Abstract
The putrescine (PUT) efficacy in preserving the postharvest quality, regulating the bioactive compounds and internal browning (IB) was examined during ambient storage of pear fruit. To reduce the internal browning and preserve the colour of pear fruit during ambient storage, preharvest PUT @ 1 mM, 2 mM and 3 mM application was given 14 days before harvest and fruit were stored at ambient conditions (31 ± 2 °C, 78 ± 5% RH) for 15 days. PUT at 2 mM & 3 mM delayed the IB and reduced the polyphenoloxidase enzymatic activity compared with the control fruit. PUT also maintained total phenolics content and enhanced the peroxidase enzymatic activity. These treatments preserved chlorophyll content and suppressed the carotenoids synthesis led to delay in colour changes as compared with control. Results suggest that 2 mM & 3 mM PUT reduced IB incidence and PPO activity and maintained the pear fruit colour during ambient storage.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Pear (Pyrus spp.) is one of the important and economically feasible fruit crop and its semi-soft cultivars are commercially grown in the north-western plains of India. However, pear matured in mid-summer, when temperature and humidity are very high. After harvest IB in pear fruit during storage is triggered by high accumulation of CO2 and ethylene by respiration due to the climacteric nature of fruit that limits the shelf life [1]. IB is an important disorder that is visible only at the end of storage when fruit cut into two halves, as the outer surface of the fruit not altered; even brown coloured tissues are widely extended from the core to the flesh of the fruit [2]. Therefore, it is difficult to investigate the symptoms of IB externally. Since pear fruit affected with IB are undesirable to consume, so it is an urgent need to study the effective method to reduce IB during storage of pear.
Enzymatic oxidation is closely associated with fruit tissue browning. IB is mainly attributed to the PPO activity in the core and cortex tissue of pear, which oxidized the phenolic compounds to highly active o-quinones [3], which formed a brown polymer that subsequently formed brown tissues [4]. PPO activity is highest in the core tissue and increases during the storage period. Since PPO and polyphenols are present in separate cell compartments and browning process needs intercellular membrane breakdown [5]. On the other hand, antioxidants like phenols, CRTs and activity of antioxidant POD enzyme is a potential tool to prevent enzymatic browning [6].
Cell structure breakdown during storage leads to a rapid decline in TPC, while polyamines (PAs) play a significant role in maintaining the TPC in fruit [7]. PAs strengthen the cell wall and kept TPC and PPO in separate compartments results in mitigating IB during storage [8]. Ripening stage of fruit can be indexed with changes in peel colour from green to yellow. PAs have been used to alleviate the discolouration of fruit peel due to their anti-senescence properties which preserved the Chls content by increasing the RNAase and protease activity [9].
In general, PUT is a predominant PA is closely associated with maintaining fruit quality during storage [10]. Although, the application of PAs especially PUT to mitigate the IB and retention of fruit peel colour is well documented in fruit like apricot [8] and pomegranate [11]; To the best of our knowledge, no information about the role of PUT in mitigating the IB and fruit peel colour preservation in sub-tropical pear during ambient storage is available and this warrants further investigation. Therefore, the objective of the current study was to study the effect of PUT treatments on reducing the IB disorder in pear cv. Punjab Beauty. The involvement of PPO, POD enzymes and TPC with respect to IB was also correlated.
Material and methods
Fruit, treatments and sampling
Preharvest PUT application at concentration (1 mM, 2 mM & 3 mM) and water spray (control) were given 14DBH to uniform and healthy plants of pear cv. Punjab Beauty at Fruit Research Farm, Punjab Agricultural University, Ludhiana (30.91°N, 75.85°E). Fruit were harvested at a mature stage (135 days after fruit set) with uniform colour and size and free from any visual defects. Harvested fruit were immediately shifted to the Post-Harvest Laboratory. Fruit were packed in three-ply corrugated fiber board (CFB) boxes (5% perforation) with paper lining after washing with 100 ppm chlorinated water and stored at ambient conditions (31 ± 2 °C, 78 ± 5% RH). One kg fruit for each replication of each treatment was packed for storage studies. Periodical observations were made on 0, 3rd, 6th, 9th, 12th and 15th day of storage.
Internal browning
Internal browning was calculated as per formula given below and expressed in term of per cent.
Total phenolics content
For estimation of total phenols, Folin Ciocalteu (FC) reagent was used [12]. 0.5 mL juice of pear was diluted with 10 mL distilled water and 0.1 mL sample was taken from the diluted solution. To this 0.1 mL diluted solution, 1.5 mL freshly prepared FC reagent (10 mL FC: 90 mL distilled water) and 4 mL saturated Na2CO3 was added and the final volume was prepared to 10 mL by using distilled water. The mixture was placed for 30 min in dark and absorbance was recorded at 738 nm using spectrophotometer (Spectronic 200+, Thermo Scientific, USA).
PPO activity
For the estimation of PPO activity, 2.5 g frozen core tissue of pear were homogenized in 10 mL of 100 mM phosphate buffer (pH 7.8) in 1.0 g polyvinylpyrrolidone (PVP), then centrifuged at 10,000 rpm for 30 min at 4 °C. The supernatant was extracted to analysis PPO activity. The protein content was estimated as one unit of PPO activity was considered against 0.01 changes in A410 per min normalized total protein content, and the PPO activity was expressed as U mg−1 protein [13].
POD activity
For estimation of POD activity, 5.0 g tissue sample of pulp was homogenised with 5 mL of 100 mM sodium acetate buffer, pH 5.5, having 1 mM polyethylene glycol (PEG-4000), 1% (v/v) Triton X-100 and 8% (v/v) PVP. The homogenate was centrifuged at 10,000 rpm for 30 min at 4 °C. The supernatant was used to analysis the POD activity and expressed as unit/min/g FW [14].
Chlorophyll ‘a’& ‘b’ and carotenoids content
For estimation of Chl and CRTs content, 0.2 g tissue was taken and dipped in 5 mL DMSO solution. The samples were kept in a water bath at 60–70 ºC for 1 h for pigment extraction. The absorbance was read at 480, 645 and 663 nm. The ‘Chl a’, ‘Chl b’ and CRTs content were calculated using the following formulas: Chl and carotenoids contents were expressed as mg/g FW tissue [15].
where W- Fresh weight of sample in grams, V- Volume of extract, A480, A645 and A663 are absorbance of samples at 480, 645 nm and 663 nm respectively.
Colour
The peel colour of pear was note down from both sides of fruit by the help of Colour Flex 45°/0° spectrophotometer (Hunter Lab Colour Flex, Hunter Associates Inc., Reston, VA, USA) [16]. The colour was expressed in CIE scale L* (lightness/darkness), a* (red/green) and b* (yellow/blue).
Statistical analysis
The experiment was conducted during the year 2016 & 2017 and laid out in Completely Randomized Design with four replicates. The data were pooled and analyzed by one-way analysis of variance (ANOVA) and means were separated by placing the alphabetical superscripts using LSD test. Different superscripts corresponding to their means were considered statistically significant at the level p ≤ 0.05 using statistical software SAS (version 9.3 for windows). Experimental data were presented as the mean ± standard error. Further, data were subjected to Pearson’s correlation analysis to assess the nature and extent of the relationship between them.
Results
Internal browning
It was exhibited from Table 1A that fruit showed symptoms of IB during storage. No IB was observed up to the 12th day of storage in all the PUT treatments as well as in control. At the end of storage, an effective incidence of IB was recorded in all the fruit, while fruit treated with 2 mM & 3 mM PUT registered 32.14% & 32.98% respectively less incidence of IB over the control. Results elicited that PUT application found to be significantly (p ≤ 0.05) reduced the incidence of IB in pear fruit during ambient storage.
Total phenolics content
Phenols have antioxidant properties and also add nutritious values to the produce. A significant reduction in the TPC was observed in pear fruit subjected to all treatments during the entire storage (Table 1B). However, PUT treatment effectively suppressed the TPC degradation compared with control. At the end of storage, the highest TPC (69.52 mg/100 g FW) was recorded in 3 mM PUT treated fruit while lowest (61.29 mg/100 g FW) in the control.
PPO activity
It was elicited from the data (Table 1C) that, PPO activity continuously increased from the beginning to the end of storage irrespective to the treatments. However, PPO activity estimated to be increased at a lower rate in the fruit treated with 2 mM & 3 mM PUT during the entire storage compared with control. At the end of storage, fruit treated with 3 mM PUT registered 16.31% less PPO activity than in control under the ambient conditions. PPO activity was inversely proportional to the concentration of PUT.
POD activity
It was found that PUT had a significant influence on the POD activity of pear fruit (Table 1D). POD showed a reduction along the storage within an interval of 3 days. POD has antioxidant properties whose activity was continuously decreased, however, 2 mM & 3 mM PUT treated fruit significantly (p ≤ 0.05) retained higher activity compared with control. The highest POD activity was recorded in 3 mM PUT treated fruit measured 29.72% higher than in control at the end of storage.
Chlorophylls content
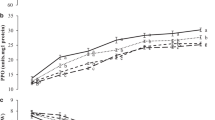
During the storage, fruit peel colour changes from green to yellowish-green depict the degradation of Chls content. A considerable reduction in ‘Chl a’ and ‘Chl b’ content of pear fruit was observed during the storage (Fig. 1a, b). However, PUT treatment significantly (p ≤ 0.05) delayed the reduction in degradation of Chls content. During the storage, effectively higher retention in Chls content was recorded in 2 mM & 3 mM PUT treated fruit over the control. At the end of storage, fruit treated with 2 mM & 3 mM PUT retained 45.46% & 46.92% higher ‘Chl a’ and 48.49% & 66.00% higher ‘Chl b’ content than in control respectively.
Carotenoids
Carotenoids content of fruit increased during storage indicate the changes in colour (Fig. 1c). However, fruit treated with PUT had a lower synthesis of CRTs compared with control during the entire storage. At the end of storage, control fruit recorded highest CRTs content and lowest in 3 mM PUT treated fruit. The content of CRTs synthesis was inversely proportional to the treatment of PUT concentration. Lowest CRTs synthesis was registered in 3 mM PUT treated fruit compared with other PUT treatments while highest in the control.
Colour (L*, a* & b*)
Colour scale ‘L*’
It was exhibited from the data that ‘L*’ value continually increased during the storage, however a significant reduction in ‘L*’ value changes is recorded in PUT treated fruit (Fig. 2a). On the 0 day of storage, no significant difference (p ≤ 0.05) was recorded among the ‘L*’ value of all the treatments. During the storage of 15 days, the PUT treated fruit maintained peel brightness over the control samples as lower ‘L*’ value was recorded in 2 mM & 3 mM PUT treated fruit while highest in the control.
Colour scale ‘ a *’
Data presented in Fig. 2b showed that colour value ‘a*’ of pear fruit continuously increased in all the fruit irrespective to the treatments depicts the green colour reduction. Increase in colour scale ‘a*’ value during storage depicts the reduction in the green colour of the fruit. However, there was a significant (p ≤ 0.05) influence of the PUT application on the colour scale ‘a*’ value of the fruit during storage. Significantly higher colour scale ‘a*’ value was retained by 2 mM & 3 mM PUT treatment compared with control during the entire storage.
Colour scale ‘ b *’
Colour scale ‘b*’ value of pear fruit effectively increased in all the treatments which revealed the improvement in the yellow colour during storage (Fig. 2c). In our experiment, PUT treatment of 2 mM & 3 mM significantly (p ≤ 0.05) reduced the changes in colour scale ‘b*’ value during the storage. There was 14.16% & 13.54% increase in colour scale ‘b*’ value recorded in 2 mM & 3 mM PUT treated fruit respectively compared with control had 16.68% increase from the initial to the end of storage.
Discussion
IB is a major postharvest disorder of pear fruit which limits the shelf life, while PUT treatment found to be effective to reduce the incidence of IB. Exogenous application of PAs maintained the membrane fluidity and thus reduced the electrolyte leakage and browning of fruit [11]. Similar to our findings, a reduction in IB of apricot cv. Alyanak was recorded during storage with PUT treatment compared with the control [17]. Disorganisation of mitochondria and chloroplast caused the flesh browning, while PAs as poly-cationic molecule strongly bind to cell membrane anionic compounds like phospholipids and stabilized the bi-layer surface which protects the membrane and reduced the incidence of IB [18]. Cell structure breakdown during storage leads to fast decline in TPC, however, anti-senescence behaviour of PUT maintained higher TPC in fruit by reduction in the cell structure breakdown in senescence phenomenon [7]. It was revealed from the correlation analysis that TPC was negatively correlated with the IB of pear fruit (Table 2; Fig. 3a). Higher retention of TPC leads to delay in the IB of fruit. Similarly in grapes, PUT treated berries retained the higher TPC and effectively reduced the rachis browning, decay incidence and cracking [19). Similarly, the reduction of TPC in plum, however, lowest degradation in TPC observed in PUT treated fruit could be due to the anti-senescence behaviour of PUT [20]. The reduction in TPC in fruit flesh during storage might be related to increase in PPO activity [21] while a moderate decrease in TPC in PUT treatments could be due to delaying the activity of PPO by PAs in line with results obtained in our study (Table 1B). PAs have antioxidant properties and linearly associated TPC [22].
PPO is a copper containing enzyme involved in tissue browning with hydroxylation of monophenols to o-diphenols and o-dihydroxyphenols to o-quinones by oxidation of phenols produce highly reactive quinines which destroy the cell wall and produced dark and brown colour tissue complex [23]. In the current study, PUT significantly suppressed the PPO activity ultimately led to a reduction in the IB of pear fruit. A significant positive correlation (0.462, R2 = 0.214) was recorded between PPO and IB (Tables 2 and 3; Fig. 3b). Reduction in PPO activity directly related to the delay and suppression in the browning of fruit. Similarly, PUT reported to reduce the PPO activity and browning of apricot during storage [8]. The important role of PUT in delaying PPO activity associated with a reduction in fruit respiration rate [21].
Peroxidase is an important antioxidant enzyme which is involved in the oxy-radical detoxification process in plant tissues [24]. PUT had a significant influence on the POD activity of pear fruit as 2 mM & 3 mM PUT treatment enhanced the POD activity than in control (Table 1D). A significant negative correlation between POD and browning (− 0.525, R2 = 0.275) depict that POD activity is inversely proportional to the IB (Tables 2 and 3; Fig. 2c), higher the POD activity lower will be the IB and vice versa (Tables 2 and 3; Fig. 3c). It is well documented that POD is an important active enzyme that has the capacity to scavenge the free-radicals and reduction in POD activity may lead to overload the ROS [25]. Excessive accumulation of ROS could cause oxidative damage, lipid peroxidation which accelerates browning [26]. PAs treatment effectively enhanced the POD activity and delayed browning in apricot similar to results obtained herein “Punjab Beauty” cultivar of pear [8].
Polyamines were reported to retard the Chl degradation and membrane deterioration by increasing the RNAase and protease activity due to their anti-senescence behaviour [9]. In the present investigation PUT treatment significantly preserved the Chl content of pear fruit and reduced the carotenoid synthesis (Fig. 1). Similar to our findings, PUT treatment found to preserves the Chl content of mango cv. Zebda [27]. PUT treatment enhanced the POD enzymatic antioxidant activity, which catalyses the quenching of ROS that prevents the cell death and helps to retain total Chls content by preserving bio-molecules such as proteins, lipids, carbohydrates and DNA [27]. Chl degradation along with CRTs synthesis changed the fruit colour from green to yellowish-green. PUT treatments of higher concentration (2 mM & 3 mM) significantly delayed the Chl degradation and CRTs synthesis during storage. The predominant index used to assess the ripening stage of fruit is an alteration of peel colour from green to yellow [28]. During storage, chlorophyll degradation along with carotenoids synthesis changed the fruit colour of banana; however, putrescine (2 mM) treated banana fruit showed a delay in CRTs synthesis compared with control [29]. Anti-senescence properties of PUT contributed to the reduction in ethylene production and delayed the degradation of Chl and synthesis of CRTs which led to delay in changes in the colour of fruit [30]. Likewise, PUT treated pear cv. Spadona reported the slower rate of conversion of fruit colour from green to yellow due to the suppressed degradation of chlorophyll content [31]. Exogenous PUT application also induced low softening and delayed the changes in colour during storage of apricot over the control [32].
Conclusion
The present studies confirmed that PUT treatments of 2 mM & 3 mM reduced the internal browning by reducing the enzymatic activity of PPO under the ambient conditions. Along with this, these treatments also maintained higher total phenolics content and POD activity over the control. Anti-senescence properties of PUT also retained the fruit peel colour and reduced the rate of carotenoids synthesis. Therefore, preharvest PUT application is an effective tool to reduce the internal browning and maintain the fruit colour during ambient storage.
References
C. Franck, J. Lammertyn, Q.T. Ho, P. Verboven, B. Verlinden, B.M. Nicolai, Browning disorders in pear fruit. Postharvest Biol. Technol. 43, 1–13 (2007)
N. Hernandez-Sanchez, B.P. Hills, P. Barreiro, N. Marigheto, An NMP study on internal browning in pears. Postharvest Biol. Technol. 44, 260–270 (2007)
N.J. Rivas, J.R. Whitaker, Purification and some properties of two polyphenol oxidases from Bartlett pears. Plant Physiol. 52, 501–507 (1973)
Y.D. Cheng, L.Q. Liu, G.Q. Zhao, C.G. Sheng, H.B. Yan, J.F. Guan, K. Yang, The effects of modified atmosphere packaging on core browning and the expression patterns of PPO and PAL genes in ‘Yali’ pears during cold storage. LWT-Food Sci. Technol. 60, 1243–1248 (2015)
C.H. Crisosto, D. Gamer, G.M. Crososto, Late harvest and delayed cooling induce internal browning of ‘Ya Li’ and ‘Seuri’ Chinese pears. HortScience 29, 667–670 (1994)
E. Pintó, I. Lentheric, M. Vendrell, C. Larrigaudière, Role of fermentative and antioxidant metabolisms in the induction of core browning in controlled-atmosphere stored pears. J. Sci. Food Agric. 81, 364–370 (2001)
M. Ghasemnezhad, M.A. Shiri, M. Sanavi, Effect of chitosan coatings on some quality indices of apricot (Prunus armeniaca L.) during cold storage. Caspian J. Environ. Sci. 8, 25–33 (2010)
M. KousheshSaba, K. Arzani, M. Barzegar, Postharvest polyamine application alleviates chilling injury and affects apricot storage ability. J. Agric. Food Chem. 60, 8947–8953 (2012)
T.E. Evans, R.L. Malmberg, Do polyamines have roles in plant developments? Ann. Rev. Plant Physiol. Plant Mol. Biol. 40, 235–269 (1989)
A.R. Dibble, P.J. Davies, M.A. Mutschler, Polyamine content of long-keeping alcobaca tomato fruit. Plant Physiol. 86, 338–340 (1988)
K. Barman, R. Asrey, R.K. Pal, Putrescine and carnauba wax pre-treatments alleviate chilling injury, enhance shelf life and preserve pomegranate fruit quality during cold storage. Sci. Hortic. 130, 795–800 (2011)
T. Swain, W.E. Hills, The phenolics constituents of Prunus domestica in the quantitative analysis of phenolic constituents. J. Sci. Food Agric. 10, 63–68 (1959)
M.M. Bradford, A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976)
H. Liu, W. Jiang, L. Zhou, B. Wang, Y. Luo, The effects of 1-methylcyclopropene on peach fruit (Prunus persica L. cv. Jiubao) ripening and disease resistance. Int. J. Food Sci. Technol. 40, 1–7 (2005)
J.D. Barnes, L. Balaguer, E. Manrique, S. Elvira, A.W. Davison, A reappraisal of the use of DMSO for the extraction and determination of chlorophylls a and b in lichens and higher plants. Environ. Exp. Bot. 32, 85–100 (1992)
S. Hunter, The Measurement of Appearance (Wiley, New York, 1975), pp. 304–305
C.E. Onursal, D. Bayindir, F. Celepaksoy, M.A. Koyuncu, Combined effects of MAP and postharvest putrescine treatment on storage life and quality of “Alyanak” apricot. Acta Hortic. 1071, 165–172 (2015)
X. Zhang, L. Shen, F. Li, Y. Zhang, D. Meng, J. Sheng, Up-regulating arginase contributes to amelioration of chilling stress and the antioxidant system in cherry tomato fruits. J. Sci. Food Agric. 90, 2195–2202 (2010)
M.A. Shiri, M. Ghasemnezhad, D. Bakhshi, H. Sarikhani, Effect of postharvest putrescine application and chitosan coating on maintaining quality of table grape cv. “Shahroudi” during long-term storage. J. Food Process. Preserv. 37, 999–1007 (2012)
G.H. Davarynejad, M. Zarei, M.E. Nasrabadi, E. Ardakani, Effects of salicylic acid and putrescine on storability, quality attributes and antioxidant activity of plum cv. “Santa Rosa.” J. Food Sci. Technol. 52, 1–10 (2013)
M.J. Jhalegar, R.R. Sharma, R.K. Pal, V. Rana, Effect of postharvest treatments with polyamines on physiological and biochemical attributes of kiwifruit (Actinidia deliciosa) cv Allison. Fruits 67, 13–22 (2012)
K. Razzaq, A.S. Khan, A.U. Malik, M. Shahid, S. Ullah, Role of putrescine in regulating fruit softening and antioxidative enzyme systems in ‘Samar Bahisht Chaunsa’ mango. Postharvest Biol. Technol. 96, 23–32 (2014)
J.C. Partington, P.G. Bolwell, Purification of polyphenol oxidase free of the storage protein patatin from potato tuber. Phytochemistry 42, 1499–1502 (1996)
G. Yuan, B. Sun, J. Yuan, Q. Wang, Effect of 1-methylcyclopropene on shelf life, visual quality, antioxidant enzymes and health-promoting compounds in broccoli florets. Food Chem. 118, 774–781 (2010)
R. Mittler, Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 7, 405–410 (2002)
P. Hariyadi, K.L. Parkin, Chilling induced oxidative stress in cucumber fruits. Postharvest Biol. Technol. 1, 33–45 (1991)
M.S. Ali, M.A. Elhamahmy, A.F. El-Shiekh, Mango trees productivity and quality as affected by Boron and Putrescine. Sci. Hortic. 216, 248–255 (2017)
J.F.S. Gomes, R.R. Vieira, F.R. Leta, Colorimetric indicator for classification of bananas during ripening. Sci. Hortic. 150, 201–205 (2013)
M.S. Hosseini, S.M. Zahedi, Z. Fakhar, Pre-storage putrescine treatment maintains quality and prolongs postharvest life of Musa acuminata L. Adv. Hortic. Sci. 30, 159–164 (2016)
S.R. Drake, P.M. Chen, Storage of ethylene treated “Anjou” and “Bosc” winter pears. Food Process. Preserv. 24, 379–388 (2000)
M.S. Hosseini, Z. Fakhar, M. Babalar, M.A. Askari, Effect of pre-harvest putrescine treatment on quality and postharvest life of pear cv Spadona. Adv. Hortic. Sci. 31, 11–17 (2017)
D. Martínez-Romero, M. Serrano, A. Carbonell, L. Burgos, F. Riquelme, D. Valero, Effects of postharvest putrescine treatment on extending shelf life and reducing mechanical damage in apricot. J. Food Sci. 67, 1706–1712 (2002)
Acknowledgements
The authors are thankful to Punjab Agricultural University, Ludhiana, India for providing the necessary research facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Singh, V., Jawandha, S.K. & Gill, P.P.S. Effect of exogenous putrescine treatment on internal browning and colour retention of pear fruit. Food Measure 15, 905–913 (2021). https://doi.org/10.1007/s11694-020-00696-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-020-00696-7