Abstract
To enhance the storage potential of pear cv. Punjab Beauty fruit, the effect of putrescine (PUT) treatments was evaluated by analysing various physico-chemical characteristics and enzymatic activities. Postharvest dip treatments of PUT (1, 2 and 3 mM) were given to uniform and healthy fruit, while tap water was used for the control fruit. Treated fruit were stored at 0–1 °C and 90–95% RH for 75 days. Evaluation of fruit quality parameters was made on the 0th, 15th, 30th, 45th, 60th, 67th and 75th days of storage. The PUT treatments (2 and 3 mM) proved to be effective to diminish the softening and enhance the storage potential with acceptable quality. These treatments also suppressed the pectin methyl esterase and cellulase activity, reduced the weight loss and spoilage compared with control. Moreover, 2 and 3 mM PUT applications delayed colour changes, retained higher soluble solids content, starch content and titratable acidity at the end of storage than in control. These findings suggested that exogenous PUT application of 2 and 3 mM could effectively maintain fruit quality and prolong the storage potential of pear cv. Punjab Beauty fruit by reducing the softening during storage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pear (Pyrus spp) is one of the much important temperate pome fruit ranked second among the fruit produced and consumed in the world (FAO 2017). Pear fruit being rich in minerals, nutrients and vitamins make an excellent dietary supplement (Mahammad et al. 2010). Pears are classified as climacteric fruit with high ethylene production (Villalobos and Mitcham 2008) and respiration rate (Lammertyn et al. 2003) after harvest, which cannot be inhibited. Ethylene is a ripening hormone associated with various physico-chemical changes in fruit like firmness, colour and sugars during storage (Abeles et al. 1992). Under sub-tropics of north-western India, pears are harvested in the mid-summer. During this period, both temperature and humidity are very high and result in the reduction of fruit shelf life. Moreover, during long-term storage pear fruit are susceptible to various postharvest disorders due to the climacteric nature (Villalobos and Mitcham 2008). So, minimizing the postharvest losses and enhancing the storage potential are the major goals for growers.
Fruit softening throughout the storage is associated with the modification of cell wall matrix and middle lamella structure. The various enzymes like PME, cellulase, β-galactosidase, etc. are linked to the cell wall modification (Fisher and Bennet 1991). Cell wall are made from the important functional cell wall components like cellulose, hemicellulose, pectin and glycoproteins (Keegstra 2010). Alteration of a cell wall structure and intercellular adhesion by depolymerization and solubilization of these polysaccharides leads to the softening of fruit (Li et al. 2010). In physico-chemical analysis, cell wall degrading enzymes activity were the principal factors in inducing fruit softening (Gwanpua et al. 2014). Changes in SSC and TA were accompanied by softening utilized as an indicant for measuring postharvest quality to assess the storability of fruit (Park et al. 2016).
Polyamines (PAs) are natural compounds and ubiquitous in all organisms and have a significant role in extending the storability with quality maintenance (Wannabussapawich and Seraypheap 2018). PAs are hormones engaged in the cell membrane stability by binding with anionic sites or phospholipids (Slocum et al. 1984). The three key forms of PAs are spermine: spermidine and putrescine have an effect on fruit physiology after harvest (Perez-Vincente et al. 2002). In general, among the various polyamines the PUT is predominant which has closs association with the fruit ripening behaviour (Dibble et al. 1998). PAs behave like an anti-senescence agent greatly helpful to delay softening in fruits like pear (Singh et al. 2019), mango (Malik and Singh 2005) and plum (Khan et al. 2007). To the outflank of our cognition, no selective information is available on the PUT role in regularizing the cell wall degrading enzymes like PME and cellulase activity in subtropical pear during low-temperature storage. Thence, the intent of this work was to determine the effect of postharvest PUT intervention on the storage potential and quality of pear fruit.
Material and methods
Plant materials and fruit treatments
Uniform, healthy and mature (Firmness: 65–70 N, SSC: 12.2–12.5%) fruit of pear cv. Punjab Beauty were picked from the Fruit Orchard, Punjab Agricultural University, Ludhiana (30.91° N, 75.85° E). Harvested fruit were immediately transported in plastic crates to the Postharvest Laboratory and dip treatments of PUT @ 1 mM, 2 mM and 3 mM were applied for 5 min, while tap water was used for the control fruit. For further storage investigations, 1 kg pear fruit for each replication of all the treatments on every storage interval were packed. The fruit were stored in cold storage (0–1 °C and 90–95% RH) after packing in three-ply CFB boxes (5% perforation) along with paper lining. The various physico-chemical and enzymatic observations regarding storage potential and fruit quality were looked into on the day of storage and on 15, 30, 45, 60, 67 and 75 days of storage.
Fruit quality measurements
Weight loss
Pear fruit were weighed using an electronic balance (AND EK-1200i, Co., LTD. Japan.) on the first day of storage. To calculate the weight loss during cold storage, fruit were weighed at each storage interval and weight loss was expressed in per cent:
Determination of fruit firmness and colour (b*)
Fruit firmness and colour during storage were determined as per the method delineated by Singh et al. (2019). Firmness was determined using a stand affixed Penetrometer (Model FT-327, USA) having steel probe (stainless) with 8 mm plunger at the opposite sites of fruit equator after peeling the fruit. Firmness was represented in the Newton (N) force units. The peel colour of fruit was noted down from both sides of fruit using Colour Flex 45°/0° spectrophotometer (Hunter Lab Colour Flex, Hunter Associates Inc., Reston, VA, USA) and presented as b* hunter colour value (Hunter 1975).
Sensory quality and spoilage
Sensory quality (SQ) of fruit in all the treatments was done by a five-judge panel. Fruit were evaluated by panellists on the basis delineated by Amerine et al. (1965) and Singh et al. (2019). The spoilage per cent of fruit was measured based on the number by counting the fruit spoiled at individual storage interval and expressed in percentage:
Estimation of SSC, TA and starch content
SSC of fruit juice of pear was calculated with the help of digital hand refractometer (ATAGO PAL-1) and expressed in terms of the per cent. TA was ascertained by titration of 2.0 ml pear juice with 0.1 N NaOH by adding phenolphthalein as indicant and registered as per cent of malic acid. Starch content of pear fruit was estimated from the previously delineated method by Stevens and Chapman (1955) and Singh et al. (2019). The starch content was estimated through glucose standard curve.
Enzyme extraction and activity assay
Pectin methyl esterase and cellulase activity
The PME activity was estimated from the fruit tissue (20 g) by following the method delineated by the Mahadevan and Sridhar (1982) and Singh et al. (2019). PME activity is uttered as mL of 0.02 N NaOH used. Enzyme extraction and estimation for the cellulase activity was similar to the enzymatic section discussed by Singh et al. (2019). The cellulase activity was represented as per cent reduction in viscosity of the substrate (Mahadevan and Sridhar 1982).
Experimental design and statistical analysis
The study was conducted in the years 2016 and 2017 and designed in Completely Randomized Design with four replications. The data were pooled and two-way analysis of variance (ANOVA) was performed and means were differentiated by the LSD test. Differences among treatments were assumed significant statistically at the p ≤ 0.05 level by using the statistical SAS software (version 9.3 for windows). Statistical data were uttered as the mean ± standard error. Further, to calculate the nature and extent of the correlation among the various parameters, data were analysed through Pearson's correlation analysis.
Results and discussion
Effect of putrescine on fruit quality measurements
Weight loss
Weight loss during storage of fruit is primarily due to the water transpiration from the surface of the fruit. As expected, weight loss was statistically (p ≤ 0.05) increased in all the treatments along the 75-day storage period (Table 1a). However, reduction in weight loss was recorded by PUT treatment and it was more obvious at the higher concentration of PUT (2 and 3 mM). At the storage end, fruit treated with 3 mM PUT registered 10.40% lower weight loss than the control. The lowest average loss in weight was also recorded in 3 mM PUT treated fruit. Water commute among inner and outer atmosphere and cellular break down after harvest causes the fruit weight loss (Ramezanian et al. 2010). Martinez et al. (2002) intimated that PUT has the ability to modify the permeability of tissue to water by stabilizing the integrity of cell membrane and cell wall properties. Stabilization of cell membrane integrity significantly limited the weight loss of fruit. Similar to our results, PUT treatment effectively reduced the weight loss during storage of pear (Singh et al. 2019), mango (Wannabussapawich and Seraypheap 2018) and kiwi fruit (Yang et al. 2016) compared with control.
Fruit firmness
Firmness is the most reliable quality and shelf life indicator for the pear fruit during storage. Fruit firmness was significantly reduced throughout the storage, irrespective of treatments (Table 1b). Results showed that fruit firmness was higher in 2 and 3 mM PUT treatments as compared to the control during the storage period. Fruit treatment with 2 and 3 mM PUT retained 34.47% and 38.03% higher firmness than untreated fruit, respectively, at the end of storage. The mean firmness recorded through the whole storage was highest in 3 mM PUT treatment. Degradation of indissoluble proto-pectin into more simple soluble pectin by enzymatic activity led to the reduction in the firmness of fruit (Abd El-Migid 1986). PAs reduced the activity of pectic acid degrading enzymes like cellulase, poly-galactronse, pectin esterase, etc. (Kramer et al. 1989). They have the ability to cross-linking to the carboxyl group of pectin substrates in the cell wall that lead to rigidification (Abbott et al. 1989). Similar to our findings, PUT treatments had been reported to maintain the higher fruit firmness of mango (Wannabussapawich and Seraypheap 2018), apricot (Martinez et al. 2002) and pear (Singh et al. 2019). Greater cell wall rigidity via electrostatic bond between PAs and the carboxylic groups of poly-galacturonic acid retained the high firmness during storage of fruit (Khosroshahi et al. 2007).
Sensory quality
Sensory quality during storage of pear fruit was significantly affected by PUT treatments (Table 1c). In 2 and 3 mM PUT treatments SQ improved and peaked at 60 days; thereafter it declined, while in control fruit the reduction in SQ was noticed after 45 storage days. At the storage end highest average SQ was registered in 2 and 3 mM PUT application and lowest in the untreated fruit. High SQ rating at end of studies in fruit treated with PUT might be due to a lesser respiration rate that led to the higher retention of polysaccharides in comparison to the control (Valero et al. 2002). Similar to our findings, Khosroshahi et al. (2007) and Singh et al. (2019) also found high consumer acceptability of PUT-treated strawberry and pear during storage, respectively. Anti-senescence action of PUT prevents the transcription, synthesis and activity of 1-aminocyclopropane-1-carboxlic acid and maintained high SQ during the storage (Valero et al. 2002). This is related to the binding of positively charged PAs to the negatively charged pectic substances during storage (Valero et al. 1999).
Spoilage
It is quite evident from data in Table 1d that no spoilage of fruit was recorded up to 60th day of low-temperature storage in all the treated fruit. However, only control fruit recorded spoilage of 3.8% on 67th day of storage (Table 1d). At the storage end, spoilage was noticed in all the treatments. The fruit treated with 3 mM PUT registered 31.46% lower spoilage compared with control. It was found that fruit treated with PUT significantly lowered the pear fruit spoilage depending upon the concentration. The lowest average spoilage was registered in 3 mM PUT-treated fruit. Similarly, in strawberry, Khosroshahi et al. (2007) reported that fruit spoilage during storage was mainly caused by fungal attack; however, postharvest dip treatment of PUT (0.3, 0.5, 1 and 2 mM) for 1 min expressed lower fungal infection compared with control and maintained good appearance up to the end of storage. The suppression of decay symptoms by PUT might be related to its anti-pathogenic properties (Walters 2003).
Fruit colour
Peel colour of pear fruit changes from green to yellowish-green during storage. Change in fruit colour is a valuable ripening indicator for the pear. In present investigations, a continuous change in colour coordinate ‘b*’ was recorded in all the treated fruit during the whole storage (Fig. 1a). PUT treatments of 2 and 3 mM had a considerable delay in a colour change of pear fruit, with a lower value of colour coordinate ‘b*’ than in control. A similar delay in colour changes was reported in PA-treated table grapes (Champa et al. 2015) and PUT-treated pear fruit (Singh et al. 2019). The catabolic pathway of chlorophyll during ripening and senescence of fruit accumulate the colourless products within the cell vacuole. The degradation of chlorophyll was considered vital during the ripening of fruit, while PUT application retarded the chlorophyll degradation and carotenoid synthesis of the fruit by reducing the hydrolytic activities of chloroplast thylakoid membranes (Malik and Singh 2006; Popovic et al. 1979).
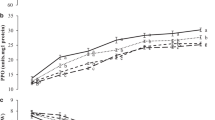
Variation in colour value b* (a), soluble solids’ content (b) and titratable acidity (c) of pear fruit during cold storage with different PUT treatments. Vertical bars represent ± SE of means for four replicates. *Values at zero day: Colour value b*—37.79, SSC (%)—12.31, TA (%)—0.33. Mean values have common superscript are statistically (*p ≤ 0.05) at par
Soluble solids content and titratable acidity
SSC and TA are important post-harvest quality attributes of climacteric fruit. SSC of pear fruit was found to be statistically (p ≤ 0.05) increased up to 67th day in fruit treated with 2 and 3 mM PUT; while only upto 45th day in the control. Afterwards, a significant reduction was recorded in all the treatments (Fig. 1b). During the initial period of storage (15th, 30th and 45th day), steady increase in SSC was noted in higher doses (2 and 3 mM) of PUT treatments, while it increased rapidly in untreated fruit. Among various treatments, no significant difference in SSC was observed on 60th and 67th day of storage. At the storage end, fruit treated with 2 and 3 mM PUT retained higher SSC compared with control might be due to the reduction in the respiration of the fruit. The minimum average SSC was registered in 3 mM PUT treated fruit. Organic acid contents depend mainly upon the fruit species and malic acid was found as major acid in pear (Hulme and Rhodes 1971). TA of the pear fruit effectively reduced in all the PUT-treated fruit throughout the storage period (Fig. 1c). However, the reduction was found at a lesser rate in 2 and 3 mM PUT-treated fruit as compared to the control. TA content reduction in pear fruit was noted to be 65.52% in untreated fruit in comparison with 3 mM PUT-treated fruit that had only a 31.03% reduction during the entire storage period.
SSC increased during storage as a result of the conversion of polysaccharides into soluble solids by the process of dehydration and hydrolysis. This impact of PUT on pear fruit for maintaining quality may be imputed to the low respiration and ethylene production and delayed in the ripening process compared with control. A similar impact of PUT on pear and kiwifruit was reported by Singh et al. (2019) and Yang et al. (2016), respectively. The organic acid consumption during storage led to a reduction in TA, while putrescine maintained it by affecting the respiration process (Zokaee et al. 2007). Similar to the findings, Singh et al. (2019) and Yang et al. (2016) revealed that PUT-treated pear and kiwifruit, respectively, retained higher titratable acidity that might be due to the reduction in the respiration rate and enzymatic activity.
Starch
It was exhibited from the present studies that the starch content of pear fruit decreased during the storage period due to the starch conversion into the soluble sugar content (Fig. 2a). The fruit treated with 3 mM PUT retained the higher average starch content. At the storage termination, fruit treatment with 3 mM PUT registered 46.67% higher starch content compared with control. The starch content reduction during the fruit storage is due to starch hydrolysis which was inversely proportional to the concentration of PUT treatment. Arthey and Philip (2005) suggested that the starch conversion to the sugars by the process of hydrolysis reduced the starch content of the fruit. Similarly, mango fruit treated with PAs (Malik and Singh 2006) and pear fruit treated with PUT (Singh et al. 2019) retained more starch content throughout the storage as compared with control. This might be due to a decrease in the transition of starch into sugars because of the low rate of respiration.
Effect of putrescine treatments on enzymatic activities
Pectin methyl esterase activity
PME activity during pear fruit storage increased significantly at a faster rate in untreated fruit up to 30th storage day followed by a sturdy enhancement to the peak on the 45th day, and thereafter it declined rapidly toward the storage termination (Fig. 2b). However, 2 and 3 mM treatment of PUT registered statistically (p ≤ 0.05) lower PME activity. In these treatments, PME activity increased with steady increment along the storage period and peaked on the 60th and 67th storage interval, respectively. At the storage last, an abrupt decline in PME activity was registered in all the PUT treatments. However, fruit treated with PUT @ 2 and 3 mM retained higher enzymatic activity of PME as than control due to more availability of SSC (described above) as primary substrates for enzymatic activity. Several biochemical and metabolic processes in harvested fruit are responsible for changes in the enzymatic activities. During the ripening and senescence, cell wall structure was affected by the pectin matrix, which is greatly influenced by PME activity (Deytieux-Belleau et al. 2008). PAs are positively charged molecules that fortified the cross linking between cell walls and carboxyl (COO−) group which reduced the enzymatic PME activity (Valero and Serrano 2010). In correspondence to these results, PA and PUT treatment reported lowest PME activity in grapes (Champa et al. 2015) and pear (Singh et al. 2019), respectively, than in control.
Cellulase activity
It was quite evident from the present study that cellulase activity increased in all the treated pear fruit during storage (Fig. 2c). However, a steady increase in cellulase activity was found in pear fruit treated with 2 and 3 mM PUT up to 67th day of storage period followed by abrupt decline to the end of storage. In control, cellulase activity increased sharply up to 30th day of storage, which slowly increased to the peak value on 45th day and then abruptly declined afterwards. At the end of storage, the highest cellulase activity was retained in PUT (2 and 3 mM) treated fruit compared with control. During the entire storage, lowest mean cellulase activity was estimated in 3 mM PUT-treated fruit. Cellulase composed of endoglucanase, exoglucanase and glucosidase is a multi-enzyme system which degrades the cellulose matrix and reduced the firmness as well as quality during storage (Li et al. 2010). Similar finding for PUT-treated pear fruit was reported by Singh et al. (2019). PA application affects the ethylene-modulated ripening enzyme like cellulase activity (Koehler et al. 1996) by reducing the ethylene production, as they both compete for the common precursor, i.e., S-adenosyl methionine decarboxylase at the binding site (Bregoli et al. 2002).
Correlation and regression analysis
To further examine the correlation between fruit quality attributes which influence the storage life of pear fruit, Pearson’s coefficient of correlations was calculated by a linear association between parameters (Table 2). Firmness was correlated with weight loss, SQ, spoilage, PME and cellulase activity. Similarly, starch was correlated with SSC. The significantly associated combinations of parameters were assessed with correlation coefficient (near to 1 or − 1) and further regression analysis was conducted to figure out the regression equation between these factors. Significant plots and their individual regression equations were found and presented in Table 3 and Fig. 3a–f.
Results of the current reporting elucidated that the weight loss enhancement led to the firmness reduction during cold storage ascertained from association analysis between weight loss and firmness (Table 2 and Fig. 3a). Firmness was observed to be correlated negatively with weight loss during storage (− 0.962). Similarly, Wannabussapawich and Seraypheap (2018) and Singh et al. (2019) reported that PUT treatment to the mango and pear fruit, respectively, reduced the loss in weight and maintained the high firmness, which supports the hypothesis that firmness of the fruit is greatly influenced by the weight loss during storage. PUT modified the permeability of tissue to water by stabilizing the cell membrane integrity and cell wall properties (Martinez et al. 2002) which retained firmness ascribed to their cross-linking capability to the carboxyl group of pectin substances of the cell wall that lead to rigidification (Abbott et al. 1989). SQ showed positive correlation with fruit firmness (0.521) (Table 2 and Fig. 3b). The higher SQ during storage in pear fruit treated with PUT may be imputed to retention of fruit firmness, SSC and lower loss in weight. The anti-senescence properties of positively charged PUT maintained the cell wall unity by adhering to negatively charged molecules which were maintained the higher SQ (Valero et al. 1999).
Spoilage of the fruit increased during storage which negatively correlated with firmness (− 0.645, R2 = 0.416) which revealed that reduction in firmness led to spoilage of the fruit (Tables 2, 3 and Fig. 3c). Arthey and Philip (2005) depicted that decrease in starch content of the fruit during storage might be due to the conversion into sugars. In the present study, a negative correlation (− 0.764, R2 = 0.583) was found indicating the reduction in starch content with increment in the sugars during storage (Tables 2, 3 and Fig. 3d). The PUT-treated fruit @ 2 and 3 mM registered higher starch content during the storage in comparison with control. Akhtar et al. (2010) also reported the hydrolysis of starch, as well as other polysaccharides into soluble sugars and water loss, led to enhance SSC of loquat fruit, while PA application maintained more starch content by lower respiration rate, resulting in a reduction in conversion of starch into sugars (Devlieghere et al. 2004)
The relationship between enzymatic activity and firmness during storage showed a statistically (p ≤ 0.05) negative correlation of firmness vs. PME activity (− 0.417) and firmness vs. cellulase activity (− 0.656) (Table 2 and Fig. 3e, f). Fruit firmness is the major postharvest quality parameter, which is greatly influenced by pectic substances. A similar result for the reduction in PME and cellulase activity along with higher firmness retention was reported in pear fruit by PUT application (Singh et al. 2019). PME and cellulase enzymes degrade or reduce the firmness as well as quality during storage (Fisher and Bennet 1991).
Conclusion
PUT treatments (2 and 3 mM) significantly retained the higher TA and starch content, delayed the colour changes and maintained the fruit firmness by reducing the activity of PME and cellulase enzymes. Therefore, it can be concluded from present findings that PUT is a potential tool to delay the softening and maintain the quality as well as extend the storage potential of pear fruit cv. Punjab Beauty up to 67 days.
References
Abbott JA, Conway SW, Sams EC (1989) Postharvest calcium chloride infiltration affects textural attributes of apples. J Am Soc Hortic Sci 114:932–936
Abd El-Migid MB (1986) Post-harvest physiological studies on Le conte and Kiefer pear fruits stored at different temperatures. Ph. D. Thesis. Alexandria University, Alexandria, Egypt
Abeles FB, Morgan PW, Saltveit ME (1992) Ethylene in plant biology. Academic Press, London
Akhtar A, Abbasi NA, Hussain A (2010) Effect of calcium chloride treatments on quality characteristics of loquat fruits during storage. Pak J Bot 42:181–188
Amerine MA, Pangborn RM, Roessler EB (1965) Principal of sensory evaluation of food. Academic Press, London, p 5
Arthey D, Philip RA (2005) Fruit processing nutrition, product and quality management, 2nd edn. Brijbasi Art Press Ltd, Noida, p 45
Bregoli AM, Scaramagli S, Costa G, Sabatini E, Ziosi V, Biondi S, Torrigiani P (2002) Peach (Prunus persica) fruit ripening: amino ethoxyvinyl glycine (AVG) and exogenous polyamines affect ethylene emission and flesh firmness. Physiol Plant 114:472–481
Champa WAH, Gill MIS, Mahajan BVC, Bedi S (2015) Exogenous treatment of spermine to maintain quality and extend postharvest life of table grapes (Vitis vinifera L.) cv. Flame seedless under low temperature storage. LWT-J Food Sci Technol 60:412–419
Devlieghere F, Vermeulen A, Debevere J (2004) Chitosan: antimicrobial activity, interactions with food components and applicability as a coating on fruit and vegetables. Food Microbiol 21:703–714
Deytieux-Belleau C, Vallet A, Doneche B, Geny L (2008) Pectin methyl esterase and polygalactouronase in the developing grape berry skin. Plant Physiol Biochem 46:638–646
Dibble AR, Davies PJ, Mutschler MA (1998) Polyamines content of long-keeping alcobaca tomato fruit. Plant Physiol 86:338–340
FAO (2017) Food and agriculture organization of the United Nations. https://www.fao.org
Fisher RL, Bennett AB (1991) Role of cell wall hydrolases in fruit ripening. Annu Rev Plant Biol 42:675–703
Gwanpua SG, Buggenhot SV, Verlinden BE, Christiaens S (2014) Pectin modifications and the role of pectin-degrading enzymes during postharvest softening of Jonagold apples. Food Chem 158:283–291
Hulme AC, Rhodes MJC (1971) Pome fruits. In: The biochemistry of fruits and their products, vol II. Academic Press, New York
Hunter S (1975) The measurement of appearance. Wiley, New York, p 30405
Keegstra K (2010) Plant cell walls. Plant Physiol 154:483–486
Khan AS, Singh Z, Abbasi NA (2007) Pre-storage putrescine application suppresses ethylene biosynthesis and retard fruit softening during low temperature storage in ‘Angelino’ plum. Postharvest Biol Technol 46:36–46
Khosroshahi MRZ, Esna-ashari M, Ershadi A (2007) Effect of exogenous putrescine on postharvest life of strawberry (Fragaria ananassa Duch.) fruit cultivar Selva. Sci Hortic 114:27–32
Koehler SM, Matters GL, Nath P, Kemmerer EC, Tucker ML (1996) The gene promoter for a bean abscission cellulase is ethylene-induced in transgenic tomato and shows high sequence conservation with a soybean abscission cellulase. Plant Mol Biol 31:595–606
Kramer GF, Wang CY, Conway WS (1989) Correlation of reduced softening and increased polyamines levels during low-oxygen storage of ‘McIntosh’ apples. J Am Soc Hortic Sci 114:942–946
Lammertyn J, Scheerlinck N, Jancsok P, Verlinden B, Nicolai B (2003) A respiration–diffusion model for ‘Conference’ pears I: model development and validation. Postharvest Biol Technol 30:29–42
Li X, Xu C, Korban SS, Chen K (2010) Regulatory mechanisms of textural changes in ripening fruits. Crit Rev Plant Sci 29:222–243
Mahadevan A, Sridhar R (1982) Methods on physiological plant pathology. Sivagami Pub, Chennai
Mahammad MU, Kamba AS, Abubakar L, Bagna EA (2010) Nutritional composition of pear fruits (Pyrus communis). Afr J Food Sci Technol 1:76–81
Malik AU, Singh Z (2005) Pre-storage application of polyamines improves shelf life and fruit quality of mango. J Hortic Sci Biotechnol 80:363–369
Malik AU, Singh Z (2006) Improved fruit retention, yield and fruit quality in mango with exogenous application of polyamines. Sci Hortic 110:167–174
Martinez RD, Serrano M, Carbonell A, Burgos OL, Riquelme F, Valero D (2002) Effect of post-harvest putrescine treatment on extending shelf life and reducing mechanical damage in apricot. J Sci Food Agric 67:1706–1712
Park Y, Lee B, Park H (2016) Observation of anatomical causes of fruit softening during growth and storage periods in ‘Wonhwang’ oriental pear (Pyrus pyrifolia). Sci Hortic 210:250–257
Perez-Vicente A, Martinez-Romero D, Carbonell A, Serrano M, Riquelme F, Guillen F, Valero D (2002) Role of polyamines in extending shelf life and the reduction of mechanical damage during plum (Prunus salicina Lindl.) storage. Postharvest Biol Technol 25:25–32
Popovic RB, Kyle DJ, Cohen AS, Zalik S (1979) Stabilization of thylakoid membranes by spermine during stress induced senescence of barley leaf discs. Plant Physiol 64:721–726
Ramezanian A, Rahemi M, Maftoun M, Bahman K, Eshghi S, Safizadeh MR, Tavallali V (2010) The ameliorative effects of spermidine and calcium chloride on chilling injury in pomegranate fruits after long-term storage. Fruits 65:169–178
Singh V, Jawandha SK, Gill PPS, Gill MS (2019) Suppression of fruit softening and extension of shelf life of pear by putrescine application. Sci Hortic. https://doi.org/10.1016/j.scienta.2019.108623
Slocum RD, Kaur SR, Galston AW (1984) The physiology and biochemistry in of polyamines in plants. Arch Biochem Biophys 235:283–303
Stevens FJ, Chapman RAG (1955) The determination of starch in meat production with the anthrone reagent. J AOAC Int 32:202–210
Valero D, Martinez-Romero D, Serrano M (2002) The role of polyamines in the improvement of the shelf life of fruit. Trends Food Sci Technol 13:228–234
Valero D, Martinez-Romero D, Serrano M, Riquelme F (1999) Polyamine roles on the postharvest of fruits. In: Pandalai S (ed) A review. In recent research developments in agricultural and food chemistry. Research Signpost, Trivandrum, pp 39–55
Valero D, Serrano M (2010) Postharvest biology and technology for preserving fruit quality. CRC-Taylor Francis Press, New York, pp 1–288
Villalobos AM, Mitcham EJ (2008) Ripening of European pears: the chilling dilemma. Postharvest Biol Technol 49:187–200
Walters DR (2003) Polyamines and plant disease. Phytochemistry 64:97–107
Wannabussapawich B, Seraypheap K (2018) Effects of putrescine treatment on the quality attributes and antioxidant activities of ‘Nam Dok Mai No.4’ mango fruit during storage. Sci Hortic 233:22–28
Yang Q, Wang F, Rao J (2016) Effect of putrescine treatment on chilling injury, fatty acid composition and antioxidant system in Kiwifruit. PLoS ONE 11:e0162159. https://doi.org/10.1371/journal.pone.0162159
Zokaee-Khosroshahi MR, Esna-Ashari M, Ershadi A (2007) Effect of exogenous putrescine on post-harvest life of strawberry (Fragaria ananassa Duch.) fruit cultivar ‘Selva’. Sci Hortic 114:27–32
Acknowledgements
The authors are greatly pleased to acknowledge Punjab Agricultural University, Ludhiana, India for the essential research amenities.
Author information
Authors and Affiliations
Contributions
VS: Corresponding author, Department of Fruit Science, Punjab Agricultural University, Ludhiana, India, 141 004. SKJ: Planned and evaluate the experiment, overview the manuscript. Department of Fruit Science, Punjab Agricultural University, Ludhiana, India, 141 004. PPPG: Analysis the data, overview the manuscript. Department of Fruit Science, Punjab Agricultural University, Ludhiana, India, 141 004.
Corresponding author
Additional information
Communicated by P. K. Nagar.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Singh, V., Jawandha, S.K. & Gill, P.P.S. Putrescine application reduces softening and maintains the quality of pear fruit during cold storage. Acta Physiol Plant 42, 28 (2020). https://doi.org/10.1007/s11738-020-3014-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-020-3014-7