with few modifications. Fresh leaves were chopped into fine pieces and added to pre-cooled 3 mL each of 0.05 M potassium phosphate buffer (pH 7.8) and 0.4 M potassium nitrate solution. Incubation was carried out in a water bath at 35 °C under darkness for 75 min. A volume of 200 μL of this mixture was added with 1 mL each of 1% sulphanilamide in 1 N HCl and 0.025% N-(1-Naphthyl)-ethylene diammonium dichloride (NEDD) in double distilled water. After 30 min, 6 mL water was added, and the absorbance was recorded at 540 nm. Enzyme activity was expressed in terms of μ moles min−1.
Assay of antioxidant enzymes
Leaves (0.5 g) were homogenized in 50 mM phosphate buffer (pH 7.0) with 1% polyvinylpyrrolidone, filtered through muslin cloth (four layers) and centrifuged at 15,000 g for 10 min. The crude enzyme extract obtained as supernatant was used for catalase (CAT; EC: 1.11.1.6) and ascorbate peroxidase (APX; EC: 1.11.1.11) antioxidant enzyme assays.
Catalase (CAT) activity
CAT catalyzes the decomposition of H2O2 to give H2O and O2, and the activity was determined by the rate of H2O2 disappearance at 240 nm. A volume of 3 mL reaction mixture contained 50 mM phosphate buffer (pH 7.0), 0.4 mL of 15 mM H2O2, 0.04 mL homogenate, and 0.04 mL of 0.1% (v/v) Triton X-100. The CAT activity was expressed as µmol H2O2 reduced min−1 g−1 at 25 ± 2 °C.
Ascorbate peroxidase (APX) activity
Ascorbate peroxidase activity was estimated as per the protocol described by Nakano and Asada (1981). APX acted upon one mole of ascorbate and H2O2 to produce one mole of dehydroascorbate. The APX activity was determined by measuring the rate of ascorbate oxidation (extinction coefficient: 2.8 mM−1 cm−1) and followed by a decrease in absorbance at 290 nm. A 3 mL reaction solution comprised of 50 mM phosphate buffer (pH 7.0), 0.4 mL of 0.1 mM H2O2, 0.4 mL of 0.5 mM ascorbic acid, 0.04 mL of 0.1 mM EDTA and 0.1 mL of enzyme extract. The enzyme activity was expressed in terms of µmol of ascorbate oxidized min−1 g−1 at 25 ± 2 °C.
Genomic DNA isolation and ISSR analysis
Plant genomic DNA was isolated using the method developed by Khanuja et al. (1999). A total of 10 ISSR primers (UBC set no. 9) were used in the study. PCR reactions were performed with a PCR master mix (TaKaRa) in a final volume of 20 μL and amplified in a thermal cycler (Applied Biosystems) using the conditions described earlier by our group (Kumar et al. 2018, 2019). Electrophoresis of amplified products was done on 1.2% agarose gel alongside a 2-log DNA ladder. Gel image was captured using the Bio-Rad universal hood II gel documentation system (Bio-Rad Laboratories Inc.) for further analysis.
High-performance liquid chromatography (HPLC) analysis.
Air-dried leaves (50 mg) of each sample were extracted with ultrasonic assisted extraction by ultrasonicator (Microclean-109, Oscar Ultrasonics) using 10 mL of methanol, and the process was repeated thrice. The extracts were pooled, concentrated under vacuum, re-dissolved in 1 mL of methanol and filtered with 0.45 µm filter prior to HPLC analysis. Quantification of andrographolide, neo-andrographolide, and 14-DDA, were performed on a reversed-phase HPLC system (Prominence-i, Shimadzu) equipped with Photodiode Array (PDA) detector and with a Phenomenex Luna C18 reverse phase column (250 × 4.6 mm internal diameter, 5 μm particle size). Solvent system for HPLC consisted of a mixture of A (water, 0.1% trifluoroacetic acid) and B (acetonitrile). Mobile phase comprised of 95:5 ratio of A:B was used for the first 18 min of run and then ramped to 55:45 for the next 7 min. The ratio was further changed to 20:80 for the next 5 min and finally to 95:5 ratio for the last 10 min. Throughout the run, flow rate was maintained at 1 mL/min, and the major bioactive constituents were detected at 223 nm. Authentic standards of andrographolide, neo-andrographolide, and 14-DDA (Sigma-Aldrich) were used for preparing the stock solution of 1 mg mL−1 concentration in methanol and were used as a reference for quantification.
Statistical data analysis
The collected data were analyzed by applying the one-way analysis of variance (ANOVA) using GraphPad Prism ver. 7.04 (GraphPad Software Inc.). Significant difference between means compared to control was done using Dunnett multiple comparison tests (Dunnett 1955) at a significance level of p ≤ 0.05 and shown as mean ± standard deviation. For GTS determination, absence (0) and presence (1) of bands were manually scored and were calculated for polymorphism. Polymorphism was calculated by comparing the banding patterns in each treated plants with control. GTS% in the treated sample was calculated using the formula \({\text{GST}}\; = \;\left( {1\; - \;Ps/TBc} \right)\; \times \;100\), where, Ps is polymorphic bands present in the treated sample compared to control, and TBc is the total number of amplified bands present in control (Genisel et al. 2015).
Results
Germination efficiency and vigour under the salt concentration
Germination percentage of A. paniculata was significantly influenced by salt stress. The germination percentage reduced gradually with increasing salt concentration (F = 39.62, p < 0.0001). The maximum (96%) germination was recorded in the control, while in the treatments, maximum germination percentage was found to occur in 100 mM (69%) followed by 50 mM (53%) salt concentration (Table 1). With increase in salt content, a gradual decrease in shoot height and tap root length was observed (F = 177.2, p < 0.0001; F = 297.3, p < 0.0001 for the shoot and tap root length, respectively). Shoot growth was observed until 100 mM salt stress condition, above which no shoot growth was observed. Maximum shoot length was observed in 10 mM (0.60 ± 0.105 cm) followed by 50 mM (0.56 ± 0.126 cm), while in control, shoot length of 0.54 ± 0.051 cm was observed. Maximum tap root length was observed in control 4.63 ± 0.290 cm, after that, there was a gradual decrease in tap root length with an increase in salt concentration (Table 1).
Morphological responses under salt stress
With the increase in salt concentration, a gradual decrease of plant height and primary branches was observed, while leaf length and width were slightly increased as compared to control (Fig. 1). The significant gradual decrease in plant height was observed in salt-treated plants as compared to control (F = 8.967, p = 0.001). Maximum average plant height of 64.5 ± 13.53 cm was recorded in control, while, a minimum of 29.0 ± 1.00 cm was recorded in 250 mM salt-treated plant. Mean differences recorded in number of primary branches were not significant (F = 2.469, p = 0.0926). A maximum of 18.66 ± 4.50 and a minimum number of 11.66 ± 1.52 primary branches were recorded in control and 250 mM salt treated plant, respectively. As salt concentration increased, leaf length (F = 1.456, p = 0.2129) and width (F = 5.192, p = 0.0003) were found to slightly increase as compared to the control. Maximum leaf length (11.10 ± 1.10 cm) and width (3.88 ± 0.48 cm) were observed for 200 mM and 250 mM salt treated plant, respectively, while the minimum was observed for control 10.10 ± 0.88 cm and 3.34 ± 0.24 cm, respectively (Fig. 2).
Effect of salt stress on photosynthetic pigments
As the salinity increases, a significant gradual reduction in photosynthetic pigments viz. total chlorophyll, chlorophyll a, chlorophyll b, and carotenoids were observed. For total chlorophyll, significant reduction was observed with increase in salt concentration (F = 13.23, p = 0.0002). A maximum of 24.22 ± 1.480 mg gFW−1 was recorded in control, while, minimum of 8.59 ± 1.468 mg gFW−1 was recorded in 250 mM salt stress condition. Chlorophyll a and b content were significantly reduced with increase in salt concentration (F = 11.59, p = 0.0003 and F = 12.74, p = 0.0002, respectively). However, as compared to the control, reduction in chlorophyll content was not significant under 50 mM salt treatment. A maximum of 16.37 ± 0.497 and 7.85 ± 1.092 mg gFW−1 of chlorophyll a and b, respectively was recorded in the control, while a minimum of 6.10 ± 1.739 and 2.49 ± 0.284 mg gFW−1 was recorded in 250 mM salt stress condition. A significant reduction was also observed in carotenoid content with increase in salt concentration (F = 9.049, p = 0.0009). Highest carotenoid content was recorded in control (4.67 ± 0.324 mg gFW−1), while minimum was recorded in 250 mM salt-treated plant (1.71 ± 0.745 mg gFW−1). Data pertaining to the photosynthetic pigments related to various salt treatments are illustrated in Fig. 3.
Effect of salt stress on biochemical markers
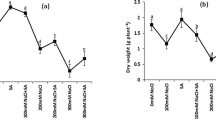
To protect themselves under high salt conditions, organic solutes like proline are synthesized by plants. In the present study, proline accumulation significantly increased with increase in salinity level (F = 261.8, p < 0.0001). In treatments, 50 mM (0.033 ± 0.001 µmoles gFW−1), 100 mM (0.035 ± 0.002 µmoles gFW−1), and 150 mM (0.037 ± 0.001 µmoles gFW−1), almost equal concentration of proline content was recorded, demonstrating same level of salinity resistance and illustrated in Fig. 4a.
Biochemical markers under salt stress. a Effect on proline accumulation, b Effect on cysteine content, c Effect on non-protein thiol accumulation, d Effect on phenol content, f Effect on activity. Values are means ± SD of three replicates. Means are significant at p < 0.5 (*); 0.01 (**); 0.001 (***) or 0.0001 (****) level as determined by ANOVA followed by Dunnett test
Under salt stress conditions, reduced level of free cysteine was recorded in A. paniculata (F = 338.6, p < 0.0001). Maximum free cysteine content was found in control (0.35 ± 0.015 nmoles gFW−1), while minimum was found in 250 mM (0.11 ± 0.003 nmoles gFW−1) salt-treated plant. Further, almost same level of cysteine was found in 50 mM (0.17 ± 0.004 nmoles gFW−1), 100 mM (0.17 ± 0.004 nmoles gFW−1), and 150 mM (0.18 ± 0.007 nmoles gFW−1) salt-treated plant (Fig. 4b).
A gradual decrease in non-protein thiol content (sulfhydryls groups) after salt stress was observed. Compared to control, non-protein thiol content was significantly decreased in A. paniculata leaves (F = 128.4, p < 0.0001). Data pertaining to the non-protein thiol content related to various salt treatments are illustrated in Fig. 4c. Compared to the control, 50 mM salt-treated plant showed non-significant non-protein thiol content (p = 0.1122), while non-protein thiol content in 100 mM (p = 0.0009), 150 mM (p < 0.0001), 200 mM (p < 0.0001), and 250 mM (p < 0.0001) were significant.
The total phenolic compounds were found to increase significantly (F = 54.45, p < 0.0001) upon salt treatments. Compared to the control, 50 mM salt-treated plant showed non-significant total phenolics content (p = 0.1122), while phenolic content in 100 mM (p = 0.0102), 150 mM (p = 0.0018), 200 mM (p = 0.0008), and 250 mM (p < 0.0001) were significant (Fig. 4d).
The significant gradual decrease in nitrate reductase activity was observed upon salt stress (F = 12.59, p = 0.0039). Maximum activity was recorded in control (12.57 ± 0.389 µmoles min−1), while the minimum was recorded in 250 mM salt treated plant (10.96 ± 0.324 µmoles min−1). Data pertaining to nitrate reductase activity related to various salt treatments are illustrated in Fig. 5.
Effect of salt stress on antioxidant enzymes
A significant increase in the CAT activity was observed with increasing salt content (F = 31.62, p = 0.0003). However, when compared with control, CAT activity was not significant at 50 mM (p = 0.2878) and 100 mM (p = 0.0637) salt stress. Moreover, 1.7 × CAT activity was increased in 50 mM, 2.1 × in 100 mM, 2.7 × in 150 mM, 3.8 × in 200 mM, and 4.1 × in 250 mM salinity conditions (Fig. 6a).
With the increase in salt stress, APX activity was also significantly increased (F = 112.5, p < 0.0001). Compare to the control, the 50 mM salt-treated plant had 2.5-fold activity followed by 100 mM (3.1-fold activity), while in 250 mM salt treated plant 5.4 fold APX activity was recorded. APX activity related to various salt treatments was illustrated in Fig. 6b.
Effect of salt stress on genomic template stability
Salt-treated plants were checked for genomic stability using 10 ISSR primers. ISSR primers used in the study were targeted for di-nucleotide (6), tri-nucleotide (2), tetra-nucleotide (1), and penta-nucleotide (1) repeats. A total of 57 bands were recorded in control, while 77 bands were obtained in 50 mM, 79 bands in 100 mM, 75 bands in 150 mM, 78 bands in 200 mM, and 68 bands in 250 mM salt-treated plants. 2–8 bands were generated from each primer with an average of 5.7 bands per primer in the control. In salt-treated plants, 5–9 bands were recorded in 50 mM, 4–11 bands in 100 mM, 4–10 bands in 150 mM, 6–10 bands in 200 mM, and 5–8 bands in 250 mM. Compared to the control, 50 mM salt-treated plants were 52.63% polymorphic, 100 mM treated plants were 59.65% polymorphic, 150 mM treated plants were 70.17% polymorphic, 200 mM treated plants were 75.44% polymorphic, and 250 mM treated plants were 78.95% polymorphic in nature. Polymorphism occurring in salt-treated plants was caused due to loss or gain of amplicons. As compared to loss of amplicons, gain of amplicons was more prominent in the treated plants. The results from the study demonstrated that 13 new bands appeared in all the salt-treated plants, while two bands were lost in all the treatments. Of the ten primers used in the study, six were able to detect the appearance of new bands in all salt-treated plants, of which UBC 855 detected a maximum of four new bands followed by UBC 862 (2 bands). Primers UBC 862 and UBC 881 detected loss of bands in salt-treated plants.
Quantitative changes in the genome were measured through genomic template stability (GTS). From the results obtained from the study, it was deduced that with an increase in salt concentration, GTS decreases significantly. For 50 mM salt-treated plants, GTS was 47.37%, for 100 mM 40.35%, for 150 mM 29.82%, for 200 mM 24.56%, and for 250 mM 21.05% was recorded. Increase in GTS was directly proportional to the polymorphism observed in treatments. A maximum of 45 polymorphic bands were recorded in 250 mM salt-treated plants, while a minimum of 30 polymorphic bands was recorded in 50 mM treated plants. With the increase in salt concentration, a maximum of 17 bands were lost in 250 mM treated plants, while 28 bands were gained. Banding patterns observed after salt treatment and in control are depicted in Table 2.
Effect of salt stress on major chemical constituents
HPLC analysis of salt-treated plants showed high variability in the studied chemical components. A HPLC chromatogram (control plant) showing the separation of its main bioactive constituents is shown in Fig. 7. Maximum andrographolide content was found in the 100 mM salt treated plant (17,600 ± 58 µg gDW−1), while the minimum was in 150 mM (4900 ± 42 µg gDW−1). A maximum of 13,900 ± 54 µg gDW−1 was recorded in control (F = 25,405, p < 0.0001). In case of neoandrographolide, maximum was recorded in the 50 mM salt-treated plant (12,600 ± 59 µg gDW−1), while the minimum was in 150 mM (1500 ± 47 µg gDW−1), with control to possess 5800 ± 50 µg gDW−1 (F = 16,325, p < 0.0001). Another important chemical constituent of A. paniculata, 14-DDA was found to be highest in control (500 ± 36 µg gDW−1), while 50 mM, 100 mM, and 150 mM salt treated plant possess 200 ± 15 µg gDW−1 of total constituents. 200 mM and 250 mM salt-treated plants were recorded to contain little higher (300 ± 17 µg gDW−1) than the less concentrated salt-treated plants (F = 96.51, p < 0.0001). Variability in chemical constituents is depicted in Fig. 8.
Discussion
Among the various abiotic stresses, salt stress is considered as one of the important ones that severely affects plant growth and metabolism, which ultimately reduces the productivity of the crops. The present study was conducted to examine the influence of salinity on seed germination, seedling vigour, morphology, biochemical indices, genomic template stability, and the main bioactive phytoconstituents of A. paniculata in an attempt to develop salt tolerant varieties. The plant seeds were viable and started germinating from the third day (control) or fourth day (in salt-treated). In general, seed germination efficiency and germination process of halophytes, as well as glycophytes, decreases with increasing salt concentration (Cordazzo 1999). The decline in the germination is mainly due to osmotic stress as well as ionic toxicity developed due to salt stress (Bajji et al. 2002). The results reported here demonstrated low germination efficiency as compared to control; however, in salt-treated seeds, maximum germination efficiency was observed in 100 mM concentration followed by 50 mM. The result was comparable with the study conducted by Talei et al. (2012), and Rajpar et al. (2007). Comparatively seedling growth is more affected than seed germination during salt stress (Cordazzo 1999). The result shows that shoot growth was not observed over 100 mM concentration, while taproot growth was observed until 240 mM beyond which, no growth was observed. This demonstrates that salinity has a higher adverse effect on shoots rather than on roots. The possible explanation for the reduced growth of seedling in saline conditions may be due to the adverse effect on water absorption, nutrient uptake, as well as biochemical processes (Talei et al. 2013a, b). Generally, salt stress has a significant effect on plant growth, reducing the overall growth of the plant with the increase in salt concentration (Parida and Das 2005). Reduction in growth can be attributed to injury caused by osmotic stress, and ion toxicity caused by the accumulation of Na+ ions, which hinders the uptake of K+ ions (Meloni et al. 2001). Also, during salt stress, plants generally focus on conserving water through the closure of stomata. As a result, low CO2 fixation occurs.
Photosynthetic pigments are well-known indicators of the adverse effects of salt stress. With the increase in salt concentration, photosynthetic pigments are degraded due to the accumulation of ROS, along with the activation of chlorophyll degrading enzyme chlorophyllase (Acemi et al. 2017). One more important factor for the reduced amount of chlorophyll pigments in salt-treated plants could be due to the suppression of specific enzymes responsible for the synthesis of chlorophyll (Zhang et al. 2014). In the present study, the photosynthetic pigments including chlorophyll a, chlorophyll b, and total chlorophyll along with carotenoids were significantly reduced. Our result is in accordance with the study carried out by Talei et al. (2015) on A. paniculata, whereby the amount of photosynthetic pigments was reduced on increasing the salt concentration.
Plants accumulate various organic solutes as a change in an external osmotic potential to cope with salt stress. Proline an organic solute is well known for its osmotic adjustment activity, and role in enhancing salt tolerance through protection of cellular membranes and enzyme integrity (Kumar et al. 2017). Our results demonstrated a significant increase in free proline content in salt-treated plants as compared to non-treated plants. Moreover, nearly equal level of proline in 50 mM, 100 mM, and 150 mM salt-treated plants shows similar adaptation against salt stress. On the other hand, phenols play an important role in maintaining cellular homeostasis during salt stress. Results from our study showed an increase in total phenolic content with increase in salt concentration. Increase in phenolic content in salt-treated plants might be due to the excessive amount of reactive oxygen species (ROS) production. Increase in phenolic content could also be attributed to the scavenging activity of phenols that might protect the plants from ROS through neutralizing free radicals, quenching singlet oxygen, and decomposing peroxides, which are inevitably produced during salt stress condition. Same type of finding was also reported from other medicinal and aromatic crops such as Rosmarinus officinalis, Ocimum basilicum, Mentha pulegium (Waskiewicz et al. 2013), where phenolic content increases with increase in salt concentration. However, unexpectedly in Nigella sativa (Bourgou et al. 2010), phenolic content decreases with increase in salt concentration.
The present study demonstrated a significantly reduced level of free cysteine and non-protein thiol content with increase in salt concentration. The result obtained was in line with the study carried out by Rohman et al. (2016), where it was stated that under salt stress, synthesis of cysteine was inhibited, which might affect the non-protein thiol synthesis. Moreover, the non-protein thiol content of the cell might be utilized as a reducing substrate in the synthesis of ascorbate. Another important reason for the low content of non-protein thiol in the salt-treated plant is that it might get consumed to protect cellular membranes from lipid peroxidation. Interestingly, Ruiz and Blumwald (2002) while studying the effect of salt stress on Brassica napus reported an increase in cysteine and non-protein thiol content. Under salt stress, nitrate reductase activity could be lowered due to inactivation of the enzyme as well as inhibition of nitrate reductase protein synthesis gene expression (Meloni et al. 2004). Decreased nitrate activity also resulted from an imbalance in minerals and nutrient uptake, especially nitrogenous compound. Results from the study showed a significant decrease in nitrate reductase activity in salt-treated plants as compared to control.
Under salt stress, plants are prone to the oxidative stress through increased production of ROS (Parida and Das 2005). The oxidative stress developed in plants can be mitigated through the potential role of antioxidant enzymes such as SOD, GPX, CAT, APX, and GR (Genisel et al. 2015). In the present study, we have evaluated the performance of CAT and APX activity. Under various salt concentrations, a significant increase in the activity of both the enzymes was observed. This increase in CAT and APX activity could be attributed to the scavenging nature of the enzymes towards ROS, thus providing improved tolerance to salt stress.
The present study used ISSR markers to check the genotoxic effect of salt stress on A. paniculata. ISSR markers have an edge over the usual RAPD markers in that they possess relatively higher annealing temperatures, thereby reducing the chances of non-specific binding (Sukumaran and Grant 2013). Another important reason for selection of ISSR primers was that the micro/minisatellite regions are prone to have a higher rate of mutations than another variable segment of genomic DNA. DNA polymorphism detected in the present study using ISSR primers was good enough to evaluate salt-induced DNA damage. GTS has been used as a quantitative parameter in several crops to check the effect of various stresses such as lead in Sesbania grandiflora (Malar et al. 2014), aluminum in Plantago species (Correia et al. 2014), cadmium on Hordeum vulgare (Liu et al. 2005), etc. It was also observed that loss of amplicons in salt-treated plants as compared to non-treated plants was more for the high molecular weight bands. This can be attributed to the fact that chances for DNA damage increase with the increased length of fragments. Similar reports were also reported by Liu et al. (2005), while studying the genotoxic effect of cadmium on H. vulgare.
Accumulation of various secondary metabolites directly depends on the responsible metabolic pathways which are again directly related to various stress conditions. Likewise, terpene biosynthesis is influenced both by genetic and environmental factors (Valifard et al. 2018). Our results showed that at 100 mM salt concentration, andrographolide content was maximum, while neoandrographolide content was maximum at 50 mM salt concentration. Similar results were also obtained for terpenoid phytoalexins production in Zea mays, where higher concentration of salts resulted in induction of zealexins while lower concentration induced kauralexins (Vaughan et al. 2015). Studies in Mentha pulegium (Mkaddem et al. 2007), Coriandrum sativum (Neffati and Marzouk 2008) also showed similar affects. Salt stress is reported to not only affect the composition but also the tissue specific induction of terpenoids. Basyuni et al. (2009) reported that under salt stress, terpenoid biosynthesis increased in both leaf and root of halophytes Kandelia candel, and only in the root of Bruguiera gymnorrhiza. The increase in specific secondary metabolites with decrease in chlorophyll content under salinity stress can be attributed to the adaptive change in the resource partitioning with carbon skeletons being diverted more towards terpene biosynthesis (Valifard et al. 2018). The major carbon source for the biosynthesis of terpenoids are the photosynthetic products and a decrease in chlorophyll content under stress may result in reduced level of photosynthesis and a consequent decrease in terpenoids (Behnke et al. 2013). However, a contrasting pattern may be observed under stress condition as observed in the present study under moderate salinity stress. Carbon sources like sugar and starch can be re-mobilized or the intermediates of the Mevalonate pathway and photorespiration can divert to isoprenoid biosynthesis when the carbon supply from photosynthesis is decreased due to stress (Ghirardo et al. 2011; Vickers et al. 2009). This may be utilized in the synthesis of protective compounds like terpenes against stress conditions (Penuelas and Llusia 2004; Vickers et al. 2009; Valifard et al. 2018). Interestingly, we also observed maximum content of 14-DDA in the control plants. Comparable differences in the composition of terpenoids with salinity stress is also reported in Salvia mirzayanii, where terpenoids like linalyl acetate, 1,8-cineole, a-terpinyl acetate were induced by salinity stress, however, bicyclogermacrene decreased with stress and was found to be higher in control plants (Valifard et al. 2018). Similar trend of change in composition and quantity of terpenoids in response to stress is also reported in Salvia officinalis and Petroselinum crispum (Aziz et al. 2013). Such variation in the composition and concentration is due to the change in demand for the essential terpenoids in plants in response to environmental stress (Valifard et al. 2018). A number of physiological and genetic factors may be affecting the biosynthesis of terpenoids under salinity stress and hence literature also records salt stress to decrease content in Salvia hispanica, and no affect in Matthiola tricuspidata (Heuer et al. 2002).
Conclusion
The present study was conducted with an attempt to obtain a detailed insight into the effect of salt stress on A. paniculata. The economic yield of the medicinal crop is directly related to the main bioactive constituents present in it. It was observed that up to 100 mM salt concentration, seed germination efficiency and seedling vigour was sustained. Moreover, at the same salt concentration, the content of its main bioactive constituents slightly increased, in comparison to the control and other treatments. The study, demonstrates that A. paniculata var. CIM-Megha could be cultivated in large scale in saline areas having up to 100 mM salinity in the soil. Furthermore, a variety with the more salt tolerant capability and higher andrographolide content will be desirable in future.
Author contribution statement
AK designed the study, conducted experiments, analyzed the data, and wrote the manuscript. VR conducted experiments, analyzed the data, and contributed to the preparation of manuscript. SV conducted experiments and analyzed the data. MS performed the chemical experiments. CH contributed to part of experiments. KS designed and performed the chemical experiments. AKS designed the study, and critically reviewed the manuscript. VS designed the study, contributed to chemicals, and critically reviewed the manuscript.
References
Acemi A, Duman Y, Karakuş YY, Kompe YO, Ozen F (2017) Analysis of plant growth and biochemical parameters in Amsonia orientalis after in vitro salt stress. Hortic Environ Biotechnol 58:231–239
Akbarsha MA, Murugaian P (2000) Aspects of the male reproductive toxicity/male antifertility property of andrographolide in albino rats: effect on the testis and the cauda epididymal spermatozoa. Phytother Res 14:432–435
Akowuah A, Zhari I, Mariam A (2008) Analysis of urinary andrographolides and antioxidant status after oral administration of Andrographis paniculata leaf extract in rats. Food Chem Toxicol 46:3616–3620
American Herbal Products Association (AHPA) (2004) Andrographis paniculata. In: McGuffin M (ed) Herbs of commerce. American Herbal Products Association, USA, p 13
Arnon D (1949) Copper enzymes isolated chloroplasts, polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15
Aziz EE, Sabry RM, Ahmed SS (2013) Plant growth and essential oil production of Sage (Salvia officinalis L.) and Curly-Leafed Parsley (Petroselinum crispum ssp. crispum L.) cultivated under salt stress conditions. World Appl Sci J 28:785–796
Bajji M, Kinet JM, Lutts S (2002) Osmotic and ionic effects of NaCl on germination, early seedling growth, and ion content of Atriplex halimus (Chenopodiaceae). Can J Bot 80:297–304
Basyuni M, Baba S, Inafuku M, Iwasaki H, Kinjo K, Oku H (2009) Expression of terpenoid synthase mRNA and terpenoid content in salt stressed mangrove. J Plant Physiol 166(16):1786–1800
Bates LS, Waldreman RP, Tear ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Behnke K, Ghirardo A, Janz D, Kanawati B, Esperschütz J, Zimmer I, Schmitt-Kopplin P, Niinemets Ü, Polle A, Schnitzler JP, Rosenkranz M (2013) Isoprene function in two contrasting poplars under salt and sunflecks. Tree Physiol 33:562–578
Bourgou S, Bettaieb I, Saidani M, Marzouk B (2010) Fatty acids, essential oil, and phenolics modifications of black cumin fruit under NaCl stress conditions. J Agric Food Chem 58:12399–12406
Calabrese C, Berman SH, Babish JG, Ma X, Shinto L, Dorr M, Wells K, Wenner CA, Standish LJ (2000) A phase I trial of andrographolide in HIV positive patients and normal volunteers. Phytother Res 14(5):333–338
Chen J, Gu W, Duan JA, Su SL, Shao J, Geng C (2014) Study on physiological characteristics and effects of salt stress in Andrographis paniculata. J Chinese Med Mat 37(8):1322–1327
Cordazzo CV (1999) Effects of salinity on seed germination, seedling growth and survival of Spartina Ciliata Breng. Acta Bot Bras 13(3):317–322
Correia S, Matos M, Ferreira V, Martins N, Gonçalves S, Romano A, Pinto-Carnide O (2014) Molecular instability induced by aluminum stress in Plantago species. Mutat Res Genet Toxicol Environ Mutagen 770:105–111
Dai P, Xiong ZT, Hung Y, Li MJ (2006) Cadmium induced changes in pigments total phenolic and phenylalanine ammonia liase activity in fronds in Azolla imbricate. Environ Toxicol 21(5):505–513
Dunnett CW (1955) A multiple comparison procedure for comparing several treatments with a control. J Am Stat Assoc 50(272):1096–1121
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
FAO (2008) Land resources, management, planning and use. http://www.fao.org/ag/agl/agll/spush
Gaitonde MK (1967) A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem J 104:627–633
Garg A, Agrawal L, Misra RC, Sharma S, Ghosh S (2015) Andrographis paniculata transcriptome provides molecular insights into tissue-specific accumulation of medicinal diterpenes. BMC Genomics 16:659
Genisel M, Erdal S, Kizilkaya M (2015) The mitigating effect of cysteine on growth inhibition in salt-stressed barley seeds is related to its own reducing capacity rather than its effects on antioxidant system. Plant Growth Regul 75:187–197
Gharsallah C, Fakhfakh H, Grubb D, Gorsane F (2016) Effect of salt stress on ion concentration, proline content, antioxidant enzyme activities and gene expression in tomato cultivars. AoB Plants 8:plw055
Ghirardo A, Gutknecht J, Zimmer I, Brüggemann N, Schnitzler JP, Beerling D (2011) Biogenic volatile organic compound and respiratory CO2 emissions after 13C-labeling: online tracing of C translocation dynamics in poplar plants. PLoS ONE 6(2):e17393
Gupta S, Yadava JNS, Tandon JS (1993) Antisecretory (antidiarrhoeal) activity of Indian medicinal plants against Escherichia coli enterotoxin induced secretion in rabbit and guinea pig ileal loop models. Int J Pharmacogn 31(3):198–204
Hageman RH, Hucklesby DP (1971) Nitrate reductase from higher plants. In: San Pietro A (ed) Methods in enzymology. Academic Press, New York, pp 491–503
Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Ann Rev Plant Biol 51:463–499
Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, Ahmad A (2012) Role of proline under changing environments: a review. Plant Signal Behav 7(11):1456–1466
Heuer B, Yaniv Z, Ravina I (2002) Effect of late salinization of chia (Salvia hispanica), stock (Matthiola tricuspidata) and evening primrose (Oenothera biennis) on their oil content and quality. Ind Crop Prod 15:163–167
Huang Z, Zhao L, Chen D, Liang M, Liu Z, Shao H, Long X (2013) Salt stress encourages proline accumulation by regulating proline biosynthesis and degradation in Jerusalem artichoke plantlets. PLoS ONE 8(4):e62085
Indian Drug Manufacturer’s Association (IDMA) (2002) Andrographis paniculata. Indian herbal pharmacopoeia. Indian Drug Manufacturer’s Association, Mumbai, pp 57–69
Jamil A, Riaz S, Ashraf M, Foolad MR (2011) Gene expression profiling of plants under salt stress. Crit Rev Plant Sci 30(5):435–458
Katiyar S, Dubey RS (1992) Influence of NaCl salinity on behaviours of nitrate reductase and nitrite reductase in rice seedlings differing in salt tolerance. J Agron Crop Sci 169:289–297
Khanuja SPS, Shasany AK, Darokar MP, Kumar S (1999) Rapid isolation of DNA from dry and fresh samples of plants producing large amounts of secondary metabolites and essential oils. Plant Mol Biol Rep 17:1–7
Kumar R, Srivastava M (2018) Study of growth and antioxidant enzymes in Andrographis paniculata (Burm f.) Wall ex Nees. as influenced by salinity and alkalinity. Int J Agric Environ Biotech 11(3):525–530
Kumar RA, Sridevi K, Kumar NV, Nanduri S, Rajagopal S (2004) Anticancer and immunostimulatory compounds from Andrographis paniculata. J Ethnopharmacol 92:291–295
Kumar A, Mishra P, Baskaran K, Shukla AK, Shasany AK, Sundaresan V (2016) Higher efficiency of ISSR markers over plastid psbA-trnH region in resolving taxonomical status of genus Ocimum L. Ecol Evol 6:7671–7682
Kumar S, Beena AS, Awana M, Singh A (2017) Physiological, biochemical, epigenetic and molecular analyses of wheat (Triticum aestivum) genotypes with contrasting salt tolerance. Front Plant Sci 8:1151
Kumar A, Rodrigues V, Mishra P, Baskaran K, Shukla AK, Shasany AK, Sundaresan V (2018) ISSR-derived species-specific SCAR marker for rapid and accurate authentication of Ocimum tenuiflorum L. Planta Med 84(2):117–122
Kumar A, Mishra P, Rodrigues V, Baskaran K, Verma RS, Padalia RC, Sundaresan V (2019) Delineation of Ocimum gratissimum L. complex combining morphological, molecular and essential oils analysis. Ind Crops Prod 139:111536
Lin F, Wu S, Lee S, Ng L (2009) Antioxidant, antioedema and analgesic activities of Andrographis paniculata extracts and their active constituent andrographolide. Phytother Res 23:958–964
Liu W, Li PJ, Qi XM, Zhou QX, Zheng L, Sun TH, Yang YS (2005) DNA changes in barley (Hordeum vulgare) seedlings induced by cadmium pollution using RAPD analysis. Chemosphere 61(2):158–167
Malar S, Manikandan R, Favas PJC, Vikram Sahi S, Venkatachalam P (2014) Effect of lead on phytotoxicity, growth, biochemical alterations and its role on genomic template stability in Sesbania grandiflora: a potential plant for phytoremediation. Ecotox Environ Safe 108:249–257
Meloni DA, Oliva MA, Ruiz HA, Martinez CA (2001) Contribution of proline and inorganic solutes to osmotic adjustment in cotton under salt stress. J Plant Nutr 24:599–612
Meloni DA, Gulotta MR, Martinez CA, Oliva MA (2004) The effects of salt stress on growth, nitrate reduction and proline and glycine betaine accumulation in Prosopis alba. Braz J Plant Physiol 16(1):39–46
Misra HO, Khanuja SPS, Lal RK, Singh A, Gupta MM, Darokar MP, Shasany AK, Jain N, Patra DD, Verma RK, Tripathi RS, Singh M, Dhawan OP, Singh AK, Bansal RP (2005) High yielding variety of Kalmegh (Andrographis paniculata) ‘CIM-Megha.’ J Med Aromat Plant Sci 27(3):525–527
Mkaddem M, Boussaid M, Fadhel NB (2007) Variability of volatiles in tunisian L. (Lamiaceae). J Essent Oil Res 19 (3):211-214
Nabi RBS, Tayade R, Hussain A, Kulkarni KP, Imran QM, Mun BG, Yun BW (2019) Nitric oxide regulates plant responses to drought, salinity, and heavy metal stress. Environ Exper Bot 161:120–133
Nanduri S, Nyavanandi VK, Thunuguntla SRC, Kasu S, Pallerla MK, SaiRam P, Rajagopal S, Ajaya Kumar R, Ramanujam R, Moses Babu J, Vyas K, Sivalakshmi Devi K, Om Reddy G, Akella V (2004) Synthesis and structure-activity relationships of andrographolide analogues as novel cytotoxic agents. Bioorg Med Chem Lett 14(18):4711–4717
Neffati M, Marzouk B (2008) Changes in essential oil and fatty acid composition in coriander (Coriandrum sativum L.) leaves under saline conditions. Ind Crops Prod 28 (2):137-142
Parida AK, Das AB (2005) Salt tolerance and salinity effects on plants: a review. Ecotox Environ Safe 60(3):324–349
Patel BB, Patel BB, Dave RS (2011) Studies on infiltration of saline–alkali soils of several parts of Mehsana and Patan districts of north Gujarat. J Appl Technol Environ Sanitation 1(1):87–92
Penuelas J, Llusia J (2004) Plant VOC emissions: making use of the unavoidable. Trends Ecol Evol 19:402–404
Qureshi M, Israr M, Abdin M, Iqbal M (2005) Responses of Artemisia annua L. to lead and salt-induced oxidative stress. Environ Exp Bot 53:185–193
Raina AP, Gupta V, Sivaraj N, Dutta M (2013) Andrographis paniculata (Burm. f.) Wall. ex Nees. (Kalmegh), a traditional hepatoprotective drug from India. Genet Resour Crop Evol 60:1181–1189
Rajpar I, Khanif YM, Saad MS (2007) Salt tolerance in Andrographis paniculata accessions. J Int Soc Southeast Asian Agric Sci 13:1–9
Rao NK (2006) Anti-hyperglycemic and renal protective activities of Andrographis paniculata roots chloroform extract. Iran J Pharm Ther 5:47–50
Rao YK, Vimalamma G, Rao CV, Tzeng YM (2004) Flavonoids and andrographolides from Andrographis paniculata. Phytochem 65:2317–2321
Rohman MM, Talukder MZA, Hossain MG, Uddin MS, Amiruzzaman M, Biswas A, Ahsan AFMS, Chowdhury MAZ (2016) Saline sensitivity leads to oxidative stress and increases the antioxidants in presence of proline and betaine in maize (Zea mays L.) inbred. Plant Omics J 9(1):35–47
Ruiz JM, Blumwald E (2002) Salinity-induced glutathione synthesis in Brassica napus. Planta 214:965–969
Saxena RC, Singh R, Kumar P, Yadav SC, Negi MPS, Saxena VS, Joshua AJ, Vijayabalaji V, Goudar KS, Venkateshwarlu K, Amit A (2010) A randomized double blind placebo controlled clinical evaluation of extract of Andrographis paniculata (KalmCold) in patients with uncomplicated upper respiratory tract infection. Phytomed 17:17178–17185
Sharma RTS, Sehgal V, Handa SS (1991) Antihepatotoxic activity of some plants used in herbal formulations. Fitoterapia 62(2):131–138
Shen YC, Chen CF, Chiou WF (2002) Andrographolide prevents oxygen radical production by human neutrophils: possible mechanism(s) involved in its anti-inflammatory effect. Brit J Pharmacol 135(2):399–406
Shrivastava P, Kumar R (2015) Soil salinity: a serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J Biol Sci 22(2):123–131
Singh PK, Roy S, Dey S (2003) Antimicrobial activity of Andrographis paniculata. Fitoterapia 74:692–694
Talei D, Yusop MK, Kadir MA, Valdiani A, Abdullah MP (2012) Response of king of bitters (Andrographis paniculata Nees.) seedlings to salinity stress beyond the salt tolerance threshold. Aust J Crop Sci 6(6):1059–1067
Talei D, Kadir MA, Yusop MK, Valdiani A, Abdullah MP (2013a) Growth indices and salinity tolerance threshold in a medicinal plant Andrographis paniculata Nees. J Med Plants Res 7(3):104–110
Talei D, Valdiani A, Yusop MK, Abdullah MP (2013b) Estimation of salt tolerance in Andrographis paniculata accessions using multiple regression model. Euphytica 189:147–160
Talei D, Valdiani A, Rafii MY, Maziah M (2014) Proteomic analysis of the salt-responsive leaf and root proteins in the anticancer plant Andrographis paniculata Nees. PLoS ONE 9(11):e112907
Talei D, Valdiani A, Maziah M, Sagineedu SR, Abiri R (2015) Salt stress-induced protein pattern associated with photosynthetic parameters and andrographolide content in Andrographis paniculata Nees. Biosci Biotechnol Biochem 79:51–58
Tan BH, Zhang A (2004) Andrographis paniculata and the cardio vascular system. In: Packer L, Ong CN, Halliwell B (eds) Herbal and traditional medicine: molecular aspects on health. CRC Press, Taipei, pp 441–456
The Indian Pharmacopoeia Commission (IPC) (2007) Kalmegh. Indian pharmacopoeia, vol III. The Indian Pharmacopoeia Commission, Ghaziabad, p 2044
Valifard M, Mohsenzadeh S, Kholdebarin B, Rowshan V, Niazi A, Moghadam A (2018) Effect of salt stress on terpenoid biosynthesis in Salvia mirzayanii: from gene to metabolite. J Hortic Sci Biotechnol 94:389–399
Vaughan MM, Christensen S, Schmelz EA, Huffaker A, Mcauslane HJ, Alborn HT, Romero M, Allen LH, Teal PE (2015) Accumulation of terpenoid phytoalexins in maize roots is associated with drought tolerance. Plant Cell Environ 38(11):2195–2207
Vickers CE, Gershenzon J, Lerdau MT, Loreto F (2009) A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat Chem Biol 5(5):283–291
Waskiewicz A, Muzolf-Panek M, Golinski P (2013) Phenolic content changes in plants under salt stress. In: Ahmad P, Azooz M, Prasad M (eds) Ecophysiology and responses of plants under salt stress. Springer, New York, pp 283–314
Wiart C, Kumar K, Yusof MY, Hamimah H, Fauzi ZM, Sulaiman M (2005) Antiviral properties of ent-labdene diterpenes of Andrographis paniculata Nees, inhibitors of herpes simplex virus type 1. Phytother Res 19(12):1069–1070
Yu BC, Hung CR, Chen WC, Cheng JT (2003) Antihyperglycemic effect of andrographolide in streptozotocin-induced diabeticrats. Planta Med 69(12):1075–1079
Zhang L, Ma H, Chen T, Pen J, Yu S, Zhao X (2014) Morphological and physiological responses of cotton (Gossypium hirsutum L.) plants to salinity. PLoS ONE 9(11):e112807
Acknowledgments
We express our sincere thanks to Director, CSIR-CIMAP, Lucknow, for his continuous support and providing the laboratory facilities required to carry out this research (CIMAP Institutional Communication No.: CIMAP/PUB/2018/79). The financial support from the Council of Scientific and Industrial Research (CSIR), New Delhi through the Mission Programme “Phytopharmaceutical Mission—(HCP-010)” is gratefully acknowledged. Financial assistance in the form of Senior Research Fellowship to AK from Indian Council of Medical Research (ICMR), New Delhi (No.: 45/28/2018/TM/BMS) is also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Renault.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumar, A., Rodrigues, V., Verma, S. et al. Effect of salt stress on seed germination, morphology, biochemical parameters, genomic template stability, and bioactive constituents of Andrographis paniculata Nees. Acta Physiol Plant 43, 68 (2021). https://doi.org/10.1007/s11738-021-03237-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-021-03237-x