Abstract
In the first step in this study, the effect of 50 mM NaCl was studied on germination percentage of five different Lepidium draba (L. draba) ecotypes, and Rafsanjan ecotype was selected as experimental material as it had the highest germination percentage. In the second step, some biochemical, physiological, and morphological traits along with content of sulforaphane (SFN) as well as the expression level of Cytochorome P450 79F1 (CYP79F1) were evaluated in 14-day-old L. draba sprouts that grew 9 days in the presence of various concentrations of NaCl including 0, 25, 50, 75, and 100 mM. According to the results of this study, germination percentage of Rafsanjan ecotype along with lengths of stem and root were declined with increasing concentrations of NaCl. Ascorbate peroxidase, guaiacol peroxidase, and superoxide dismutase enzymes activity increased up to 75 mM NaCl and then decreased. With increasing the doses of NaCl, concentrations of Na+ and Cl− increased, whereas P, Ca2+, and K+ decreased. Also, accumulation of some oxidative stress parameters including electrolyte leakage, malondialdehyde, other aldehydes, and hydrogen peroxide increased with increasing NaCl concentrations in all samples. Furthermore, contents of total phenolic, total flavonoid, total anthocyanin, total free amino acids, and total soluble carbohydrate were induced with the induction of NaCl concentrations. In this study, SFN formation increased with increasing concentration of sodium chloride up to 75 mM and decreased at higher concentration. In the last step, a partial CYP79F1 mRNA and its protein sequence were identified and registered in GenBank and then changes in the CYP79F1 gene expression levels under 0, 25, 50, 75, and 100 mM NaCl were calculated. The gene expression levels of CYP79F1 also showed the same pattern as was seen for SFN formation under salinity stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lepidium draba is a plant indigenous to Central Asia, the Black Sea, and native to Southern Europe which is now widespread throughout most of Europe (Mulligan and Frankton 1962). Wild and noxious L. draba weeds are extensively found along roadsides, in meadows, and in fields (Radonic et al. 2011). This weed, which has been shown to belong to the Brassica family, like the other Brassicaceae genus, contains glucosinolates, a unique group of secondary metabolites containing sulfur and nitrogen. This family has about 350 genders, with more than 2000 species. Some of them are commercial and have economic interest such as broccoli and watercress (Gautam et al. 2014). For humans, glucosinolates are very important as relish compounds, cancer-preventive agents, and biopesticides in agriculture (Mikkelsen et al. 2003). More than 100 different kinds of these metabolites have been identified (Fahey et al. 2001; Halkier and Gershenzon 2006), the number of which has reached more than 130 types up to now. Glucosinolates have a defensive role and are classified in two classes: phytoanticipins and phytoalexins (Pedras et al. 2009). Glucosinolates are accumulated in the cell vacuole and released in response to cell injuries or pathogens attack. At this time, when glucosinolates are exposed to myrosinase (β-thioglucoside glucohydrolase, EC. 3.2.3.1), they are hydrolysed by myrosinase and produce glucose and an unstable intermediate molecule (aglycone). According to the environmental conditions, aglycone can be converted to thiocyanates, isothiocyanates, and nitriles (Rask et al. 2000), of which isothiocyanates (ITC) can exhibit various pharmacological activities and antioxidant properties (Traka and Mithen 2009; Fahey et al. 2002). One of the most important ITCs in cruciferous vegetables is SFN (4 methylsulfonyl butyl isothiocyanate) which can be produced through glucoraphanin hydrolysis via myrosinase activity and has anti-cancer properties (Gu et al. 2012). SFN is able to remove carcinogens, stimulate detoxification, induce apoptotic cell death, and inhibit cell cycle progression and angiogenesis in diverse cancer cell types (Zhang et al. 1992; Clarke et al. 2008).
Lately, several studies have shown that salt stress increases the glucosinolate content in broccoli florets, significantly (López-Berenguer et al. 2008, 2009). Every year, salinity stress, which is one of the major abiotic stresses, extremely affects crop production worldwide (Kumar et al. 2007). Various events such as reactive oxygen species (ROSs) production, prevention of enzymatic activities, decreasing plant production and photosynthetic speed, and postponement of the plant growth occur when plants are exposed to salinity stress (Lee et al. 2013). For prevention of ROSs damage, plants use antioxidant defense metabolites such as carotenoids, flavonoid, anthocyanin, and other secondary metabolites (Ahmad et al. 2010b; Núñez et al. 2003; Yasar et al. 2008; Gengmao et al. 2015).
Cytochromes P450, CYP79F1, and CYP79F2 catalyze the short- and long-chain aliphatic glucosinolates from chain-elongated methionines (Chen et al. 2003a, b; Reintanz et al. 2001). CYP79F1 can take both short- and long-chain methionine derivatives, while CYP79F2 solely metabolizes the long-chain methionine derivatives (Penta- and hexahomomethionine) (Chen et al. 2001). Most probably, CYP79F1 and CYP79F2 play different roles in plant growth and extension (Hansen et al. 2001).
When plants face a variety of abiotic stresses such as salinity and drought, reactive oxygen species are produced in these conditions, and finally, normal growth of plants is disturbed in response to these stresses (Sharma et al. 2019a). Salinity stress in plants leads to hyperosmotic stress and ionic imbalance, and this condition is able to induce reactive oxygen species including hydrogen peroxide (H2O2), superoxide (O2.−) and hydroxyl radicals (OH.) which can expose oxidative stress (Bose et al. 2013). These reactions could extremely harm the lipids, photosynthetic pigments, nucleic acids, cell membranes, and proteins (Ahmad et al. 2010a). H2O2, which is a diffusible molecule, can enter the cell membrane and harm the cells (Kordrostami et al. 2017). In 2013, Gil et al. proved that conformity to salinity stress is a complex process and requires the selective ion uptake to hold a suitable Na+, Cl− , and K+ balance (Gil et al. 2013).
It is specified that exposure to different stress conditions is able to disturb the plant metabolism, which results in an increase in the reactive oxygen species (ROS) levels (Foyer and Noctor 2005; Hussain et al. 2016). In plants, ROS scavenging happens using antioxidative defense system, which results in reducing negative effects of oxidative stress (Sharma et al. 2015; Tanveer et al. 2018). Using two ways including non-enzymatic antioxidants defense system (carotenoids, flavonoids, anthocyanin, and so on) and enzymatic antioxidant defense system [superoxide dismutase (SOD), peroxidase (POD), ascorbate peroxidase (APX), and guaiacol peroxidase (GPX)], plants are able to alleviate oxidative damage caused by ROS (Gengmao et al. 2015; Wei et al. 2015).
One of the flavonoids is anthocyanin which is also called the flavylium (2-phenylchromenylium) ion. A positive charge at the oxygen atom of the C-ring of basic flavonoid construction exists in anthocyanins. Osmotic stresses, such as salinity, light, pH, and temperature effect on the stability of anthocyanin considerably (Khoo et al. 2017). Purple, red, and blue pigments in fruits, flowers, and tubers are anthocyanin that in alkaline and acidic conditions emerge as blue and red pigment, respectively. (Laleh et al. 2006). Moreover, plants are able to increase their tolerance with changes in their level of secondary metabolites. One of the main secondary metabolites in plants is total phenolic compounds, which act as plant advocacy mechanisms versus salinity stress and are able to reduce the destructive effect of salinity (Lim et al. 2012). The main objectives of the present study were to measure the activity of APX, GPX, and SOD enzymes, the concentrations of Na+, Cl−, P, Ca2+ , and K+, the accumulation of electrolyte leakage (EL), malondialdehyde (MDA), other aldehydes, and hydrogen peroxide (H2O2), the contents of total phenolic, total flavonoid, total anthocyanin, total free amino acids, total soluble carbohydrate, and SFN along with expression level of CYP79F1 gene.
Material and Methods
Germination Percentage
Matured L. draba seeds were gathered from different parts of Kerman province in Iran including: Lalezar, Baft, Rafsanjan, Kerman, and Zarand at the end of May and in early June 2018. For disinfection, seeds were immersed in 2/5% sodium hypochlorite for 15 min, followed by washing three times with distilled water. Next, the seeds were exposed to 70% ethanol for 1 min and again washed several times with distilled water. After disinfection of seeds, Whatman papers were put inside the Petri dishes and 100 L. draba seeds were placed in each Petri dish, and 5 ml of 50 mM sodium chloride solution was added to it. To determine the germination percentage, germinated seeds in each Petri dish till the 14th day were counted.
Quite similar to the previous method (effect of 50 mM NaCl on the germination percentage of different ecotypes of L. draba), after selection of the L. draba ecotype with the highest germination percentage, it was exposed to different concentrations of NaCl including 0, 25, 50, 75, and 100 mM to assess the germination percentage of this ecotype in response to the different concentrations of NaCl.
Seeds Germination
Lepidium draba ecotype which had the highest germination percentage was selected as the experimental material and after disinfection, it was gently placed on the surface of the 0.8% agar (pH 7) in a 90 mm Petri dish and transferred to the incubator. Seeds were cultured in complete darkness for 3 days at temperature of 28 ± 1 °C and 55% relative humidity in incubator.
Plant Culture
Germinated L. draba seeds were cultured in containers containing ½ Hoagland nutrient solution (Hoagland and Arnon 1950) with pH 6.2, and these dishes were placed in the germinator at 25 °C with 16-h light and 8-h dark photoperiod (450–550 lmol m−2 s−1 flux density) and 55% relative humidity for 2 days. After 2 days, Hoagland solution containers became empty and new Hoagland solutions with different concentrations of NaCl (0 as control, 25, 50, 75, and 100 mM) were prepared. The dishes were filled with 180 cc Hoagland nutrient solution and were put in the germinator with previous conditions. During this time, an aquarium pump was used to aerate the Hogland nutrient solution. In order to measure the electrolyte leakage, lipid peroxidation, and hydrogen peroxide and contents of total phenolic, flavonoid, anthocyanin, and gene expression, some of the 14-day-old sprouts were immediately harvested and frozen in aluminum paper in liquid nitrogen and kept in polyethylene bags at − 80 °C. The rest of the sprouts were used for the evaluation of ion concentrations and SFN content with the following procedure.
Measurement of Stem and Root Lengths
Lengths of stem and root in 14-day-old L. draba plant were evaluated by ruler and reported in cm.
Preparation of Enzyme Extract
Lepidium draba sprouts (0.5 g) in attendance of liquid nitrogen were eroded and by 5 ml of 50 mM sodium phosphate buffer (pH 7.0) containing 0.2 mM ethylenediamine-tetraacetic acid (EDTA) and 1% polyvinylpyrrolidone (PVPP) was homogenated. This solution for 20 min at 17,600×g (4 °C) was centrifuged and then its supernatant was applied to calculate the enzymes’ activity.
Estimation of Ascorbate Peroxidase Enzyme Activity
Lepidium draba sprouts (1 g) were eroded in a mortar by using liquid nitrogen with 0.1 mM EDTA, 2% PVPP, 1 mM phenylmethylsulfonyl fluoride (PMSF) and 10 mM dithiothreitol (DTT) in 50 mM Na–P buffer, pH 7.5. Also, the extraction buffer contained 2 mM ascorbate for ascorbate peroxidase analysis. The activity of ascorbate peroxidase was measured by monitoring the decline in absorbance at the 290 nm wavelength (as for 3 min, ascorbate (ε = 2.8 M−1 cm−1) was oxidized) (Asada and Nakano 1981). The assay medium contained 200 μl of the sample, 200 μl ascorbate, 200 μl EDTA, 1200 μl of 50 mM potassium phosphate buffer (pH 7.0), and 200 μl of 0.1 mM H2O2.
Estimation of Guaiacol Peroxidase Enzyme Activity
Guaiacol peroxidase enzyme activity was measured by a reaction medium containing 50 mM phosphate buffer (pH 7), 19 mM H2O2, and 9 mM guaiacol described by (Lin and Kao 1999). Wavelength of 470 nm was applied to measure the kinetic evolution of solution during 1 min. 26.6 mM−1 cm−1 extinction coefficient was used to measure peroxidase enzyme activity. One unit of peroxidase is the amount of enzyme that leads to the constitution of 1 mM of tetraguaiacol per 1 min.
Estimation of Superoxide Dismutase Enzyme Activity
McCord and Fridovich method by using Xanthine, Cytochrome C, and Xanthine Oxidase was employed to assess the SOD activity (McCord and Fridovich 1969) using the following assay medium: EDTA 0.1 mM, ferricytochrome C 1 × 10−5 M, 100 mM phosphate buffer pH 7.5, xanthine oxidase 0.004 U/assay, and xanthine 5 × 10−5 M. The velocity of absorbance variations at 550 nm was 0.025 Au min−1 with the above reactants; enough plant extract was appended to the above to try to decrease the latter rate by 50%. Purified SOD (from human erythrocytes, Sigma) was diluted to give the equivalent of 1 U/assay (decreased the reference rate of O2−. production by 50%) and was used as control for this assay.
Measurement of the Mineral Nutrient
The concentrations of Na+, P, Ca2+ , and K+ were determined under 0, 25, 50, 75, and 100 mM NaCl for 14-day-old sprouts, and for this intention, 1 g of frozen samples at 550 °C for 10 h was pulverized and ashed and then the ash was completely dissolved in HCl. Flame photometer was used to assess the concentrations of Na+, P, Ca2+ , and K+ in the solution. In order to calculate the Cl− concentrations under 0, 25, 50, 75, and 100 mM NaCl in 14-day-old sprouts of this plant, coulometric–amperometric titration with AgNO3 was used (Kamiab et al. 2014).
Measurement of the Electrolyte Leakage
In this research, Hamed et al. method was applied to determine the amount of electrolyte leakage (Hamed et al. 2007), and for this purpose, 300 mg of plant samples was put in plastic test tubes in the presence of 15 ml of ultra-pure water. These plastic test tubes were incubated in a water bath at 25 °C for 3 h, and then, the elementary electrical conductivity was evaluated by an EC meter. In the further step, the plant samples were heated for 20 min at 96 °C to release all the electrolytes. The electrolyte leakage was calculated using the following formula:
Measurement of Lipid Peroxidation and Other Aldehydes
In this study, Heath and Packer method with slight modification was used to measure the level of lipid peroxidation (Heath and Packer 1968), and to this aim, 0.2 g of sprouts was homogenized in 3 ml of 50 mM phosphate buffer with pH 7.0. The homogenate was centrifuged at 16,000×g for 20 min, and then, to 1.0 ml aliquot of the supernatant, 2.0 ml of 0.5% 2-thiobarbituric acid (TBA) in 20% trichloracetic acid (TCA) was appended. The water bath was used to heat the mixture at 96 °C for 35 min, and then, the mixture was cooled in an ice bath. After centrifugation at 11,000 g for 15 min, 532 nm wavelength was applied to record the absorbance of the supernatant. Also, the quantity for nonspecific absorption at 600 nm was recorded and this value was reduced from the absorbance recorded at 532 nm. The extinction coefficient of 155 mM−1 cm−1 was used to calculate the MDA concentration. MDA content was expressed as nmol g−1 fresh weight tissue. Other aldehydes concentrations were calculated using the extinction coefficient of 457 mM−1 cm−1 (Meir et al. 1992).
Measurement of the Hydrogen Peroxide
Velikova et al. method with slight modification was used to determine the quantity of H2O2 (Velikova et al. 2000), and to this aim, 0.25 g of sprouts was ground in 3 ml of 5% TCA with 0.1 g activated charcoal, and 13,000×g for 16 min was applied to centrifuge the homogenate. To 0.5 ml of the supernatant, 0.75 ml of 1 M potassium iodide (KI) and 0.5 ml of 10 mM potassium phosphate buffer were appended. 390 nm wavelength was used to calculate the content of H2O2 using the extinction coefficient of 0.28 M−1 cm−1 and presented as µmol g−1 fresh weight tissue.
Plant Extract Preparation
An aqueous extract of L. draba sprouts was obtained according to the Fattahi et al. method (Fattahi et al. 2013). For this intention and briefly, 15 g of plant sprouts was completely powdered and extracted with 300 ml of 5% ethanol by boiling for 16 min. After filtration of this homogenate, the filtrate was evaporated and kept at − 20 °C for subsequent analysis (Fattahi et al. 2014).
Measurement of the Total Phenolic Content
To measure the total phenolics content of L. draba sprouts, Folin–Ciocalteu reagent was applied (Chun et al. 2003). 0.5 ml of Folin–Ciocalteu reagent was mixed with 0.5 ml of the plant extract, and this solution was kept at 25 °C for 8 min. After 8 min, 2 ml of sodium carbonate solution (7.5%) was added to this solution, and then, the volume of this solution was adjusted to 8 ml with water. After 2 h, 725 nm wavelength was used to measure the total phenolic content. Calibration curve was drawn using the gallic acid as standard. To express the total phenolic content, mg gallic acid equivalents per gram of sample was used.
Extraction and Measurement of Total Flavonoids
Measurement of total flavonoids was calculated according to the aluminum chloride method described by (Zhishen et al. 1999), using quercetin as reference standard developed by (Vattem et al. 2004). To this aim, 1 ml of extract or standard solution of quercetin (20, 40, 60, 80, and 100 μg ml−1) was appended to a 10 ml volumetric flask including 4 ml of distilled water. 0.30 ml of 5% NaNO2 was appended to the flask and after 5 min, 0.3 ml of 10% AlCl3 was added. 2 ml of 1 M NaOH after 5 min was appended and the volume with distilled water was made up to 10 ml. 510 nm wavelength was used to measure the total flavonoids content. Amounts of total flavonoids were reported as mg quercetin equivalent (mg QE/100 g FW).
Extraction and Measurement of Total Anthocyanins
By using Wagner et al. protocol (Wagner 1979) and with a slight modification, 0.1 g of L. draba sprouts in 10 ml of acidic methanol (1% acidified methanol) was rubbed and centrifuged at 4000×g for 10 min. The supernatant was placed in darkness for 24 h, then, its absorption was read at wavelength of 550 nm using Carry-50 spectrophotometer device. Anthocyanins concentration was calculated using extinction coefficient of 33,000 M−1 cm−1 (ε = 33,000 M−1 cm−1).
Measurement of Total Free Amino Acids
Yemm and Cocking method was used to calculate the total free amino acids using ninhydrin assay with some modifications (Yemm et al. 1955). Sprouts (500 mg) were extracted in 5 ml of 80% ethanol and centrifuged at 17,500×g for 40 min. Total free amino acids were measured using 7.6 ml of ninhydrin reagents containing 1% ninhydrin in 0.5 M citrate buffer pH 5.5, glycerol (87%), and 0.5 M citrate buffer pH 5.5 in a ratio of 5:12:2 using 0.1 ml of the test extract. After 2 min of violent shaking, a water bath was used to heat the contents for 16 min at 100 °C. After this step, the contents were cooled, and then, 570 nm wavelength was applied to calculate the amounts of free amino acids using glycine standard curve.
Measurement of Total Soluble Carbohydrate
Wardlaw and Willenbrink method with slight modification was used to calculate the total soluble carbohydrates (Wardlaw and Willenbrink 1994). L. draba sprouts (0.5 g) were finely homogenized using 5 ml of 95% ethanol, and then, 0.1 ml of alcoholic extract was mixed with 3 ml of anthrone (150 mg anthrone, 100 ml of 72% sulphuric acid, W/W). After this step, a water bath at 100 °C for 15 min was used to heat the homogenate solution. After cooling, 625 nm was used to measure the quantity of total soluble carbohydrates using a glucose standard curve.
SFN Standard Solution Preparation and SFN Extraction and Quantification
SFN standard (Sigma-Aldrich) solution was made according to the (Liang et al. 2006) method, so 5 mg of SFN was solved in 10 ml acetonitrile and kept at − 80 °C until injection. Also, with some slight modification, extraction and determination of SFN were carried out according to the (Liang et al. 2006) and (Riahi-Madvar et al. 2014) methods; therefore, 0.3 g of the fresh tissue was eroded with a mortar and pestle and mixed with 1 ml of acidic water (pH 5). After 2.5-h incubation at 43 °C, 5 ml of acetonitrile was added to the mixture, and it was sonicated for 3 min. The resulting mixture was centrifuged at 11,000 rpm for 13 min at 4 °C. Finally, the supernatant was passed through 0.2 m syringe filter and the SFN content was measured using high-performance liquid chromatography [HPLC, Agilent 1100 series, C18 column (25 × 0.46 cm)]. Moving phase solvent including acetonitrile/H2O (60/40 v/v) and a flow rate of 1 ml min−1 under room temperature were used in order to separate SFN. The retention time was compared with authentic standard detected at 254 nm in order to identify the SFN peak.
Total RNA Extraction
In order to remove RNase enzyme, all microtubes, pipette tips, falcons, mortars, and utensils were put in 0.1% diethyl pyrocarbonate (DEPC) treated water for 24 h and then were dried in an oven at 80 °C. After that, they were sterilized two times in an autoclave. Also, DEPC-treated water was used for the preparation of all solutions. The following steps were performed for all treatments and replicates. Initially, 100 mg of plant tissue was crushed in the presence of liquid nitrogen and transferred to a 1.5 ml tube, and 1 ml cold RNX_ plus solution (Cinnagen, Tehran, Iran) was added to the tubes and vortexed for 30 s. Tubes were put on ice for 20 min, during this time the tubes were gently shaken every 5 min. 200 µl of chloroform was added to the tubes and they were shaken, strongly. Tubes were placed on ice for 5 min. Following this step, the tubes were centrifuged (4 °C, 12,000 rpm) for 15 min. Supernatant liquid was discarded and transferred to a new microtube and the same amount of isopropyl alcohol was added. Tubes were gently shaken and were put on ice for 15 min and again centrifuged with previous conditions. The supernatant liquid was discarded, and 1000 ml of 75% ethanol was added to the pellet. Tubes were centrifuged (4 °C, 7500 rpm for 4 min), supernatant liquid was removed, and the tubes were placed in reverse on sterile paper until they were dried. Next, 30 ml DEPC solution was added to the tubes and they were kept at − 80 °C. In order to survey the quality and quantity of total RNA, 1% agarose gel (photography was done under UV light) and spectrophotometer (260 nm) were used, respectively.
CDNA Synthesis
For cDNA synthesis, 1 µg of total RNA, 1 µl 10 × buffer, 1 µl DNase ,and 1 µl deionized water were poured in 0.2 ml microtubes and they were kept at 37 °C for 30 min. 2 µl EDTA (50 mM) was added to the microtubes and they were kept at 65 °C for 10 min. In the next step, 0.5 µl oligo (dt) 18 primer and 0.5 µl random hexamer were appended to the microtubes and they were maintained at 65 °C for 5 min. Eventually, 1 µl RNase Inhibitor, 4 µl RT buffer (10×), 1 µl dNTP, 1 µl Riverse Transcriptase enzyme, and 2 µl deionized water were, respectively, added to the microtubes and they were kept at 42 °C for 60 min. In order to prevent reverse transcriptase enzyme activity, microtubes were kept at 85 °C for 5 min.
CYP79F1 Gene Identification and Sequences Analysis
Because of the unavailability of L. draba CYP79F1 gene sequence in databases, its sequence was derived by PCR. For this purpose, amplification of cDNA was carried out in the presence of specific primers (forward and reverse) using Taq DNA polymerase. The forward (5ʹ-TTACCATACCCTTTTCACATCCTACTAGTC-3) and reverse (5ʹ-TGCCGGATTATCAATCGCTGCTATAC-3ʹ) primers were designed using Gene Runner (version 3.05) software according to the CYP79F1 gene sequence of Arabidopsis thaliana, Brassica rapa, Brassica oleracea, Raphanus sativus, and Brassica napus (GenBank accession No. 838,211, 103,872,429, 106,343,089, 108,812,420, 106,367, and 453, respectively), which belong to the Brassicaceae family like L. draba. 25 µl reaction mixture was considered for CYP79F1 gene amplification. 0.5 µl of the cDNA, 1.25 mM MgCl2, 10 mM TriseHCl pH 8.4, 1.25 U Taq polymerase (Invitrogen, Carlsbad, USA), 0.1 µM each primer, 25 mM potassium chloride (KCl) and 62.5 µM dNTPs were used to extend CYP79F1 gene. cDNA amplification of CYP79F1 gene was done under the following conditions: initial denaturation at 95 °C for 4 min, 35 cycle amplification (95 °C for 1 min, 68 °C for 30 s, and 72 °C for 1 min) and a final extension for 10 min at 72 °C. Observation and separation of amplified product was performed using 1% agarose gel electrophoresis. Agarose gel image was viewed using the UV Gel Document. The size of the bands was estimated based on 100-bp DNA marker. After electrophoresis, the product of the PCR in 1% agarose gel, a solid single-band PCR product, and non-elongation were sent to be sequenced by an automatic sequencer (23 ABI 3730XLs, Macrogen, Korea) according to the specific forward and reverse primers. After determining the sequence of the desired mRNA, files related to the sequenced fragment were changed to Fasta format using the Chromas v.1.95 software. Similarity search for nucleotide sequence was obtained using Blast online software in NCBI gene database. Also, similarity and identity percentages were extracted from protein and nucleotide blast in NCBI.
Dendrogram Analyses and Estimates of Evolutionary Divergence for CYP79F1 mRNA
In order to study the relationship and compute pairwise distances of L. draba CYP79F1 mRNA with other plants CYP79F1 mRNAs that were derived from a common ancestor, dendrogram was drawn and evolutionary divergence was estimated using MEGA7 software.
Analysis of CYP79F1 Gene Expression
The sequences of the primers related to CYP79F1 as the target gene and GAPDH as internal control gene were designed from the registered sequences in NCBI. Goharrizi et al. showed that GAPDH is a stable internal control gene under salinity stress (Goharrizi et al. 2018). Through alignment of the reported sequences, conserved sequences were determined and primers were designed using Gene Runner software (Table 1). In order to achieve the best conditions, amplification was performed at different temperatures in gradient PCR and 57 °C was chosen as the best annealing temperature for CYP79F1 and GAPDH genes. Amplicon length for CYP79F1 and GAPDH, 180 and 177 base pairs were calculated, respectively.
PCRs were performed with a ROTOR GENE 3000 REAL-TIME PCR MACHINE using SYBR® Green to detect dsDNA synthesis. Reactions were done in 20 μl volumes containing 0.6 µl of each primer (0.1 µM), 2 μl cDNA, 10 μl SYBR Green Master Mix Reagent (Takara), 0.4 µl ROX, and 6.4 µl distilled water. Reactions were run using the following conditions, 94 °C for 4 min, 40 cycles of 95 °C for 30 s, 57 °C for 30 s, and finally 75 °C for 10 s. After seeing a single peak in real-time PCRs, the threshold line was drawn by LinRegPCR program (https://www.hartfaalcentrum.nl/index.php?main=files&fileName=LinRegPCR.zip&sub=LinRegPCR) and Ct of CYP79F1 and GAPDH genes was calculated by the threshold line. 2−ΔCT method was used for calculating CYP79F1 gene expression. Delta Ct is gotten by calculating the difference between Ct of the CYP79F1 gene and GAPDH gene. Negative control in all steps confirmed the accuracy of the amplification.
Statistical Analysis
Experiments were conducted in completely randomized design in three replicates. Data were analyzed by one-way ANOVA, followed by Duncan multiple comparison tests. The values were reported as means with their standard error for all results. Also, statistical differences were considered significant at P < 0.05.
Results
Germination Percentage
Among the collected seeds (Lalezar, Baft, Rafsanjan, Kerman, and Zarand), the seeds that were gathered around Rafsanjan and Baft showed the highest and lowest germination percentage under 50 mM NaCl, respectively (Fig. 1a). The seeds that were collected around Rafsanjan were selected for other analyses. Also, Rafsanjan L. draba ecotype seeds that were exposed to the 5 different levels of NaCl showed a decreasing trend in their germination percentage with increasing concentrations of NaCl (Fig. 1b).
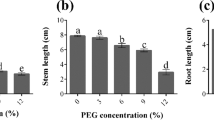
Germination percentage of different ecotypes of L. draba seeds under 50 mM NaCl (a) and germination percentage of Rafsanjan L. draba ecotype seeds under 0, 25, 50, 75, and 100 mM NaCl (b). Bars with different letters are significantly different at P < 0.05, according to Duncan's multiple range tests
Stem and Root Lengths
According to our results, stem and root lengths were declined with induction of NaCl in all samples (Fig. 2). For stem length, 0.92-, 0.83-, 0.79-, and 0.7-fold down-regulation and for root length, 0.91-, 0.87-, 0.72-, and 0.63-fold down-regulation were calculated under 25, 50, 75, and 100 mM NaCl compared to normal environment, respectively (Fig. 2a, b).
Ascorbate Peroxidase, Guaiacol Peroxidase, and Superoxide Dismutase Enzymes Activities
Ascorbate peroxidase enzyme activity was generated by inducing the NaCl concentrations up to 75 mM and then decreased. 1.09-, 1.37-, 1.9-, and 0.65-fold change were calculated under 25, 50, 75, and 100 mM NaCl compared to normal environment, respectively (Fig. 3a). According to the results of this study, guaiacol peroxidase activity increased with increasing concentrations of NaCl up to 75 mM and after that decreased (Fig. 3b). 1.67-, 2.4-, 2.62-, and 0.54-fold change were assessed under 25, 50, 75, and 100 mM NaCl in comparison with 0 mM environment, respectively (Fig. 3b). Our results showed that the amount of superoxide dismutase enzyme activity was elevated by elevating the NaCl concentrations up to 75 mM and then decreased (Fig. 3c). 1.18-, 1.33-, 1.42-, and 1.14-fold variations were evaluated for 25, 50, 75, and 100 mM NaCl compared to 0 mM, respectively (Fig. 3c).
Measurement of the Mineral Nutrient Content
The amounts of Na+, Cl−, P, Ca2+ , and K+ accumulation under different doses of NaCl in the sprouts of this plant were assessed to recognize the impact of this stress on plants’ physiology. As shown in Fig. 4, the results of this study showed that the Na+ and Cl− concentrations with an increase in NaCl concentrations increase, while the amounts of P, Ca2+ , and K+ ions decrease, significantly (Fig. 4).
Measurement of the Electrolyte Leakage, Lipid Peroxidation, Other Aldehydes, and Hydrogen Peroxide Contents
Our results showed that the amounts of electrolyte leakage in 14-day-old sprouts of L. draba are considerably enhanced in all samples by increasing concentrations of NaCl (Fig. 5a). 1.13-, 1.71-, 1.97-, and 2.14-fold up-regulation in electrolyte leakage contents were estimated under 25, 50, 75, and 100 mM NaCl in comparison with the normal condition, respectively (Fig. 5a). Lipid peroxidation in the 14-day-old sprouts of L. draba plant was measured as MDA content and is shown in Fig. 5b. In this study, increasing NaCl concentrations led to the increasing MDA contents in all samples, significantly (Fig. 5b). Under different concentrations of NaCl including 25, 50, 75, and 100 mM, enhancement of 1.2-, 2.04-, 2.74-, and 4.38-fold was calculated for lipid peroxidation compared to normal environment in this plant, respectively (Fig. 5b). Also, 1.39-, 1.46-, 1.81-, and 2.16-fold up-regulations were observed for other aldehydes contents under 25, 50, 75, and 100 mM NaCl in comparison with 0 mM, respectively (Fig. 5c). Increasing NaCl levels increased the H2O2 concentration in sprouts of this plant in all samples (Fig. 5d). 1.13-, 1.32-, 1.41-, and 2.06-fold up-regulations were observed for H2O2 concentrations under 25, 50, 75, and 100 mM NaCl compared to normal condition, respectively (Fig. 5d).
Measurement of the Total Phenolic Content
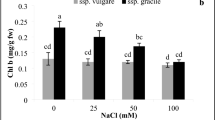
In this study, total phenolic content is enhanced by enhancement of NaCl concentrations in all samples (Fig. 6a). Enhancement of 1.2-, 2.24-, 2.55-, and 3.52-fold was calculated for 25, 50, 75, and 100 mM NaCl relative to the normal condition, respectively (Fig. 6a). Under NaCl stress, flavonoid content increased with increasing concentrations of sodium chloride in all samples (Fig. 6b). Also, under 100 mM NaCl concentration, the highest concentration of flavonoids was measured (Fig. 6b). Additionally, between all NaCl concentrations, a significant difference was observed in all samples between flavonoids content (Fig. 6b). Up-regulation of 1.1-, 1.13-, 1.15-, and 1.67-fold was estimated for flavonoid content under 25, 50, 75 and 100 mM NaCl in comparison with the 0 mM, respectively (Fig. 6b). Under different levels of salinity stress, with enhancing NaCl concentration, anthocyanin content increased in all samples, but a significant difference was not observed between 0 and 25 mM of NaCl (Fig. 6c). Regarding these results, anthocyanin content was improved by increasing levels of NaCl in comparison with the control condition (Fig. 6c). Enhancement of 1.08-, 1.33-, 2.04-, and 2.33-fold was calculated for anthocyanin content under 25, 50, 75, and 100 mM NaCl in comparison with the 0 mM, respectively (Fig. 6c). As shown in Fig. 6d, in a dose-dependent manner, the contents of total free amino acids increased with induction of NaCl in all samples (Fig. 6d). 1.1-, 1.22-, 2.02-, and 2.74-fold up-regulations were observed for the free amino acids contents under 25, 50, 75, and 100 mM NaCl compared to 0 mM, respectively (Fig. 6d). Contents of total soluble carbohydrate in a dose-dependent manner were elevated by increasing concentrations of NaCl in all samples (Fig. 6e). Enhancement of 1.28-, 1.71-, 2.01-, and 2.62-fold was calculated for total soluble carbohydrate under 25, 50, 75, and 100 mM NaCl in comparison with 0 mM, respectively (Fig. 6e). The retention time of SFN was about 4 min after injection into the column. Variance analysis showed significant differences between NaCl treatments, and means comparison showed the following results (Fig. 6f). Under salt stress in 14-day-old L. draba sprouts, SFN content was increased by increasing concentrations of sodium chloride up to 75 mM and after that it was decreased (Fig. 6f). Also, in all NaCl concentrations, a significant difference was observed between SFN contents (Fig. 6f). 18.72, 22.76, 24.69, 29.8, and 23.53 µg g−1 FW were computed for SFN content under 0, 25, 50, 75, and 100 mM NaCl, respectively (Fig. 6f).
Effect of different NaCl levels on the total phenolic (a), flavonoid (b), anthocyanin (c), total free amino acids (d), total soluble carbohydrate (e), and sulforaphane (f) contents in 14-day-old L. draba sprouts. Bars with different letters are significantly different at P < 0.05, according to Duncan's multiple range tests
Amplification, Sequences Alignment, and Expression Level of CYP79F1 Gene
For CYP79F1 gene amplification, cDNA library was used as a template in the presence of specific primers. The amplified fragment (928 bp) was observed on 1% agarose gel (Fig. 7a). mRNA sequence identified as 491 nucleotides related to the codon region of this enzyme was accurately recognized and recorded in GenBank with KT626603 accession number. Amino acid sequence (168 amino acids) encoded by this sequence was also extracted and recorded with KT626603 accession number in GenBank. The recorded sequence of L. draba CYP79F1 mRNA showed 85% and 84% identity with CYP79F2 mRNA sequence of Arabidopsis lyrata and Arabidopsis thaliana, respectively. Also, Blast results showed that the CYP79F1 protein sequence of L. draba has 83%, 84%, and 82% identity with the CYP79F1 protein sequence of Brassica napus, Brassica oleracea and Arabidopsis lyrata, respectively. In this study, 2^−ΔCt method was applied to calculate the CYP79F1/GAPDH mRNA ratio. Variance analysis showed highly significant differences between NaCl treatments. Expression of CYP79F1 gene under salinity stress conditions was studied in 5 levels, and means comparison showed the following results. Expression of CYP79F1 gene was increased by increasing concentrations of sodium chloride up to 75 mM and decreased at higher concentration (Fig. 7b). In order to validate the PCR product (real-time PCR product), separation of amplified product was performed using 1% agarose gel (Fig. 7c).
CYP79F1 amplification band (928-base pair band was observed on a 1% agarose gel after PCR amplification) (a), expression level of CYP79F1 gene relative to the GAPDH as internal control (b), and real-time quantitative RT-PCR amplification fragments’ specificity (amplified fragments obtained after qRT-PCR were separated by agarose gel electrophoresis) (c) under different concentrations of NaCl in 14-day-old L. draba sprouts. Bars with different letters are significantly different at P < 0.05, according to Duncan's multiple range tests
Dendrogram Analyses and Estimates of Evolutionary Divergence for CYP79F1 mRNA
Dendrogram analyses and pairwise distances computation of CYP79F1 mRNA were drawn by MEGA7 software. The results showed that L. draba with Arabidopsis thaliana have a common ancestor (Fig. 8). The number of base substitutions per site between sequences was shown in Table 2. Analyses were conducted using the Maximum Composite Likelihood model (Tamura et al. 2004). Codon positions included were 1st + 2nd + 3rd + Noncoding. All positions containing gaps and missing data were eliminated. There were a total of 206 positions in the final dataset. Evolutionary analyses were conducted in MEGA7 (Kumar et al. 2016).
Discussion
In this study, five different L. draba ecotypes of Kerman province in Iran including: Lalezar, Baft, Rafsanjan, Kerman, and Zarand had various reactions to germination under 50 mM NaCl. Rafsanjan and Kerman ecotypes, which grew in warmer temperatures, had higher germination percentage, respectively. Also, germination percentage of Rafsanjan ecotype, which was selected as the experimental material of this study, decreased with increasing concentrations of NaCl. In sweet sorghum genotypes that were exposed to the different doses of salinity stress, germination percentage was declined with increasing levels of salinity (Almodares et al. 2007). One of the most important proceedings in plant extension is seed germination that can be declined by abiotic stresses, such as salinity and drought. Salinity decreases germination percentage, seedling growth, and the number of seedlings. Also, it considerably increases dispersion of germination (Almansouri et al. 2001; Kaya et al. 2006). It is specified that when plants are exposed to salinity stress, seed establishment, seed germination, leaf area, and plant biomass production reduce under the destructive effects of salinity (Tanveer et al. 2018). Low temperature increases abscisic acid (ABA) content during seed development, and several studies reported seeds that grow and develop at cooler temperatures are generally more dormant and have less germination than those that grow and develop at warmer temperature, such as wheat (Biddulph et al. 2007), weedy rice (Gu et al. 2006), and Arabidopsis (Donohue et al. 2008) and these results are quite similar to our results.
Application of NaCl significantly reduced shoot and root lengths in L. draba plants when compared to the control environment. Several studies have shown that NaCl stress decreased the shoot and root lengths of various plants in comparison with the normal condition (Ahmad et al. 2019, 2017; Kaur et al. 2018; Bai et al. 2011). Plant development decreases under abiotic stresses by affecting various physiological and biochemical mechanisms such as hormonal signaling, photosynthesis, and antioxidant systems (Sharma et al. 2016). Reduction of stem and root lengths occurs either directly via the decline in the level of growth by reduction of the leaf surface and then reducing the turgor (Bouchabke et al. 2006), inhibiting cell division (Granier et al. 2000), and declining the total biomass (Labdelli et al. 2014), or indirectly by reducing the number of leaf-bearing organs (Labdelli et al. 2014).
According to the results of this study, APX, GPX, and SOD enzymes activity increased up to 75 mM NaCl and then decreased. Numerous researches have demonstrated that in different plants treated with NaCl, activities of APX, SOD, CAT (catalase), and GR (glutathione reductase) increased compared with those in the control plants (Ahmad et al. 2016, 2018, 2019), SOD and APX in mustard and maize (Ahmad et al. 2017; Bai et al. 2011), and GPX, SOD, and CAT in UCB-1 (Goharrizi et al. 2019). Also, another study has proven that salinity stress causes the expression levels of SOD, CAT, and APX genes to increase, significantly (Kaur et al. 2018). Also, it is proved that GPX activity increased in maize under salinity stress (de Azevedo Neto et al. 2006). Furthermore, many studies have reported the increased activity of antioxidant enzymes in other plants like Morus alba (Ahmad et al. 2014), C. aeritinum (Ahmad et al. 2016), and Brassica juncea (Ahmad et al. 2015). When plants are exposed to salinity stress, ROS accumulation increases and leads to lack of balance between ROS production and scavenging system, which results in cellular oxidative damage. Plants have evolved an effective defense system of scavenging ROS including enzymatic (APX, SOD, CAT, GPX) and non-enzymatic (carotenoids, flavonoids, anthocyanin, and so on) antioxidants defense systems to protect versus cellular detriment and retain the useful action of ROS-mediated signal transduction which operates in a normal plant (Shi et al. 2014; Gengmao et al. 2015; Wei et al. 2015). Most likely, APX, GPX, and SOD in this study increased in order to decrease the produced ROS under salinity stress.
In this study, the results of Na+, Cl−, P, Ca2+ , and K+ ions accumulation under 0, 25, 50, 75, and 100 mM NaCl showed that Na+ and Cl− ions increased with increasing the concentrations of NaCl, unlike with the P, Ca2+ , and K+ ions that reduced. Ahmad et al. showed that in mung bean treated with 50 and 100 mM NaCl, the concentration of Na+ increased, while Ca2+ and K+ decreased compared with those in the control plants, significantly (Ahmad et al. 2019). A previous research has shown that in roots and leaves of pistachio rootstocks, Na+ and Cl− increase, while K+ decreases, significantly (Akbari et al. 2018). Also, another research showed that P ion decreases under NaCl stress in faba bean plant (Tavakkoli et al. 2010). Furthermore, it has been shown that the Ca2+ concentration in the roots of two Canola (Brassica napus L.) was declined with NaCl application (Bahrani 2013). Previous researches have shown the distinguished importance of Na+ for several plant species. These researches have proved that Na+ acts as a reason for ion-specific detriment during salinity tension. The high amounts of Na+ in metabolic processes lead to competition between the Na+ and K+ ions and consequently, causes enzymes to become inactive (Amtmann and Sanders 1998; Munns and Tester 2008; Tester and Davenport 2003). Elimination of Na+ and/or Cl− minimizes ion toxicity in plants, but precipitates water deficit. Also, osmotic adjustment can be facilitated by absorption of Na+ and/or Cl−, but can also lead to nutritional imbalance and specific ion toxicity. Salinity caused by high levels of NaCl reduces plant growth by the accumulation of high doses of both Na+ and Cl− ions. To defeat the harm of salt stress, plants have the ability to reduce uptake or transport Cl− and Na+ from the roots to the leaves to maintain ion homeostasis (Li et al. 2014). In this research, possibly induced NaCl causes Na+ and Cl− ions to increase and P, Ca2+ , and K+ to decrease in 14-day-old L. draba sprouts. Most probably, high absorption of Na+ and Cl− by L. draba plant root is the main reason for the lower level of P, Ca2+ and K+ in sprouts of this plant.
Our data showed that electrolyte leakage content significantly increased with increasing NaCl concentrations, and these data are completely similar to the previous research by (Hniličková et al. 2019). Many studies have shown that the increasing NaCl content results in increasing electrolyte leakage content in mung bean, tomato, chickpea, and mustard plants, significantly (Ahmad et al. 2016, 2017, 2018, 2019; Kaur et al. 2018). Also, Mahmoudi et al. in (2011) have proven that NaCl increases electrolyte leakage in lettuce (Mahmoudi et al. 2011). The preliminary site of ion-specific salinity damage is plasma membrane (Mansour and Salama 2004). Therefore, electrolyte leakage content can be considered as one of the most important indexes for plants҆ resistance (Ashraf and Ali 2008). In this study, most likely, NaCl stress causes the electrolyte leakage content to increase with increasing NaCl levels.
MDA, which is an index for lipid peroxidation, and other aldehydes contents in the sprouts of L. draba plant were measured. The results of this study showed that both MDA and other aldehydes contents increase under NaCl stress in 14-day-old L. draba sprouts up to 100 mM. Several studies have shown that in green bean, mung bean, chickpea, tomato, and mustard, MDA content increases in response to salinity stress, significantly (Yasar et al. 2008; Ahmad et al. 2016, 2017, 2018, 2019; Kaur et al. 2018). In 2019, Goharrizi et al. showed that in UCB-1, 100 mM NaCl increased the content of MDA, significantly (Goharrizi et al. 2019). Also, it is proved that when MDA content increases, the plants’ growth decreases (Cavalcanti et al. 2004). Kabiri et al. showed that other aldehydes contents increase under salinity stress, significantly (Kabiri et al. 2014). ROS generation in response to salinity stress is unavoidable (Abogadallah 2010), and ROS causes membrane lipid peroxidation in plants. MDA and other aldehydes contents are generally two indexes for resistance of plants under unfavorable conditions of stresses such as salinity and drought (Abogadallah 2010). For example, Taïbi et al. have proved that salt-tolerance plants have less membrane lipid peroxidation (Taïbi et al. 2016). According to our data, it can be concluded that increasing MDA and other aldehydes contents are oxidative damages of salinity stress to the 14-day-old L. draba sprouts which occur because of the enhancement in NaCl levels.
According to our results, enhancement of H2O2 occurs with increasing doses of NaCl in all samples. Several researchers have shown that the content of H2O2 increases in different plants under salinity stress, significantly (Wang et al. 2016; Ahmad et al. 2016, 2017, 2018, 2019; Kaur et al. 2018; Bai et al. 2011). NaCl can impair plant productivity in several various ways (Meloni et al. 2003). One of the detriments induced by salinity stress is the growth in reactive oxygen species (ROS) such as hydroxyl radical (.OH), hydrogen peroxide (H2O2), and superoxide (O2–) (Yen et al. 2013). Higher plants generate ROS under stress conditions and excessive accumulation of ROS in response to salinity stress can cause oxidative stress (Yen et al. 2013; Czegeny et al. 2014) and extremely disrupt natural metabolism by membrane lipids peroxidation (Fridovich 1986; Wise and Naylor 1987), nucleic acid mutation (Fridovich 1986; Imlay and Linn 1988), and protein destruction (Davies 1987). Enhancement of H2O2 content in this study occurs because of the destructive effects of salinity in this plant.
In this study, enhancement of total phenolic content was observed under salinity stress. Our results showed that total phenolic content was increased by increasing NaCl levels. A previous research declared that by increasing the concentrations of salinity, the amount of total phenolic content significantly increases (Akbari et al. 2018). Also, it has been shown that salinity stress can also change the plants phenolic compounds which depend on the salt sensibility of plants (Yuan et al. 2010). The accumulation of total phenolic content under salinity stress might be related to its synthesis pathway. Phenolic compounds in the plant are generally generated by the phenylpropanoid pathway, and this pathway can be induced under salinity tension conditions (Lim et al. 2012). Under salinity stress conditions, plants induce several phytohormones, such as methyl jasmonic acid and jasmonic acid, in which they induce phenylalanine ammonia lyase. In plants, phenylalanine ammonia lyase is the main enzyme in the phenolic metabolism, resulting in the phenolic compounds accumulation (Rebey et al. 2017).
In this research, flavonoid content was evaluated under different concentrations of salinity and with increasing concentration of NaCl, flavonoid content increased in all samples. Additionally, variance analysis showed a significant difference between flavonoid content in all NaCl concentrations. The results of flavonoid content are completely similar to the previous results reported by (Gengmao et al. 2015; Ahmad et al. 2017). Gengmao et al. showed that with increasing concentration of salinity stress up to 150 mM NaCl, flavonoid content has increased in Safflower plant (Gengmao et al. 2015). Also, it was specified that under abiotic stress environments, plants increase the synthesis of flavonoids to fight against the unfavorable conditions of stress (Sharma et al. 2019b). In the same article, Sharma et al. showed that salinity, drought, cold, UV, and heavy metal stresses are able to stimulate the phenolic biosynthetic pathways, which finally result in enhanced abiotic stress tolerance (Sharma et al. 2019b). In response to salinity stress, plants use secondary metabolites to eliminate and enervate the damaging effects of salt stress. Oxidative defense metabolism, compatible solutes, inorganic nutrients, hormonal regulation as well as secondary metabolites are used in response to salinity stress by plants (Parida and Das 2005; Duan et al. 2008; Ashraf 2009; Noreen et al. 2010; Mittler 2002). Flavonoids are one of the most important secondary metabolites which reduce destructive effects of osmotic stress such as salinity (Buer et al. 2010). Flavonoids accumulate in vacuoles, chloroplasts, and nuclei and vacuole flavonoids interfere in detoxification of hydrogen peroxide (Shoeva et al. 2016).
In this research, variance analysis showed that the effect of NaCl < 100 mM on anthocyanin content is meaningful and the comparison between means proved that with increasing concentration of NaCl, anthocyanin content increased in all treatments. Numerous researches proved our results (Piao et al. 2001; Parida and Das 2005; Hughes et al. 2010; Keutgen and Pawelzik 2007; Eraslan et al. 2008). One of the flavonoids is anthocyanin, which has a positive charge at the oxygen atom of the C-ring of basic flavonoid construction (Khoo et al. 2017). Also, Anthocyanins are a large class of water‐soluble flavonoid pigments that meaningfully influence how leaves reply to abiotic stresses. Anthocyanins are involved in tolerance to stresses such as drought, salinity, heavy metal, UV‐B, as well as resistance to biotic stresses like herbivores and pathogens (Landi et al. 2015). Also, anthocyanin is one of the major plant pigments of which its content presents a valuable index of the plant's physiological condition especially under osmotic stress in leaves and sprouts (Gitelson and Merzlyak 2004). Anthocyanins are involved in photoprotection of chlorophyll and in response to osmotic stresses such as salinity in plants (Gould et al. 2000; Landi et al. 2015). Also, in a review paper, Sharma et al. concluded that in plants, tolerance increasing is correlated with the multiple functions of polyphenols, commonly including capacity of several polyphenol classes to preserve the plant from visible light (anthocyanins) and excessive light such as UV (flavonoids) and/or in their ROS scavenging ability (Sharma et al. 2019b).
In this study, total free amino acids content increased with increasing concentrations of NaCl in all samples. Cusido et al. in a study have shown that in Nicotiana rustica L. and under salinity stress, the contents of free amino acids increased, significantly (Cusido et al. 1987). In this study, probably protein synthesis disruption is the main reason of total free amino acids increasing under salinity stress, and most likely, total free amino acids increasing occurs for osmotic adjustment under Na+ toxicity and low water potential.
Total soluble carbohydrate was elevated by salinity stress in 14-day-old L.draba sprouts. Under salinity stress in rice, content of sugar increased compared with those in the control plants, significantly (Amirjani 2011; Pattanagul and Thit
isaksakul 2008). Also in chickpea plants, total soluble carbohydrate increased under salinity stress (Ahmad et al. 2016). It is proven that plants in order to decrease the osmotic stress produced under salinity stress, increase the contents of sugars along with other compatible solutes (Dubey and Singh 1999), which allow the plants to support their fundamental metabolism under stressed conditions (Hurry et al. 1995). Likely, increasing total soluble carbohydrate in this study can help L. draba to synthesize more protein and enhance its growth under stress conditions.
In the current study, the SFN content and expression of CYP79F1 gene were investigated under different concentrations of NaCl. Our results showed that NaCl treatment has a significant effect on SFN content as well as the expression level of CYP79F1 gene. SFN content and CYP79F1 gene expression increased with enhancement of NaCl levels up to 75 mM and after that decreased. Guo et al. reported that SFN content in concentration of 100 mM NaCl in broccoli sprouts has a significant increase in comparison with the control condition which is very similar to the results of this study (Guo et al. 2013). No report is available for expression of CYP79F1 under salinity stress, but CYP79F1 gene that is involved in biosynthesis of aliphatic glucosinolates was induced by methyl jasmonate (Mikkelsen et al. 2003). Additionally, the same article showed that after methyl jasmonate treatment, the concentration of long-chain aliphatic glucosinolates increases (Mikkelsen et al. 2003; Borek et al. 1998).
Cytochromes are a big gene family that has the ability to metabolize insecticides and poisonous secondary metabolites (Feyereisen 1999; Mao et al. 2011). Cytochromes P450 are key players in the biosynthesis of glucosinolates (Nafisi et al. 2006). Glucosinolates have a defensive role in plants (Pedras et al. 2009) and are accumulated in the cell vacuole and released in reaction to cell injuries or pathogens attack. When glucosinolates are exposed to myrosinase, they are hydrolyzed by myrosinase and produce glucose and aglycone, and aglycone can be converted to thiocyanates, isothiocyanates, and nitriles (Rask et al. 2000). One of the most important isothiocyanates is SFN, which can be produced through glucoraphanin hydrolysis via myrosinase activity (Gu et al. 2012). Five cytochrome P450 (CYP) gene products, CYP79F1 and CYP79F2, CYP79B2 and CYP79B3, CYP79A2, have been shown to catalyze the conversion of short- and long-chain-elongated methionine substrates (Chen et al. 2003b; Hansen et al. 2001), tryptophan (Mikkelsen et al. 2000; Hull et al. 2000), and phenylalanine (Wittstock and Halkier 2000) to the corresponding aldoximes, respectively. Also, CYP79F2 and CYP79F1 play distinct roles in physiological effects of the accumulation of short- and long-chain derivatives of methionine in the mutants (Hansen et al. 2001). SFN that is derived from glucoraphanin involves in many mechanisms including inhibition of phase 1 enzymes (Clarke et al. 2008), and induction of phase 2 enzymes that detoxify carcinogens (Fahey et al. 2001). Also, Devi and Thangam (2012) showed that SFN possesses anti-proliferative effects. This increase of total aliphatic glucosinolate under salt stress could be due to the osmotic adjustment under low water potential (Qasim et al. 2003) and maybe increasing levels of aliphatic glucosinolate occur as a result of the decline in the compensation of indole glucosinolate. Therefore, it may be concluded that changes in SFN content can be affected by myrosinase activity and/or glucoraphanin content by NaCl. Also, in this study, increasing CYP79F1 gene expression, which was induced by NaCl treatment, could be a part of response to make the plant more resistant to stress.
Conclusion
The results of this study proved that salinity stress is able to reduce the germination percentage of different ecotypes, but ecotypes which grow in warmer temperatures have higher germination percentage. Also, our results showed that shoot and root lengths reduce in response to the salinity stress. Based on our results, in response to salinity stress, enzymatic antioxidant defense system including the activities of APX, GPX, and SOD and non-enzymatic antioxidant defense systems including the contents of total phenolic, total flavonoid, total anthocyanin, total free amino acids, total soluble carbohydrate, and SFN are extremely activated to reduce the destructive effects of salinity in 14-day-old L.draba sprouts. From the results of this study, it can be concluded that increasing Na+ and Cl− concentrations are the main reasons of decreasing P, Ca2+ , and K+ ions in this plant. Increasing contents of some oxidative stress parameters such as electrolyte leakage, malondialdehyde, other aldehydes, and hydrogen peroxide in this study showed the destructive effects of sodium toxicity and osmotic stress in the 14-day-old L. draba sprouts. According to the results, SFN content increasing under salt stress may be due to the osmotic adjustment under low water potential and sodium toxicity by aliphatic glucosinolate degradation via myrosinase activity. Also, similar patterns of SFN content and CYP79F1 expression level were observed upon salinity stress in this plant. Finally, according to the previous and present studies, it can be concluded that the amount of sodium chloride, the time of treatment and ecotype have a remarkable effect on the contents of valuable plant components.
References
Abogadallah GM (2010) Insights into the significance of antioxidative defense under salt stress. Plant Signal Behav 5(4):369–374. https://doi.org/10.4161/psb.5.4.10873
Ahmad P, Jaleel CA, Salem MA, Nabi G, Sharma S (2010a) Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit Rev Biotechnol 30(3):161–175. https://doi.org/10.3109/07388550903524243
Ahmad P, Jaleel CA, Sharma S (2010b) Antioxidant defense system, lipid peroxidation, proline-metabolizing enzymes, and biochemical activities in two Morus alba genotypes subjected to NaCl stress. Russ J Plant Physiol 57(4):509–517. https://doi.org/10.1134/S1021443710040084
Ahmad P, Ozturk M, Sharma S, Gucel S (2014) Effect of sodium carbonate-induced salinity–alkalinity on some key osmoprotectants, protein profile, antioxidant enzymes, and lipid peroxidation in two mulberry (Morus alba L.) cultivars. J Plant Interact 9(1):460–467. https://doi.org/10.1080/17429145.2013.855271
Ahmad P, Hashem A, Abd-Allah EF, Alqarawi AA, John R, Egamberdieva D, Gucel S (2015) Role of Trichoderma harzianum in mitigating NaCl stress in Indian mustard (Brassica juncea L.) through antioxidative defense system. Front Plant Sci 6:868. https://doi.org/10.3389/fpls.2015.00868
Ahmad P, Abdel Latef AA, Hashem A, Abd Allah EF, Gucel S, Tran L-SP (2016) Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Front Plant Sci 7:347–347. https://doi.org/10.3389/fpls.2016.00347
Ahmad P, Ahanger MA, Alyemeni MN, Wijaya L, Egamberdieva D, Bhardwaj R, Ashraf M (2017) Zinc application mitigates the adverse effects of NaCl stress on mustard [Brassica juncea (L.) Czern & Coss] through modulating compatible organic solutes, antioxidant enzymes, and flavonoid content. J Plant Interact 12(1):429–437. https://doi.org/10.1080/17429145.2017.1385867
Ahmad P, Abass Ahanger M, Nasser Alyemeni M, Wijaya L, Alam P, Ashraf M (2018) Mitigation of sodium chloride toxicity in Solanum lycopersicum L. by supplementation of jasmonic acid and nitric oxide. J Plant Interact 13(1):64–72. https://doi.org/10.1080/17429145.2017.1420830
Ahmad P, Ahanger MA, Alam P, Alyemeni MN, Wijaya L, Ali S, Ashraf M (2019) Silicon (Si) supplementation alleviates NaCl toxicity in Mung bean [Vigna radiata (L.) Wilczek] through the modifications of physio-biochemical attributes and key antioxidant enzymes. J Plant Growth Regul 38(1):70–82. https://doi.org/10.1007/s00344-018-9810-2
Akbari M, Mahna N, Ramesh K, Bandehagh A, Mazzuca S (2018) Ion homeostasis, osmoregulation, and physiological changes in the roots and leaves of pistachio rootstocks in response to salinity. Protoplasma 255(5):1349–1362. https://doi.org/10.1007/s00709-018-1235-z
Almansouri M, Kinet JM, Lutts S (2001) Effect of salt and osmotic stresses on germination in durum wheat (Triticum durum Desf.). Plant Soil 231(2):243–254. https://doi.org/10.1023/A:1010378409663
Almodares A, Hadi M, Dosti B (2007) Effects of salt stress on germination percentage and seedling growth in sweet sorghum cultivars. J Biol Sci 7(8):1492–1495. https://doi.org/10.3923/jbs.2007.1492.1495
Amirjani MR (2011) Effect of salinity stress on growth, sugar content, pigments and enzyme activity of rice. Int J Bot 7(1):73–81
Amtmann A, Sanders D (1998) Mechanisms of Na+ uptake by plant cells. In: Callow JA (ed) Advances in botanical research, vol 29. Academic Press, New York, pp 75–112. https://doi.org/10.1016/S0065-2296(08)60310-9
Asada K, Nakano Y (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22(5):867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232
Ashraf M (2009) Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol Adv 27(1):84–93. https://doi.org/10.1016/j.biotechadv.2008.09.003
Ashraf M, Ali Q (2008) Relative membrane permeability and activities of some antioxidant enzymes as the key determinants of salt tolerance in canola (Brassica napus L). Environ Exp Bot 63(1):266–273. https://doi.org/10.1016/j.envexpbot.2007.11.008
Bahrani A (2013) Effect of salinity on growth, ions distribution, accumulation and chlorophyll concentrations in two canola (Brassica napus L.) cultivars. Am-Eurasian J Agric Environ Sci 13(5):683–689. https://doi.org/10.5829/idosi.wasj.2013.27.08.642
Bai X, Yang L, Yang Y, Ahmad P, Yang Y, Hu X (2011) Deciphering the protective role of nitric oxide against salt stress at the physiological and proteomic levels in maize. J Proteome Res 10(10):4349–4364. https://doi.org/10.1021/pr200333f
Biddulph TB, Plummer JA, Setter TL, Mares DJ (2007) Influence of high temperature and terminal moisture stress on dormancy in wheat (Triticum aestivum L.). Field Crops Res 103(2):139–153. https://doi.org/10.1016/j.fcr.2007.05.005
Borek V, Elberson LR, McCaffrey JP, Morra MJ (1998) Toxicity of isothiocyanates produced by glucosinolates in Brassicaceae species to black vine weevil eggs. J Agric Food Chem 46(12):5318–5323. https://doi.org/10.1021/jf9805754
Bose J, Shabala S, Rodrigo-Moreno A (2013) ROS homeostasis in halophytes in the context of salinity stress tolerance. J Exp Bot 65(5):1241–1257. https://doi.org/10.1093/jxb/ert430
Bouchabke O, Tardieu F, Simonneau T (2006) Leaf growth and turgor in growing cells of maize (Zea mays L.) respond to evaporative demand under moderate irrigation but not in water-saturated soil. Plant Cell Environ 29(6):1138–1148. https://doi.org/10.1111/j.1365-3040.2005.01494.x
Buer CS, Imin N, Djordjevic MA (2010) Flavonoids: new roles for old molecules. J Integr Plant Biol 52(1):98–111. https://doi.org/10.1111/j.1744-7909.2010.00905.x
Cavalcanti FR, Oliveira JTA, Martins-Miranda AS, Viégas RA, Silveira JAG (2004) Superoxide dismutase, catalase and peroxidase activities do not confer protection against oxidative damage in salt-stressed cowpea leaves. New Phytol 163(3):563–571. https://doi.org/10.1111/j.1469-8137.2004.01139.x
Chen S, Petersen BL, Olsen CE, Schulz A, Halkier BA (2001) Long-distance phloem transport of glucosinolates in Arabidopsis. Plant Physiol 127(1):194–201. https://doi.org/10.1104/pp.127.1.194
Chen KM, Gong HJ, Chen GC, Wang SM, Zhang CL (2003a) Up-regulation of glutathione metabolism and changes in redox status involved in adaptation of reed (Phragmites communis) ecotypes to drought-prone and saline habitats. J Plant Physiol 160(3):293–301. https://doi.org/10.1078/0176-1617-00927
Chen S, Glawischnig E, Jørgensen K, Naur P, Jørgensen B, Olsen CE, Hansen CH, Rasmussen H, Pickett JA, Halkier BA (2003b) CYP79F1 and CYP79F2 have distinct functions in the biosynthesis of aliphatic glucosinolates in Arabidopsis. Plant J 33(5):923–937. https://doi.org/10.1046/j.1365-313X.2003.01679.x
Chun OK, Kim DO, Lee CY (2003) Superoxide radical scavenging activity of the major polyphenols in fresh plums. J Agric Food Chem 51(27):8067–8072. https://doi.org/10.1021/jf034740d
Clarke JD, Dashwood RH, Ho E (2008) Multi-targeted prevention of cancer by sulforaphane. Cancer Lett 269(2):291–304. https://doi.org/10.1016/j.canlet.2008.04.018
Cusido RM, Palazon J, Altabella T, Morales C (1987) Effect of salinity on soluble protein, free amino acids and nicotine contents in Nicotiana rustica L. Plant Soil 102(1):55–60. https://doi.org/10.1007/BF02370900
Czegeny G, Wu M, Der A, Eriksson LA, Strid A, Hideg E (2014) Hydrogen peroxide contributes to the ultraviolet-B (280–315 nm) induced oxidative stress of plant leaves through multiple pathways. FEBS Lett 588(14):2255–2261. https://doi.org/10.1016/j.febslet.2014.05.005
Davies KJ (1987) Protein damage and degradation by oxygen radicals I. General aspects. J Biol Chem 262(20):9895–9901
de Azevedo Neto AD, Prisco JT, Enéas-Filho J, Abreu CEBd, Gomes-Filho E (2006) Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ Exp Bot 56(1):87–94. https://doi.org/10.1016/j.envexpbot.2005.01.008
Devi JR, Thangam EB (2012) Mechanisms of anticancer activity of sulforaphane from Brassica oleracea in HEp-2 human epithelial carcinoma cell line. Asian Pac J Cancer Prev 13(5):2095–2100. https://doi.org/10.7314/APJCP.2012.13.5.2095
Donohue K, Heschel MS, Butler CM, Barua D, Sharrock RA, Whitelam GC, Chiang GC (2008) Diversification of phytochrome contributions to germination as a function of seed-maturation environment. New Phytol 177(2):367–379. https://doi.org/10.1111/j.1469-8137.2007.02281.x
Duan J, Li J, Guo S, Kang Y (2008) Exogenous spermidine affects polyamine metabolism in salinity-stressed Cucumis sativus roots and enhances short-term salinity tolerance. J Plant Physiol 165(15):1620–1635. https://doi.org/10.1016/j.jplph.2007.11.006
Dubey RS, Singh AK (1999) Salinity induces accumulation of soluble sugars and alters the activity of sugar metabolising enzymes in rice plants. Biol Plant 42(2):233–239. https://doi.org/10.1023/A:1002160618700
Eraslan F, Inal A, Pilbeam DJ, Gunes A (2008) Interactive effects of salicylic acid and silicon on oxidative damage and antioxidant activity in spinach (Spinacia oleracea L. cv. Matador) grown under boron toxicity and salinity. Plant Growth Regul 55(3):207. https://doi.org/10.1007/s10725-008-9277-4
Fahey JW, Zalcmann AT, Talalay P (2001) The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 56(1):5–51. https://doi.org/10.1016/S0031-9422(00)00316-2
Fahey JW, Haristoy X, Dolan PM, Kensler TW, Scholtus I, Stephenson KK, Talalay P, Lozniewski A (2002) Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo [a] pyrene-induced stomach tumors. Proc Natl Acad Sci 99(11):7610–7615. https://doi.org/10.1073/pnas.112203099
Fattahi S, Ardekani AM, Zabihi E, Abedian Z, Mostafazadeh A, Pourbagher R, Akhavan-Niaki H (2013) Antioxidant and apoptotic effects of an aqueous extract of Urtica dioica on the MCF-7 human breast cancer cell line. Asian Pac J Cancer Prev 14(9):5317–5323. https://doi.org/10.7314/APJCP.2013.14.9.5317
Fattahi S, Zabihi E, Abedian Z, Pourbagher R, Motevalizadeh Ardekani A, Mostafazadeh A, Akhavan-Niaki H (2014) Total phenolic and flavonoid contents of aqueous extract of stinging nettle and in vitro antiproliferative effect on hela and BT-474 cell lines. Int J Mol Cell Med 3(2):102–107
Feyereisen R (1999) Insect P450 enzymes. Annu Rev Entomol 44:507–533. https://doi.org/10.1146/annurev.ento.44.1.507
Foyer CH, Noctor G (2005) Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ 28(8):1056–1071. https://doi.org/10.1111/j.1365-3040.2005.01327.x
Fridovich I (1986) Biological effects of the superoxide radical. Arch Biochem Biophys 247(1):1–11. https://doi.org/10.1016/0003-9861(86)90526-6
Gautam M, Ge X-H, Li Z-Y (2014) Brassica. In: Pratap A, Kumar J (eds) Alien gene transfer in crop plants: achievements and impacts, vol 2. Springer, New York, pp 207–229. https://doi.org/10.1007/978-1-4614-9572-7_10
Gengmao Z, Yu H, Xing S, Shihui L, Quanmei S, Changhai W (2015) Salinity stress increases secondary metabolites and enzyme activity in safflower. Ind Crops Prod 64:175–181. https://doi.org/10.1016/j.indcrop.2014.10.058
Gil R, Boscaiu M, Lull C, Bautista I, Lidón A, Vicente O (2013) Are soluble carbohydrates ecologically relevant for salt tolerance in halophytes? Funct Plant Biol 40(9):805–818. https://doi.org/10.1071/FP12359
Gitelson AA, Merzlyak MN (2004) Non-destructive assessment of chlorophyll carotenoid and anthocyanin content in higher plant leaves: principles and algorithms.
Goharrizi KJ, Wilde HD, Amirmahani F, Moemeni MM, Zaboli M, Nazari M, Moosavi SS, Jamalvandi M (2018) Selection and validation of reference genes for normalization of qRT-PCR gene expression in wheat (Triticum durum L.) under drought and salt stresses. J Genet 97(5):1433–1444. https://doi.org/10.1007/s12041-018-1042-5
Goharrizi KJ, Baghizadeh A, Kalantar M, Fatehi F (2019) Assessment of changes in some biochemical traits and proteomic profile of UCB-1 pistachio rootstock leaf under salinity stress. J Plant Growth Regul. https://doi.org/10.1007/s00344-019-10004-3
Gould KS, Markham KR, Smith RH, Goris JJ (2000) Functional role of anthocyanins in the leaves of Quintinia serrata A Cunn. J Exp Bot 51(347):1107–1115. https://doi.org/10.1093/jexbot/51.347.1107
Granier C, Inze D, Tardieu F (2000) Spatial distribution of cell division rate can be deduced from that of p34(cdc2) kinase activity in maize leaves grown at contrasting temperatures and soil water conditions. Plant Physiol 124(3):1393–1402
Gu X-Y, Kianian SF, Foley ME (2006) Dormancy genes from weedy rice respond divergently to seed development environments. Genetics 172(2):1199–1211. https://doi.org/10.1534/genetics.105.049155
Gu Z-X, Guo Q-H , Gu Y-J (2012) Factors influencing glucoraphanin and sulforaphane formation in Brassica plants: a review. J Integr Agric 11(11):1804–1816. https://doi.org/10.1016/S2095-3119(12)60185-3
Guo R-f, Yuan G-f, Wang Q-m (2013) Effect of NaCl treatments on glucosinolate metabolism in broccoli sprouts. J Zhejiang Univ Sci B 14(2):124. https://doi.org/10.1631/jzus.B1200096
Halkier BA, Gershenzon J (2006) Biology and biochemistry of glucosinolates. Annu Rev Plant Biol 57:303–333. https://doi.org/10.1146/annurev.arplant.57.032905.105228
Hamed KB, Castagna A, Salem E, Ranieri A, Abdelly C (2007) Sea fennel (Crithmum maritimum L.) under salinity conditions: a comparison of leaf and root antioxidant responses. Plant Growth Regul 53(3):185–194. https://doi.org/10.1007/s10725-007-9217-8
Hansen CH, Wittstock U, Olsen CE, Hick AJ, Pickett JA, Halkier BA (2001) Cytochrome P450 CYP79F1 from Arabidopsis catalyzes the conversion of dihomomethionine and trihomomethionine to the corresponding aldoximes in the biosynthesis of aliphatic glucosinolates. J Biol Chem 276(14):11078–11085. https://doi.org/10.1074/jbc.M010123200
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125 (1):189–198. https://doi.org/10.1016/0003-9861(68)90654-1
Hniličková H, Hnilička F, Orsák M, Hejnák V (2019) Effect of salt stress on growth, electrolyte leakage, Na+ and K+ content in selected plant species. Plant Soil Environ 65(2):90–96. https://doi.org/10.17221/620/2018-PSE
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Circ Calif Agric Exp Stn 347 (2nd edit)
Hughes NM, Reinhardt K, Feild TS, Gerardi AR, Smith WK (2010) Association between winter anthocyanin production and drought stress in angiosperm evergreen species. J Exp Bot 61(6):1699–1709. https://doi.org/10.1093/jxb/erq042
Hull AK, Vij R, Celenza JL (2000) Arabidopsis cytochrome P450s that catalyze the first step of tryptophan-dependent indole-3-acetic acid biosynthesis. Proc Natl Acad Sci 97(5):2379–2384. https://doi.org/10.1073/pnas.040569997
Hurry VM, Strand A, Tobiaeson M, Gardestrom P, Oquist G (1995) Cold hardening of spring and winter wheat and rape results in differential effects on growth, carbon metabolism, and carbohydrate content. Plant Physiol 109(2):697–706
Hussain S, Khan F, Cao W, Wu L, Geng M (2016) Seed priming alters the production and detoxification of reactive oxygen intermediates in rice seedlings grown under sub-optimal temperature and nutrient supply. Front Plant Sci 7:439. https://doi.org/10.3389/fpls.2016.00439
Imlay JA, Linn S (1988) DNA damage and oxygen radical toxicity. Science (New York, NY) 240(4857):1302–1309
Kabiri R, Nasibi F, Farahbakhsh H (2014) Effect of exogenous salicylic acid on some physiological parameters and alleviation of drought stress in Nigella sativa plant under hydroponic culture. Plant Prot Sci 50(1):43–51. https://doi.org/10.17221/56/2012-pps
Kamiab F, Talaie A, Khezri M, Javanshah A (2014) Exogenous application of free polyamines enhance salt tolerance of pistachio (Pistacia vera L.) seedlings. Plant Growth Regul 72(3):257–268. https://doi.org/10.1007/s10725-013-9857-9
Kaur H, Sirhindi G, Bhardwaj R, Alyemeni MN, Siddique KHM, Ahmad P (2018) 28-homobrassinolide regulates antioxidant enzyme activities and gene expression in response to salt- and temperature-induced oxidative stress in Brassica juncea. Sci Rep 8(1):8735. https://doi.org/10.1038/s41598-018-27032-w
Kaya MD, Okçu G, Atak M, Çıkılı Y, Kolsarıcı Ö (2006) Seed treatments to overcome salt and drought stress during germination in sunflower (Helianthus annuus L.). Eur J Agron 24(4):291–295. https://doi.org/10.1016/j.eja.2005.08.001
Keutgen AJ, Pawelzik E (2007) Modifications of strawberry fruit antioxidant pools and fruit quality under NaCl stress. J Agric Food Chem 55(10):4066–4072. https://doi.org/10.1021/jf070010k
Khoo HE, Azlan A, Tang ST, Lim SM (2017) Anthocyanidins and anthocyanins: colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr Res 61(1):1361779. https://doi.org/10.1080/16546628.2017.1361779
Kordrostami M, Rabiei B, Hassani Kumleh H (2017) Biochemical, physiological and molecular evaluation of rice cultivars differing in salt tolerance at the seedling stage. Physiol Mol Biol Plants 23(3):529–544. https://doi.org/10.1007/s12298-017-0440-0
Kumar V, Shriram V, Jawali N, Shitole MG (2007) Differential response of indica rice genotypes to NaCl stress in relation to physiological and biochemical parameters. Arch Agron Soil Sci 53(5):581–592. https://doi.org/10.1080/03650340701576800
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 70 for bigger datasets. Mol Biol Evol 33(7):1870–1874. https://doi.org/10.1093/molbev/msw054
Labdelli A, Adda A, Halis Y, Soualem S (2014) Effects of water regime on the structure of roots and stems of durum wheat (Triticum durum Desf.). J Bot. https://doi.org/10.1155/2014/703874.
Laleh G, Frydoonfar H, Heidary R, Jameei R, Zare S (2006) The effect of light, temperature, pH and species on stability of anthocyanin pigments in four Berberis species. Pak J Nutr 5(1):90–92. https://doi.org/10.3923/pjn.2006.90.92
Landi M, Tattini M, Gould KS (2015) Multiple functional roles of anthocyanins in plant-environment interactions. Environ Exp Bot 119:4–17. https://doi.org/10.1016/j.envexpbot.2015.05.012
Lee MH, Cho EJ, Wi SG, Bae H, Kim JE, Cho JY, Lee S, Kim JH, Chung BY (2013) Divergences in morphological changes and antioxidant responses in salt-tolerant and salt-sensitive rice seedlings after salt stress. Plant Physiol Biochem 70:325–335. https://doi.org/10.1016/j.plaphy.2013.05.047
Li J, Jia H, Wang J, Cao Q, Wen Z (2014) Hydrogen sulfide is involved in maintaining ion homeostasis via regulating plasma membrane Na+/H+ antiporter system in the hydrogen peroxide-dependent manner in salt-stress Arabidopsis thaliana root. Protoplasma 251(4):899–912. https://doi.org/10.1007/s00709-013-0592-x
Liang H, Yuan QP, Dong HR, Liu YM (2006) Determination of sulforaphane in broccoli and cabbage by high-performance liquid chromatography. J Food Compos Anal 19(5):473–476. https://doi.org/10.1016/j.jfca.2005.11.005
Lim JH, Park KJ, Kim BK, Jeong JW, Kim HJ (2012) Effect of salinity stress on phenolic compounds and carotenoids in buckwheat (Fagopyrum esculentum M.) sprout. Food Chem 135(3):1065–1070. https://doi.org/10.1016/j.foodchem.2012.05.068
Lin CC, Kao CH (1999) NaCl induced changes in ionically bound peroxidase activity in roots of rice seedlings. Plant Soil 216(1):147. https://doi.org/10.1023/A:1004714506156
López-Berenguer C, Martínez-Ballesta MC, García-Viguera C, Carvajal M (2008) Leaf water balance mediated by aquaporins under salt stress and associated glucosinolate synthesis in broccoli. Plant Sci 174(3):321–328. https://doi.org/10.1016/j.plantsci.2007.11.012
Lopez-Berenguer C, Martinez-Ballesta Mdel C, Moreno DA, Carvajal M, Garcia-Viguera C (2009) Growing hardier crops for better health: salinity tolerance and the nutritional value of broccoli. J Agric Food Chem 57(2):572–578. https://doi.org/10.1021/jf802994p
Mahmoudi H, Kaddour R, Huang J, Nasri N, Olfa B, M’Rah S, Hannoufa A, Lachaâl M, Ouerghi Z (2011) Varied tolerance to NaCl salinity is related to biochemical changes in two contrasting lettuce genotypes. Acta Physiol Plant 33(5):1613–1622. https://doi.org/10.1007/s11738-010-0696-2
Mansour MMF, Salama KHA (2004) Cellular basis of salinity tolerance in plants. Environ Exp Bot 52(2):113–122. https://doi.org/10.1016/j.envexpbot.2004.01.009
Mao Y-B, Tao X-Y, Xue X-Y, Wang L-J, Chen X-Y (2011) Cotton plants expressing CYP6AE14 double-stranded RNA show enhanced resistance to bollworms. Transgenic Res 20(3):665–673. https://doi.org/10.1007/s11248-010-9450-1
McCord JM, Fridovich I (1969) Superoxide dismutase: an enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244(22):6049–6055
Meir S, Philosoph-Hadas S, Aharoni N (1992) Ethylene-increased accumulation of fluorescent lipid-peroxidation products detected during senescence of parsley by a newly developed method. J Am Soc Hortic Sci 117(1):128–132. https://doi.org/10.21273/JASHS.117.1.128
Meloni DA, Oliva MA, Martinez CA, Cambraia J (2003) Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ Exp Bot 49(1):69–76. https://doi.org/10.1016/S0098-8472(02)00058-8
Mikkelsen MD, Hansen CH, Wittstock U, Halkier BA (2000) Cytochrome P450 CYP79B2 from Arabidopsis catalyzes the conversion of tryptophan to indole-3-acetaldoxime, a precursor of indole glucosinolates and indole-3-acetic acid. J Biol Chem 275(43):33712–33717. https://doi.org/10.1074/jbc.M001667200
Mikkelsen MD, Petersen BL, Glawischnig E, Jensen AB, Andreasson E, Halkier BA (2003) Modulation of CYP79 genes and glucosinolate profiles in arabidopsis by defense signaling pathways. Plant Physiol 131(1):298–308. https://doi.org/10.1104/pp.011015
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7(9):405–410. https://doi.org/10.1016/S1360-1385(02)02312-9
Mulligan GA, Frankton C (1962) Taxonomy of the genus cardaria with particular reference to the species introduced into North America. Can J Bot 40(11):1411–1425. https://doi.org/10.1139/b62-136
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681. https://doi.org/10.1146/annurev.arplant.59.032607.092911
Nafisi M, Sønderby IE, Hansen BG, Geu-Flores F, Nour-Eldin HH, Nørholm MH, Jensen NB, Li J, Halkier BA (2006) Cytochromes P450 in the biosynthesis of glucosinolates and indole alkaloids. Phytochem Rev 5(2–3):331–346. https://doi.org/10.1007/s11101-006-9004-6
Noreen Z, Ashraf M, Akram NA (2010) Salt-induced regulation of some key antioxidant enzymes and physio-biochemical phenomena in five diverse cultivars of turnip (Brassica rapa L.). J Agron Crop Sci 196(4):273–285. https://doi.org/10.1111/j.1439-037X.2010.00420.x
Núñez M, Mazzafera P, Mazorra LM, Siqueira WJ, Zullo MAT (2003) Influence of a brassinosteroid analogue on antioxidant enzymes in rice grown in culture medium with NaCl. Biol Plant 47(1):67–70. https://doi.org/10.1023/A:1027380831429
Parida AK, Das AB (2005) Salt tolerance and salinity effects on plants: a review. Ecotoxicol Environ Saf 60(3):324–349. https://doi.org/10.1016/j.ecoenv.2004.06.010
Pattanagul W, Thitisaksakul M (2008) Effect of salinity stress on growth and carbohydrate metabolism in three rice (Oryza sativa L.) cultivars differing in salinity tolerance. Indian J Exp Biol 46(10):736–742
Pedras MSC, Okinyo-Owiti DP, Thoms K, Adio AM (2009) The biosynthetic pathway of crucifer phytoalexins and phytoanticipins: de novo incorporation of deuterated tryptophans and quasi-natural compounds. Phytochemistry 70(9):1129–1138. https://doi.org/10.1016/j.phytochem.2009.05.015
Piao HL, Lim JH, Kim SJ, Cheong GW, Hwang I (2001) Constitutive over-expression of AtGSK1 induces NaCl stress responses in the absence of NaCl stress and results in enhanced NaCl tolerance in Arabidopsis. Plant J 27(4):305–314. https://doi.org/10.1046/j.1365-313x.2001.01099.x
Qasim M, Ashraf M, Ashraf M, Rehman S-U, Rha E (2003) Salt-induced changes in two canola cultivars differing in salt tolerance. Biol Plant 46(4):629–632. https://doi.org/10.1023/A:1024844402000
Radonic A, Blazevic I, Mastelic J, Zekic M, Skocibusic M, Maravic A (2011) Phytochemical analysis and antimicrobial activity of Cardaria draba (L.) Desv volatiles. Chem Biodivers 8(6):1170–1181. https://doi.org/10.1002/cbdv.201000370
Rask L, Andréasson E, Ekbom B, Eriksson S, Pontoppidan B, Meijer J (2000) Myrosinase: gene family evolution and herbivore defense in Brassicaceae. Plant Mol Biol. https://doi.org/10.1023/A:1006380021658
Rebey IB, Bourgou S, Rahali FZ, Msaada K, Ksouri R, Marzouk B (2017) Relation between salt tolerance and biochemical changes in cumin (Cuminum cyminum L.) seeds. J Food Drug Anal 25(2):391–402. https://doi.org/10.1016/j.jfda.2016.10.001
Reintanz B, Lehnen M, Reichelt M, Gershenzon J, Kowalczyk M, Sandberg G, Godde M, Uhl R, Palme K (2001) Bus, a bushy Arabidopsis CYP79F1 knockout mutant with abolished synthesis of short-chain aliphatic glucosinolates. Plant Cell 13(2):351–367. https://doi.org/10.1105/tpc.13.2.351
Riahi-Madvar A, Mohammadi M, Pourseyedi S (2014) Elicitors induced sulforaphane production in Lepidium draba. Asian J Biomed Pharm Sci 4(35):64. https://doi.org/10.15272/ajbps.v4i35.561
Sharma A, Kumar V, Singh R, Thukral AK, Bhardwaj R (2015) 24-Epibrassinolide induces the synthesis of phytochemicals effected by imidacloprid pesticide stress in Brassica juncea L. J Pharmacognosy Phytochem 4(3):60–64.
Sharma A, Thakur S, Kumar V, Kanwar MK, Kesavan AK, Thukral AK, Bhardwaj R, Alam P, Ahmad P (2016) Pre-sowing seed treatment with 24-epibrassinolide ameliorates pesticide stress in Brassica juncea L through the modulation of stress markers. Front Plant Sci 7:1569. https://doi.org/10.3389/fpls.2016.01569
Sharma A, Shahzad B, Kumar V, Kohli SK, Sidhu GPS, Bali AS, Handa N, Kapoor D, Bhardwaj R, Zheng B (2019a) Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules 9(7):285. https://doi.org/10.3390/biom9070285
Sharma A, Shahzad B, Rehman A, Bhardwaj R, Landi M, Zheng B (2019b) Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 24(13):2452. https://doi.org/10.3390/molecules24132452
Shi H, Wang X, Ye T, Chen F, Deng J, Yang P, Zhang Y, Chan Z (2014) The cysteine2/histidine2-type transcription factor zinc finger of Arabidopsis thaliana6 modulates biotic and abiotic stress responses by activating salicylic acid-related genes and C-repeat-binding factor genes in Arabidopsis. Plant Physiol 165(3):1367–1379. https://doi.org/10.1104/pp.114.242404
Shoeva OY, Mock H-P, Kukoeva TV, Börner A, Khlestkina EK (2016) Regulation of the flavonoid biosynthesis pathway genes in purple and black grains of Hordeum vulgare. PLoS ONE 11(10):e0163782. https://doi.org/10.1371/journal.pone.0163782
Taïbi K, Taïbi F, Ait Abderrahim L, Ennajah A, Belkhodja M, Mulet JM (2016) Effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidant defence systems in Phaseolus vulgaris L. South African Journal of Botany 105:306–312. https://doi.org/10.1016/j.sajb.2016.03.011
Tamura K, Nei M, Kumar S (2004) Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci USA 101(30):11030–11035. https://doi.org/10.1073/pnas.0404206101
Tanveer M, Shahzad B, Sharma A, Biju S, Bhardwaj R (2018) 24-Epibrassinolide; an active brassinolide and its role in salt stress tolerance in plants: a review. Plant Physiol Biochem 130:69–79. https://doi.org/10.1016/j.plaphy.2018.06.035
Tavakkoli E, Rengasamy P, McDonald GK (2010) High concentrations of Na+ and Cl- ions in soil solution have simultaneous detrimental effects on growth of faba bean under salinity stress. J Exp Bot 61(15):4449–4459. https://doi.org/10.1093/jxb/erq251
Tester M, Davenport R (2003) Na+ tolerance and Na+ transport in higher plants. Ann Bot 91(5):503–527
Traka M, Mithen R (2009) Glucosinolates, isothiocyanates and human health. Phytochem Rev 8(1):269–282. https://doi.org/10.1007/s11101-008-9103-7
Vattem DA, Lin YT, Labbe RG, Shetty K (2004) Phenolic antioxidant mobilization in cranberry pomace by solid-state bioprocessing using food grade fungus Lentinus edodes and effect on antimicrobial activity against select food borne pathogens. Innov Food Sci Emerg Technol 5(1):81–91. https://doi.org/10.1016/j.ifset.2003.09.002
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151(1):59–66. https://doi.org/10.1016/S0168-9452(99)00197-1
Wagner GJ (1979) Content and vacuole/extravacuole distribution of neutral sugars, free amino acids, and anthocyanin in protoplasts. Plant Physiol 64(1):88–93. https://doi.org/10.1104/pp.64.1.88
Wang Y, Li X, Li J, Bao Q, Zhang F, Tulaxi G, Wang Z (2016) Salt-induced hydrogen peroxide is involved in modulation of antioxidant enzymes in cotton. Crop J 4(6):490–498. https://doi.org/10.1016/j.cj.2016.03.005
Wardlaw IF, Willenbrink J (1994) Carbohydrate storage and mobilisation by the culm of wheat between heading and grain maturity: the relation to sucrose synthase and sucrose-phosphate synthase. Funct Plant Biol 21(3):255–271. https://doi.org/10.1071/PP9940255
Wei P, Yang Y, Wang F, Chen H (2015) Effects of drought stress on the antioxidant systems in three species of Diospyros L. Hortic Environ Biotechnol 56(5):597–605. https://doi.org/10.1007/s13580-015-0074-5
Wise RR, Naylor AW (1987) Chilling-enhanced photooxidation : evidence for the role of singlet oxygen and superoxide in the breakdown of pigments and endogenous antioxidants. Plant Physiol 83(2):278–282. https://doi.org/10.1104/pp.83.2.278
Wittstock U, Halkier BA (2000) Cytochrome P450 CYP79A2 from Arabidopsis thaliana L catalyzes the conversion of L-phenylalanine to phenylacetaldoxime in the biosynthesis of benzylglucosinolate. J Biol Chem 275(19):14659–14666. https://doi.org/10.1074/jbc.275.19.14659
Yasar F, Ellialtioglu S, Yildiz K (2008) Effect of salt stress on antioxidant defense systems, lipid peroxidation, and chlorophyll content in green bean. Russ J Plant Physiol 55(6):782. https://doi.org/10.1134/S1021443708060071
Yemm E, Cocking E, Ricketts R (1955) The determination of amino-acids with ninhydrin. Analyst 80(948):209–214. https://doi.org/10.1039/AN9558000209
Yen WJ, Chyau CC, Lee CP, Chu HL, Chang LW, Duh PD (2013) Cytoprotective effect of white tea against H2O2-induced oxidative stress in vitro. Food Chem 141(4):4107–4114. https://doi.org/10.1016/j.foodchem.2013.06.106
Yuan G, Wang X, Guo R, Wang Q (2010) Effect of salt stress on phenolic compounds, glucosinolates, myrosinase and antioxidant activity in radish sprouts. Food Chem 121(4):1014–1019. https://doi.org/10.1016/j.foodchem.2010.01.040
Zhang Y, Talalay P, Cho CG, Posner GH (1992) A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci USA 89(6):2399–2403. https://doi.org/10.1073/pnas.89.6.2399
Zhishen J, Mengcheng T, Jianming W (1999) The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 64(4):555–559. https://doi.org/10.1016/S0308-8146(98)00102-2
Acknowledgements
The authors gratefully acknowledge the Barafza Keshavarz Pars Company for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jamshidi Goharrizi, K., Riahi-Madvar, A., Rezaee, F. et al. Effect of Salinity Stress on Enzymes’ Activity, Ions Concentration, Oxidative Stress Parameters, Biochemical Traits, Content of Sulforaphane, and CYP79F1 Gene Expression Level in Lepidium draba Plant. J Plant Growth Regul 39, 1075–1094 (2020). https://doi.org/10.1007/s00344-019-10047-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-019-10047-6