Abstract
Nanotechnology applications are increasingly utilized to improve crops. Besides their use as antifungal and antimicrobial agents, silver nanoparticles (AgNPs) are currently exploited to improve seed germination, plant development, and photosynthetic efficiency. In the current study, we evaluated the effect of biosynthesized AgNPs in the seedlings of Zea mays L., Trigonella foenum-graecum L., and Allium cepa L. AgNPs were biosynthesized in the blue gum (Eucalyptus globules) leaves and characterized by UV–Visible spectra, Fourier Transform Infrared (FTIR), and Scanning Electron Microscopic analyses. The biosafety of the AgNPs was tested by cytotoxicity assay, antibacterial activity, and determination of MIC and MBC. The effects of biogenic AgNPs application at different concentrations (25, 50, 75 < and 100 mg L−1) on seed germination, seedling growth, oxidative stress status, and antioxidant enzyme activities were studied. Applications of AgNPs significantly improved seed germination and growth of Z. mays L., T. foenum-graecum L., and A. cepa L. (p < 0.05). Notably, growth was stimulated by an increase in the concentration of AgNPs. Applications of AgNPs also enhanced the activity of antioxidant enzymes, including catalase, peroxidase, and ascorbate peroxidase as well as glutathione and ascorbate contents, whereas the malondialdehyde content was reduced by increasing the concentration of AgNPs. The expression levels of antioxidant enzymes were upregulated in AgNP-treated seedlings compared with those of the control. Our study demonstrated that the application of silver nanoparticles significantly enhanced seed germination and antioxidant machinery and improved the early growth characteristics in both monocot and dicot crops.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Worldwide crop production is facing several challenges, like climatic change, urbanization, atmospheric pollutants, and extreme usage of agrochemicals, pesticides, and fertilizers (Hu et al. 2014; Ditta et al. 2015; Toraih et al. 2019; Hetta et al. 2020). The unprecedented rise in food demand is exaggerating these problems (Vwioko et al. 2017; Abdelaal et al. 2019; Elkelish et al. 2020). Global changes in the agricultural sector at the structural level occur due to the rapid development of technological innovations (Elkeilsh et al. 2019; Soliman et al. 2020b). In this connection, developments in technologies are made that could influence the quality and quantity of the crop and ultimately protect the environment (Elkelish et al. 2019c; Moustafa-Farag et al. 2020b). Such techniques include the application of cost-effective and reduced agricultural inputs with concomitant enhancement in farming production (Elkelish et al. 2019a; Habib et al. 2020). Biostimulants improve plant growth via triggering natural processes that mediate nutrient uptake and tolerance to abiotic stresses (Soliman et al. 2018; Elkelish et al. 2019b). In this era, nanotechnology, one of the most promising fields of science, brings unlimited possibilities of development to many areas, including agriculture and horticulture. Recently, metallic nanoparticles have attracted the interests of the researchers due to their unique physicochemical and biological properties (Prasad et al. 2017; Zahran et al. 2018; Abdel-Azeem et al. 2019; Soliman et al. 2019).
Knowing the essence of nanoparticles–plant interactions is critical in determining plant exposure to nanoparticles and assessing their absorption, distribution, morphological, and physiological changes and its toxicity (Chen and Yada 2011; Saleem et al. 2020). Pradhan and Mailapalli (2017) stated that the productivity and growth of the plant are being enhanced by the newly developed nanomaterials through controlling their metabolism. Among all the noble metal nanoparticles, nanosilver exhibits properties that differ significantly from those of bulk materials, resulting in increased biological activity, chemical reactivity, catalytic behavior, and, most importantly antimicrobial and anti-inflammatory activities (Abdel-hameed et al. 2016; Hetta 2016; Lediga et al. 2018). Several methods for preparing metal nanoparticles have been developed. However, biogenic synthesis is the best choice with advantages over chemical and physical methods as it is time saving, cost-efficient, and eco-friendly. Biogenic synthesis does not require any culture preparation and does not leave hazardous residues to pollute the atmosphere (Kannan et al. 2013; Batiha et al. 2020).
Many reports investigate the effect of silver nanoparticles; however, the formulation of the plant-based silver nanoparticles is still needed further studies (Nowack 2010). The concentration and the size of the synthesized silver AgNPs are a limiting factor in the effect that appeared in the plant, moreover, the dosage and extent of exposure. For instance, barley is totally inhibited from germination after exposure to 10 mg/L AgNPs; and a significant reduction in flax shoot took place under the same concentration (El-Temsah and Joner 2012). Recent studies depict that exposure to optimal levels of AgNPs can increase plant growth (Geisler-Lee et al. 2012; Qian et al. 2013). Many reports showed that antioxidant machinery and the ameliorative effect of ROS elimination are highly provoked after exposure of the plant to the optimum concentration of nanoparticle. These effects are being achieved by controlling the microRNAs expression, which is responsible for the plant in the morphophysiological level (Sigamoney et al. 2016; Siddiqi and Husen 2016; Soliman et al. 2020a).
Africa is regarded as a key center of agricultural production because of the availability of arable land (Manby 2001; El-Esawi et al. 2020; Habib et al. 2020). Several crops need more attention to enhance gross production in order to face the massive population growth in Africa. These crops include maize, onion, and fenugreek. Maize, commonly known as corn, was initially domesticated approximately 5000–10,000 years ago in Africa and is mainly among the three most critical grains across the world (Adiaha 2017). For instance, the Onion (Allium cepa L.) is an essential and prevalent species that expanded all over the world (Pavlović et al. 2016). It is actually among the list of 15 most commonly grown vegetables in the world (Pavlović et al. 2016). In comparison, fenugreek (Trigonella foenum-graecum L.) is used medicinally in different countries and is a source of many potent drugs (Abdel-Daim et al. 2015).
The impact of nanoparticles in the agriculture sector is still unclear. Therefore, in the present study, we used monocot crops, like maize and onion, and the dicot crops, like fenugreek, to test their response to the biogenic silver nanoparticles. Silver nanoparticles were biosynthesized and characterized using the blue gum (Eucalyptus globules) leaf extract and were then evaluated for their biosafety and potential effects on seed germination, seedling growth, and antioxidant systems in the above crops.
Materials and Methods
Plant materials
Fresh leaves of blue gum (Eucalyptus globules) were collected from the Botanical garden of the Faculty of Agriculture, Suez Canal University, Ismailia, Egypt, and seeds of Zea mays L. cv. Giza 2, Trigonella foenum-graecum L. cv. Giza-2, and Allium cepa L. cv. Giza-6 were purchased from the Agriculture Research Centre, Giza, Egypt.
Biogenic synthesis of silver nanoparticles (AgNPs)
Preparation of plant extract
Fresh leaves of E. globules were collected and cleaned thoroughly with deionized water and cut into small pieces. 10 g finely cut pieces were boiled in 100 mL deionized water for 15 min at 80 °C. After cooling, the mixture was filtered through Whatman No. 1 filter paper (Oakland, California, USA) and the filtrate was stored at 4 °C until used for the next stage.
Synthesis of silver nanoparticles
One millimolar silver nitrate was prepared and used for the synthesis of silver nanoparticles. 10 mL, of E. globules leaf extract, was mixed with 90 mL of AgNO3 solution and conducted under dark conditions at room temperature for 1 day. The formation of AgNPs was observed as a conversion in color from pale green to brown. The AgNPs obtained by E. globules leaf extract was centrifuged for 25 min at 13,000 rpm and subsequently dispersed in sterile distilled water to eliminate any uncoordinated biological materials. The AgNPs extract was stored at 4 °C until further analysis.
Characterization of AgNPs
UV–visible spectroscopy
The optical absorbance of the synthesized silver nanoparticles was determined using a UV–visible spectrophotometer (UV-1601PC; Shimadzu, Tokyo, Japan) between the wavelengths of 300 and 700 nm. A sample volume of 0.1 mL was diluted with 2 mL of deionized water in the cuvette. The reduction of pure Ag+ ions was monitored and measured using a UV-1700 spectrophotometer.
Scanning Electron Microscopic (SEM) Analysis
Scanning electron microscopic (SEM) analysis was performed using an SEM model JEOL JSM-840 electron probe with a microanalyzer attached to an EDX unit with acceleration voltage 30 kV, Magnification 10 × to 400 × and resolution for w. (3.5 mm). Thin films of synthesized and stabilized AgNPs were prepared on a carbon-coated copper grid by dropping only a minimal amount of the sample on the grid, and the sample was analyzed for morphology and size of the silver nanoparticles.
Fourier transform infrared (FTIR)
Fourier transform infrared (FTIR) spectral measurements were performed to identify various potential biomolecules in the E. globulus leaf extract with green-biosynthesized silver nanoparticles. FTIR spectral measurements were performed using a Shimadzu infrared FT-IR system model 8300 in the band from 500 to 4000 cm−1.
Cytotoxicity assay (MTT) against human cancer cell lines
To evaluate the toxicity effect of AgNPs on the viability of cancer cell lines (HepG2 and MCF7 cell lines) using MTT assay, as described by Mosmann (1983), cells were exposed to serial concentrations of AgNPs (10, 15 and 20 µg/mL) compared with 5-fluorouracil (5-Fu), then incubated at 37 °C in the incubator for 72 h. After incubation, 20 µL of MTT solution incubated for 3 h. The effective anticancer activity of the extract was investigated by multi-model plate reader (BioTek, Germany) at 570 nm.
Antibacterial activity and determination of MIC and MBC
The antibacterial activity of silver nitrate and silver nanoparticles (25, 50, 75, 100 µg/mL) were evaluated against Escherichia coli (ATCC 8739), Bacillus subtilis (ATCC 6633), Staphylococcus aureus (DSMZ 3463), and Pseudomonas aeruginosa by Agar well diffusion method according to Bauer et al. (1966). The minimum inhibitory concentration (MIC) of prepared AgNO3 and AgNPs were determined by a serial twofold dilution of test samples with different concentrations (25, 50, 75, and 100 µg/mL) in autoclaved distilled water (Andrews 2001). However, the minimum bactericidal concentration (MBC) was determined following Ayala-Núñez et al. (2009).
Experimental conditions for the effect of AgNPs
Suspensions of AgNPs (25, 50, 75, and 100 mg L−1) were prepared daily with deionized water and dispersed with a sonicator (JL-360; Shanghai, China) for 20 min. Healthy and uniform-sized grains of Z. mays, T. foenum-graecum, and A. cepa were used in this study. Sterilized seeds were soaked in silver nanoparticles at concentrations of 25, 50, 75, and 100 mg L−1 for 2 h, while control was immersed in distilled water for 2 h.
Germination and growth parameters
Seed germination
Primed seeds were sown in Petri dishes on filter paper wetted with 10 mL of distilled water. Fifteen grains of maize and 20 seeds of T. foenum-graecum and A. cepa were in each petri dish. The seeds were incubated in a complete randomized block design with four replicates for each treatment in a germinator with an average temperature of 25/15 ± 1 °C for 8/16 h in darkness and light, respectively. Ten days after treatment, seedlings were harvested. Some leaves were frozen in liquid nitrogen and stored at − 80 °C for analysis of the activity of selected antioxidant enzymes and biochemical assays.
Germination and growth parameters
A number of germinated seeds were counted from the 1st day after germination when roots appeared until the 9th day. Germination data were recorded on triplicate petri dish and the germination parameters were presented as a mean or three replicas. The emergence of the radical was considered as an indicator of germination, and germination percentage was determined according to the method recommended by ISTA (1985). Vigor index was determined following the method of Abdul-Baki and Anderson (1973), and seedling vigor index (SVI) was calculated with the following formula:
Shoot length (SL), root length (RL), and seedling fresh weight (SFW) were measured at the end of the experiment.
Biochemical assays
Plant preparation and extraction
0.5 g sample of fresh tissue was homogenized in an adequate amount of deionized water using a sonicator (JL-360; Shanghai, China). The homogenate was centrifuged, and supernatant preserved at − 80 °C until used for the biochemical analyses. Protein concentration was determined, according to Bradford (1976).
Lipid peroxidation assay
Malondialdehyde (MDA) was analyzed by measuring the production of thiobarbituric acid reactive substances (TBARS), according to Ohkawa et al. (1979), using a TBARS assay kit (Catalog no. 10009055; Cayman, USA).
Determination of non-enzymatic antioxidants
Glutathione (GSH) and ascorbic acid (AsA) were determined in plant tissues using kits (Catalog no. NWK-GSH01 and NWK-Vit C01, respectively) purchased from Northwest Life Science Specialties (NWLSS), Vancouver, Canada. GSH was assayed, according to Beutler et al. (1963) and AsA, according to McHenry and Graham (1935).
Assay of antioxidant enzyme activities
Activities of catalase (CAT) and peroxidase were determined according to Aebi (1984) and Gregory (1966), respectively. Ascorbic peroxidase (APX) activity was assayed following the method of Nakano and Asada (1981). For taking absorbance UV–visible spectrophotometer (UV-1601PC; Shimadzu, Japan) was employed.
Molecular analysis of antioxidant genes
Total RNA was extracted from fresh seedlings on the 9th day using RNA Reagent (Tri-Pure, Fermentas) as described by Xu et al. (2010). The RNA samples were digested with DNase I, and the first strand of cDNA was synthesized from 5 μg of total RNA from each sample using M-MLV reverse transcriptase (Toyobo, Osaka, Japan). The specific primer sequences of antioxidant enzyme genes SOD, CAT, and APX2 for each plant were designed, and the internal standard was β-actin gene. The qRT-PCR was conducted using a Rotor-Gene thermocycler and SYBR Green I Master Mix according to the manufacturer’s protocol, under the following PCR conditions: 95 °C for 1 min, followed by 40 cycles at 95 °C for 15 s, 60 °C for 15 s, and 72 °C for 20 s. The relative expression of the antioxidant enzyme genes was calculated using the 2−ΔΔCT comparative CT method (Livak and Schmittgen 2001), and the results are presented as n-fold differences in expression.
Statistical analyses
Data presented are mean of three replicates. Statistical analyses were performed using the IBM-SPSS ver. 23 statistical software package for Mac OS. Differences between AgNPs treatments and among species were assessed using two-way analysis of variance (ANOVA). Duncan’s multiple range tests (DMRTs) (Duncan 1955) were performed to compare means of different treatments. Spearman’s rank correlation was also shown to evaluate the correlation between increasing concentrations of AgNPs and manifold measured germination, growth, and biochemical parameters.
Results
Silver nanoparticle characterization
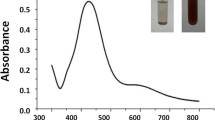
The green synthesis of AgNPs that were biologically synthesized using fresh leaves of Eucalyptus globulus from a silver nitrate solution (AgNO3) was elucidated. Mixing and incubating the solution of silver nitrate with E. globulus leaf extract at room temperature for a whole day, the extract changed from colorless to brown to dark brown (Fig. 1). The PH was 8.12 for AgNPs. The AgNPs were characterized by their surface area, shape, disparity, and size.
Characterization of green-synthesized AgNPs from E. globulus leaf extract (a) UV–Vis absorption spectra, b–e SEM micrograph of silver nanoparticles from fresh leaves of E. globules; µm is the measurement unit of the apparatus used, f FTIR spectral analysis of green-synthesized AgNPs showing the biosynthesized silver nanoparticles (black line) and E. globulus leaf extract (blue line)
UV–Visible spectra
The nanoparticles of the noble metal silver exhibited unique optical properties because of their surface plasmon resonance. The establishment of AgNPs was settled based on the change in UV–Visible absorbance peak associated with the surface plasmon resonance; UV–Visible spectroscopic analysis conducted as a factor at room temperature of the reaction time (Fig. 1a). The analysis showed a peak absorbance at approximately 450 nm (Fig. 1a) that is specific for AgNPs. The UV–Vis absorption band in the present visible light reaction (420–450 nm) was an indication of the surface plasmon resonance of the AgNPs.
SEM
SEM analysis of AgNPs was present and evenly distributed throughout the biomass of the AgNO3-incubated culture. The aspherical shape of silver nanoparticles with various particles (Fig. 1b–e). AgNPs ranged in size from 0.066 to 0.56 µm. Scanning electron microscopy (SEM) micrographs of silver nanoparticles at different magnification are presented in Fig. 1b–e for the detailed characterization of nanoparticles.
FTIR
To categorize different potential biomolecules in the E. globulus leaf extract, the FTIR spectral measurements were performed with green-biosynthesized silver nanoparticles (Fig. 1f). The Spectral data presented the current molecular environment of organic silver nanoparticles. FTIR analysis for the green-synthesized AgNPs of our study showed different intense bands at 3757.46, 3738.17, 3315.74, 2910.68, 1637.62, 1637.62, 1394.58, 1274.99, 1055.10, and 545.87 cm−1 (Fig. 1f). Figure 1f shows the FTIR spectrum revealing possible biomolecules in E. globulus leaf extract, reducing silver ions and interacting with AgNPs. A significant difference was observed among peaks (t = 5.255; p < 0.001) and among peak intensities (t = 7.269; p < 0.001). The significant difference observed between IR positions in the extract of E. globulus, and those of green-synthesized AgNPs was due to the reduction process.
The IR spectra of AgNPs from E. globulus were consistent with the same bands in the same regions with shifts varying from 10 to 100 nm (cm−1), which indicated good interactions of OH, C=O, and NH in the extract of E. globulus leaves.
A peak was observed at 3414 cm−1and identified as hydroxyl groups (Junior et al. 2013; Sharaf et al. 2013). A band of stretching vibration of C = C bonds appears at 1645 cm−1(Barud et al. 2013) due to aromatic fragments (Franca et al. 2014); the stretching vibration of C=O and of C=C due to flavonoids and amino acids; the asymmetric bending vibration of N–H due to C=O stretching vibration (Sharaf et al. 2013); or the stretching vibration of C=O due to lipids or flavonoids (Junior et al. 2013) (Fig. 1f). The band at 1251 cm−1 was due to vibration of the C–O bond of polyols. A band at 1066 cm−1 was identified with secondary alcohols (Sharaf et al. 2013) (Fig. 1f).
Cytotoxicity assay (MTT) against human cancer cell lines
MTT cytotoxicity assay to test cell viability (%) of HepG-2 human liver cancer and MCF-7 human breast cancer cell line post-treatment with AgNO3 and biogenic AgNPs. Figure 2a, b shows the percentage viability of the cells treated with AgNPs. The assay was done with several concentrations of silver nitrate (AgNO3) and biogenic Nano silver (AgNPs) (10, 15, and 20 µg/mL) compared with 5-fluorouracil (5-Fu) as a positive control. The results showed that the percentage of cancer inhibition of HepG-2 and MCF-7 relies on the concentration of the AgNPs used. The greater inhibition in HepG-2 was recorded at the highest concentration (20 µg/mL) (p < 0.05; Fig. 2a). However, the MCF-7 showed the highest inhibition percentage at the moderate concentration of AgNPs (15 µg/mL) (p < 0.05; Fig. 2b). On the other hand, the silver nitrate showed no significant inhibition percentage in both HepG-2 and MCF-7 cell line compared to the untreated cell.
Percentage of cell viability determined by MTT cytotoxicity exposed to silver nitrate (AgNO3) and biogenic silver nanoparticles AgNPs (10,15, and 20 µg/mL) a in HepG-2 cells, b in MCF-7 cells. Data represented are the mean of three replicates ± standard deviations. Variations in viability between different AgNO3 and AgNPs concentrations were evaluated by univariate analyses followed by post hoc analysis. Means with the same letters are not significantly different according to Duncan’s multiple range tests (DMRTs)
Antibacterial activity, and MIC and MBC
The obtained results for antibacterial activity of silver nitrate (AgNO3) and biogenic nanosilver (AgNPs) synthesized by natural biogenic extract were investigated against E. coli, B. subtilis, S. aureus, and P. aeruginosa. The diameter of inhibition zones (mm) around each well is represented in Fig. 3. Both silver nitrate and biogenic nanosilver (AgNPs) showed a potent antibacterial activity. They significantly (p < 0.05) growth inhibition of the E. coli, B. subtilis, S. aureus, and P. aeruginosa especially in concentration of 100 µg/mL (Fig. 3).
The comparative results of antibacterial activities of silver nitrate and biogenic AgNPs (25 µg/mL, 50 µg/mL, 75 µg/mL, and 100 µg/mL) by agar well diffusion technique. Ampicillin (Am) was used as positive controls. Data represented as mean of triplicate. Means with different letters are significantly different according to Smarts at p < 0.05
The growth inhibition effect of silver nitrate (AgNO3) and biogenic nanosilver (AgNPs) against bacteria was measured also by MIC and MBC (Fig. 4a, b). The AgNPs showed a lower value in both the MIC and MBC than AgNO3. The lowest values of MIC and MBC were observed with AgNO3 and AgNPs in Escherichia coli and Pseudomonas aeruginosa, respectively. Thus, the biogenic silver nanoparticles were found to be a more potent bactericidal agent in terms of size.
Growth and germination
Various concentrations of AgNPs affect growth performance and germination of the three crop plants (T. foenum-graecum, Z. mays and A. cepa), as shown in Table 1. The growth and germination of the three crop plants significantly increased (p < 0.001***) by increasing concentrations of AgNPs (Table 1). The three species significantly showed different responses to AgNPs revealed by two-way ANOVA (p < 0.001***). The 2-way ANOVA indicated that the influence of different AgNPs concentrations was significant for all germination and growth parameters studied (Table 1). Germination and growth parameters (germination %, seedling vigor index, shoot length, root length, and seedling fresh weight) increased significantly (p < 0.001) with increased AgNPs concentration; however, root fresh weight of Z. mays showed a different trend with AgNPs, as revealed by Spearman’s correlation. Root fresh weight showed a non-significant decrease with increasing AgNPs concentrations.
Lipid peroxidation (oxidative damage)
Oxidative stress intensity in T. foenum-graecum, Z. mays, and A. cepa under the effect of different AgNPs concentrations was assessed as a measure of membrane lipid peroxidation determined in terms of malondialdehyde content (Fig. 5a). Data of membrane lipid peroxidation revealed that AgNPs significantly decreased the oxidative stress damage on T. foenum-graecum, Z. mays, and A. cepa (p < 0.0001) (Fig. 5a). The membrane lipid peroxidation in the untreated controls showed an average of 3.94 ± 0.002, 2.30 ± 0.0017, and 4.33 ± 0.0011 μmol g−1 FW; however, after treatment with 100 mM AgNPs, membrane lipid peroxidation decreased significantly (p < 0.001***) to 3.62 ± 0.001, 2.14 ± 0.0011, and 3.25 ± 0.0023 μmol g−1 FW in T. foenum-graecum, Z. mays, and A. cepa, respectively (Fig. 5a).
a Membrane lipid peroxidation as MDA (μmol g−1 FW) and b glutathione contents (μmol g−1 FW) in three plant species, T. foenum-graecum, Z. mays, and A. cepa, under the effect of different concentrations of green-synthesized AgNPs (25, 50, 75, and 100 mM). Data represented are the mean of three replicates ± standard deviations. Variations in MDA between different species and different AgNP concentrations were evaluated by univariate analyses followed by post hoc analysis. Means with the same letters are not significantly different according to Duncan’s multiple range tests (DMRTs)
Antioxidant enzyme activity assays
In general, for all tested seedlings, GSH and AsA increased significantly (p < 0.001) with the increase in the concentration of biosynthesized AgNPs from 0 to 100 mM. AgNPs application resulted in a significant reduction in MDA levels relative to control levels. The maximum increase in GSH and AsA was 18.45 ± 0.002 and 89.80 ± 0.002 μmol g−1 FW, respectively, and was recorded in T. foenum-graecum (Figs. 5b, 6a). By contrast, the lowermost intensification was found in Z. mays and A. cepa seedlings.
a Ascorbic acid content (ASA; mg g−1 FW), b ascorbic acid peroxidase (APX; unit g−1 FW min−1), c catalase activities (CAT; unit g−1 FW min−1) and d peroxidase activities (POD; unit g−1 FW min−1) in three plant species, T. foenum-graecum, Z. mays, and A. cepa, under the effect of different concentrations of green-synthesized AgNPs (25, 50, 75, and 100 mM). Data represented are the mean of three replicates ± standard deviations. Means with the same letters are not significantly different according to Duncan’s multiple range tests (DMRTs)
Cellular glutathione levels in untreated control groups averaged 16.94 ± 0.012, 1.94 ± 0.0017, and 3.85 ± 0.0023 μmol g−1 FW; however, after treatment with 100 mM AgNPs, levels increased significantly (p < 0.001) to 20.76 ± 0.001, 4.03 ± 0.0011, and 5.89 ± 0.002 μmol g−1 FW in T. foenum-graecum, Z. mays, and A. cepa, respectively (Fig. 5b). Moreover, the ascorbic acid levels in untreated control groups averaged 70.08 ± 0.025, 2.31 ± 0.0023, and 4.035 ± 0.002 mol g−1 FW; however, after treatment with 100 mM AgNPs, levels increased significantly (p < 0.001) to 89.80 ± 0.002, 3.43 ± 0.0017, and 5.95 ± 0.0035 mol g−1 FW in T. foenum-graecum, Z. mays, and A. cepa, respectively (Fig. 6a).
The exogenous AgNPs affected the activities of antioxidant enzymes in A. cepa, T. foenum-graecum and Z. mays seedlings (Fig. 6b–d). AgNPs induced a significant increase in CAT, POD, and APX activities with increased concentrations of AgNPs over the control. A significant improvement over controls was observed for all antioxidative enzymes in all treatments except APX in T. foenum-graecum. In the case of APX, compared to control, activity decreased at 25 and 50 mg/L AgNPs and increased to 100 mg/L.
Expression of antioxidant genes
The transcript levels of antioxidant (SOD, CAT, and APX2) genes analyzed using quantitative real-time PCR for T. foenum-graecum, Z. mays, and A. cepa seedlings exhibited an upregulation after 9 days of exposure to 100 mg/L AgNPs. The relative expression was calculated according to the fold change using the R =2−ΔΔCT equation (Figs. 7a–c). For the relative expression level of the SOD gene, fold change due to treatment was 22-, 14-, and 2-fold for SOD in T. foenum-graecum, Z. mays, and A. cepa seedlings, respectively, over the control gene (β-actin) (Fig. 7a–c). However, for CAT the observed fold change was a 0.2-, 15-, and 5-fold in T. foenum-graecum, Z. mays, and A. cepa, respectively, over the control (Fig. 7b). Additionally, the fold change in upregulation for the APX2 gene was 38-, 14-, and 2-fold in T. foenum-graecum, Z. mays, and A. cepa, respectively (Fig. 7c).
Fold change of gene expression of a SOD, b CAT, and c APX2 genes using quantitative real-time PCR for T. foenum-graecum, Z. mays, and A. cepa seedlings after 9 days of exposure to 100 mg/L AgNPs and was calculated according to the fold change using the R = 2−ΔΔCT equation. Data represented are the mean of three replicates ± standard deviation. Means with the same letters are not significantly different according to Duncan’s multiple range tests (DMRTs)
Discussion
Crops are affected by many factors that lead to a significant decline in their potential productivity. Hence, a need for introducing novel technologies for agricultural improvement has emerged. Among these, nanotechnology has become fundamental and one of the emerging tools in agronomy for enhancing the production by suppressing the ill factors (Prasad et al. 2017; Moustafa-Farag et al. 2020a; Rashad et al. 2020). However, numerous reports showed the adverse physiological effects of nano-metals in various crops. For instance, a high AgNP level reduces multiple plant growth based on the penetration of nanoparticles and transportation into plant tissues (Nair and Chung 2015; Rastogi et al. 2017; Çekiç et al. 2017). However, utilizing plants for the synthesis of silver nanoparticles is the attainable and safe approach also provides a full variability of metabolites that helps in the reduction of silver nitrate (Logeswari et al. 2015; Ahmed et al. 2016). The novelty in our manuscript is the usage of leaf extract of E. globulus as a reducing agent for the biogenic synthesis of AgNPs. The surface plasmon is responsible for the rapid conversion of color to yellowish-brown and confirms the completion of the reaction and formation of colloidal AgNPs (Zheng et al. 2005; Prasad et al. 2017; Domingo et al. 2019). We found that 427 nm is the broad absorption band for silver nanoparticles, which implied that the synthesized particles were discrete correctly and were free of any aggregation (Singhal et al. 2011).
Moreover, the concentration of surface-active molecules, like flavonoids and terpenoids, in E. globulus was high, making the reduction and stabilization of AgNPs (Dubey et al. 2009; Ayepola 2008). Furthermore, the spectra of the FTIR released details on the present molecular environment of the silver nanoparticles organic molecules. The results of FTIR analysis for the green-synthesized AgNPs of our study showed different intense bands that were identified in accordance with Gutierrez-Goncalves and Marcucci (2009), and Junior et al. (2013). To evaluate the safety of biosynthesized silver nanoparticles in comparison with silver nitrate, the MTT cytotoxicity and antibacterial activity showed a significant inhibition for cell line and potent antibacterial activity. The range of biosafety of the biogenic silver nanoparticles is highly acceptable in comparison with other similar synthesis methods. These result in accordance to with Mukherjee and Mahapatra (2010) that Ag nanoparticle triggers the level of oxidative stress, and intensively disrupt the biological membrane, that consequently triggering the apoptosis (Hashim et al. 2020). Furthermore, AgNPs resulted in high expression of ROS, which activate mitochondrial apoptotic pathways (Zhang et al. 2014; Zamin et al. 2019). Many reports interpreting the potent antibacterial efficiency of AgNPs. Dorau et al. (2004) the AgNPs have been assumed to be crucial for lysis of bacterial cell through inhibition of cell-bound membrane enzymes. This has also been shown to disrupt the respiratory and cell chain and/or increase the rate of formation of ROS, including hydroxyl and superoxide (Rai et al. 2009; Duncan 2011; Durán et al. 2016).
Treatment of the Z. mays, T. foenum-graecum, and A. cepa with the biosynthesized AgNPs resulted in different responses regarding the germination and seedling growth, biochemical attributes, and gene expression. It has been reported that the concentration and the size of nanoparticles are highly limiting factors considering the effect that appeared upon the tested plant (Ma et al. 2010; Thuesombat et al. 2014). Such impacts of AgNPs application are consistent with the findings of Parveen and Rao (2015), who reported a significantly increased rate of germination in B. ovalifoliolata seeds treated with 50 mg/mL AgNPs. Recent research investigations have reported enhancement in the growth performance of soybean exposed to flooding by exogenous treatment with nanoparticles (Judy et al. 2011; Hossain et al. 2015). Zheng et al. (2005) showed increased water absorption by seeds, nitrate reductase, and antioxidant activity (Lu et al. 2002). In addition, Arora et al. (2012) reported significant improvement in Brassica due to a synergistic effect with the phytohormones after the treatment with nanoparticles. Moreover, many research reports have reported improvement in plant growth owing to the nanoparticles application, which enhanced the activities of antioxidant machinery (guaiacol peroxidase, APX, and SOD) (Lei et al. 2008; Jasim et al. 2017). However, specific contradictory reports depicting inhibition of germination in Vicia faba (Ahmed et al. 2013) and in Arabidopsis thaliana (Geisler-Lee et al. 2012) might been attributed to the capacity of nanoparticles aggregated on the surfaces of the seedling root. The excessive aggregation of NPs, including those of AgNPs brings a reduction in water availability and hydraulic conductivity, delimited plant development, and decreased transpiration (Harris and Bali 2008; Yin et al. 2012; Vannini et al. 2014; Nair and Chung 2015). The initial contact or association may be triggered by electrostatic adsorption, mechanical adhesion, or hydrophobic affinity of particular NPs, and the ROS stress exerted by AgNPs is increased. (Yin et al. 2012). Moreover, at high concentrations, AgNPs induced an inhibitory effect in cucumber and Oryza sativa (Zheng et al. 2005).
More common growth parameters and physiological endpoints can be more informative about the interactions between NPs and crop types (Thiruvengadam et al. 2018). The most common physiological parameters are antioxidants enzymes, which are well known for scavenging ROS through their increased activity under various stresses (Velmurugan et al. 2018; Baskar et al. 2018; Alhaithloul et al. 2019; Moustafa-Farag et al. 2020a). ROS has many fundamental roles in gene expression, especially those genes regulating several key developmental processes, like cell cycle, response to stresses, and systemic signaling. However, their overproduction leads to oxidative stress through the collapse of the antioxidant defense system of cells by inhibiting SOD, CAT, GPX, and GR activities concomitant with the reduction of GSH and ASA levels (Chung et al. 2016). Additionally, ROS induced lipid peroxidative product, MDA, which is a polyunsaturated fatty acid produced during the decomposition of bio-membranes and a primary indicator of oxidative injury (Demiral and Turkan 2005; El-Esawi et al. 2018). In the present study, MDA concentrations were measured as oxidative stress indicators in the leaves, and the MDA content significantly decreased (p < 0.05) when AgNPs were applied to a range of 75 mg/L (Fig. 4). In the AgNP-treated plants, reduction of ROS and MDA levels may be attributed to upregulated antioxidative mechanisms preventing the electron leakage from cells (Hatami and Ghorbanpour 2013). The functioning of the antioxidant machinery in the AgNP-treated plant cells determines the amount of ROS being produced and accumulated in them, which was much evident (Çekiç et al. 2017). High concentrations of AgNPs severely damage the cell membrane and significantly increase the level of lipid peroxidation (Ghanati and Bakhtiarian 2013; El-Esawi 2017), and such concentration-dependent oxidative damage by AgNPs has been suggested to indicate their threshold level for membrane protection (Heidari et al. 2018; Moustafa-Farag et al. 2020b).
In our results, CAT, POD, and APX activities increased with increasing AgNPs concentrations up to a certain level in the three crops (Figs. 6b, 7a, b). We found that a moderate level of AgNPs (75 mg/l) imparted a defensive strategy for improving the activities of ROS scavenging enzymes. Greater activities of CAT and POD reduce the ROS production, decreasing the chances of oxidative stress and the nanotoxicity in plants (Krishnaraj et al. 2012; El-Esawi and Alayafi 2019). Earlier upregulation of the antioxidant system due to AgNPs has been reported to impart quick elimination of H2O2, hence leading to growth maintenance (Gopinath et al. 2012; Priyadarshini et al. 2013). Antioxidant enzyme activities in the three crops reached the maximal value in AgNP-treated plants. Our results are in consonance with Lei et al. (2008) and Farrag (2016), who also demonstrated increased activities of CAT, SOD, AXP, and POD due to AgNPs and TiO2 treatment. Recently, Hossain et al. (2015) confirm that ZnONPs significantly increased the activity of antioxidant enzymes in treated faba bean seedlings. Yasur and Rani (2013) reported that the SOD activity of L. esculentum and R. communis increased due to AgNP’s treatment. Moreover, wheat seedlings treated with biogenic AgNPs exhibited an increment in the actions of CAT and POD (Hossain et al. 2015).
In the present experiment, the concentrations of AsA and GSH increased significantly (p < 0.05) in all the three crops (Figs. 5, 6a). The high levels of endogenous AsA in treated plants were important in order to compensate for oxidative stress by promoting a metabolic process with the enzymatic antioxidant system. (Manke et al. 2013; Dakal et al. 2016). The applications of CeO2 and In2O3 NPs have been reported to induce the gene expression responsible for the regulation of sulfur assimilation and the biosynthesis of GSH in Arabidopsis (Vwioko et al. 2017; Yang et al. 2017; Elsayed et al. 2018). Transcriptomic responses to AgNPs include the enhancement of gene regulation, including mitigation of oxidative stress (Begum et al.,2011).
The real-time PCR data indicated that exposure of T. foenum-graecum, Z. mays, and A. cepa seedlings to AgNPs led to significant (p < 0.05) upregulation of the expression of antioxidant genes (SOD, CATc, and APX2) compared with that of control plants. Increased expression levels of antioxidant genes might have induced the tolerance to oxidative stress by mediating efficient elimination of toxic ROS. The highest fold change due to AgNPs treatment was for APX2 gene, followed by CAT gene, and the lowest fold change was for SOD gene. Similar to the results of this study, aquaporin expression (NtPIP1) gene and marker genes were significantly upruled in Nicotiana xanthi cells with exposure to MWCNTs, and there was evidence that the increase in cell growth is linked to water absorption and division (Khodakovskaya et al. 2012). The action of biogenic silver nanoparticles on T. foenum-graecum, Z. mays, and A. cepa are summarized in Fig. 8.
Conclusions
The results of the current study reveal that biogenic silver nanoparticle is a promising within a different type of nanoparticle synthesis. The biosafety of the synthesized nanoparticles is highly suitable for large-scale applications. The exogenous treatment with AgNPs increased seed germination and improved early growth characteristics. The T. foenum-graecum, Z. mays, and A. cepa responded to the biosynthesized AgNPs with higher seed germination percentage than that of the controls. The current study determined that the best level of AgNPs in improving the overall growth profile which is 75 mL/L AgNPs, causing maximum stimulation of the antioxidant enzyme system, compared with the other responses at other concentrations. The response of the dicot crop (T. foenum-graecum) was better than that of the two monocot crops (Zea mays and Allium cepa). Our findings confirm that AgNPs may be introduced effectively to defend crops against environmental stresses and increase crop growth during the early stages.
Author contribution statement
Conceptualization, MS, and AE; Formal analysis, AA, AE, and SQ; Investigation, AA, AE, and ME; Methodology, MS, HA, AA and ME; Molecular Analysis, SQ and AE; Supervision, MS, AE and SQ; Writing–original draft, AE, AA and ME; Writing–review & editing, SQ, HA, MS, AA, AE and ME.
References
Abdelaal KA, EL-Maghraby LM, Elansary H et al (2019) Treatment of sweet pepper with stress tolerance-inducing compounds alleviates salinity stress oxidative damage by mediating the physio-biochemical activities and antioxidant systems. Agronomy 10:26. https://doi.org/10.3390/agronomy10010026
Abdel-Azeem A, Nada A, O’Donovan A et al (2019) Mycogenic silver nanoparticles from endophytic trichoderma atroviride with antimicrobial activity. J Renew Mater 7:171–185. https://doi.org/10.32604/jrm.2020.08960
Abdel-Daim MM, Abd Eldaim MA, Hassan AGA (2015) Trigonella foenum-graecum ameliorates acrylamide-induced toxicity in rats: roles of oxidative stress, proinflammatory cytokines, and DNA damage. Biochem Cell Biol Biochim Biol Cell 93:192–198. https://doi.org/10.1139/bcb-2014-0122
Abdel-hameed EA, Rouster SD, Ji H et al (2016) Evaluating the role of cellular immune responses in the emergence of HCV NS3 resistance mutations during protease inhibitor therapy. Viral Immunol 29:252–258. https://doi.org/10.1089/vim.2015.0093
Abdul-Baki AA, Anderson JD (1973) Vigor determination in soybean seed by multiple criteria 1. Crop Sci 13:630–633. https://doi.org/10.2135/cropsci1973.0011183X001300060013x
Adiaha MS (2017) Economic value of Maize (Zea mays L.) in Nigeria and its impacts on the global food production. Int J Sci World 6:27. https://doi.org/10.14419/ijsw.v6i1.8771
Aebi H (1984) [13] Catalase in vitro. Methods in enzymology. Academic Press, New York, pp 121–126
Ahmed E, Azeem A, Elsayed B (2013) Phytotoxicity of silver nanoparticles on Vicia faba seedlings. N Y Sci J 6:148–156
Ahmed S, Ahmad M, Swami BL, Ikram S (2016) A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J Adv Res 7:17–28. https://doi.org/10.1016/j.jare.2015.02.007
Alhaithloul HA, Soliman MH, Ameta KL et al (2019) Changes in ecophysiology, osmolytes, and secondary metabolites of the medicinal plants of Mentha piperita and Catharanthus roseus subjected to drought and heat stress. Biomolecules 10:43. https://doi.org/10.3390/biom10010043
Andrews JM (2001) Determination of minimum inhibitory concentrations. J Antimicrob Chemother 48:5–16. https://doi.org/10.1093/jac/48.suppl_1.5
Arora S, Sharma P, Kumar S et al (2012) Gold-nanoparticle induced enhancement in growth and seed yield of Brassica juncea. Plant Growth Regul 66:303–310. https://doi.org/10.1007/s10725-011-9649-z
Ayala-Núñez NV, Lara Villegas HH, del Carmen Ixtepan Turrent L, Rodríguez Padilla C (2009) Silver nanoparticles toxicity and bactericidal effect against methicillin-resistant Staphylococcus aureus: nanoscale does matter. NanoBiotechnology 5:2–9. https://doi.org/10.1007/s12030-009-9029-1
Ayepola Y (2008) The antibacterial activity of leaf extracts of Eucalyptus camaldulensis (Myrtaceae). J Appl Sci Res 4(11):1410–1413
Barud HDS, Júnior DA, Miguel A, et al (2013) Antimicrobial Brazilian Propolis (EPP-AF) Containing biocellulose membranes as promising biomaterial for skin wound healing. In: Evid. Based Complement. Alternat. Med. https://www.hindawi.com/journals/ecam/2013/703024/. Accessed 8 Apr 2018
Baskar V, Nayeem S, Kuppuraj SP et al (2018) Assessment of the effects of metal oxide nanoparticles on the growth, physiology and metabolic responses in in vitro grown eggplant (Solanum melongena). 3 Biotech 8:362. https://doi.org/10.1007/s13205-018-1386-9
Batiha GE-S, Magdy Beshbishy A, Adeyemi OS et al (2020) Phytochemical screening and antiprotozoal effects of the methanolic Berberis vulgaris and acetonic Rhus coriaria extracts. Molecules 25:550. https://doi.org/10.3390/molecules25030550
Bauer AW, Kirby WM, Sherris JC, Turck M (1966) Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol 45:493–496
Begum P, Ikhtiari R, Fugetsu B (2011) Graphene phytotoxicity in the seedling stage of cabbage, tomato, red spinach, and lettuce. Carbon 49:3907–3919
Beutler E, Duron O, Kelly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Çekiç FÖ, Ekinci S, Inal MS, Ünal D (2017) Silver nanoparticles induced genotoxicity and oxidative stress in tomato plants. Turk J Biol 41:700–707. https://doi.org/10.3906/biy-1608-36
Chen H, Yada R (2011) Nanotechnologies in agriculture: new tools for sustainable development. Trends Food Sci Technol 22:585–594. https://doi.org/10.1016/j.tifs.2011.09.004
Chung I-M, Park I, Seung-Hyun K et al (2016) Plant-Mediated synthesis of silver nanoparticles: their characteristic properties and therapeutic applications. Nanoscale Res Lett. https://doi.org/10.1186/s11671-016-1257-4
Dakal TC, Kumar A, Majumdar RS, Yadav V (2016) Mechanistic basis of antimicrobial actions of silver nanoparticles. Front Microbiol. https://doi.org/10.3389/fmicb.2016.01831
Demiral T, Turkan I (2005) Comparative lipid peroxidation, antioxidant defense systems and proline content in roots of two rice cultivars differing in salt tolerance. Environ Exp Bot 53:247–257. https://doi.org/10.1016/j.envexpbot.2004.03.017
Ditta A, Arshad M, Ibrahim M (2015) Nanoparticles in sustainable agricultural crop production: applications and perspectives. Nanotechnology and plant sciences. Springer, Berlin, pp 55–75
Domingo G, Bracale M, Vannini C (2019) Chapter 8—phytotoxicity of silver nanoparticles to aquatic plants, algae, and microorganisms. In: Tripathi DK, Ahmad P, Sharma S et al (eds) Nanomaterials in plants, algae and microorganisms. Academic Press, New York, pp 143–168
Dorau B, Arango R, Green F (2004) An investigation into the potential of ionic silver as a wood preservative. In: Proc Woodframe Hous Durab Disaster Issues Conf Oct 4–6 2004 Las Vegas Nev USA Madison WI For Prod Soc 2004, pp 133–145
Dubey M, Bhadauria S, Kushwah BS (2009) Green synthesis of nanosilver particles from extract of Eucalyptus hybrida (safeda) leaf. Dig J Nanomater Biostruct 4(3):537–543
Duncan DB (1955) Multiple range and multiple F tests. Biometrics 11:1–42. https://doi.org/10.2307/3001478
Duncan TV (2011) Applications of nanotechnology in food packaging and food safety: barrier materials, antimicrobials and sensors. J Colloid Interface Sci 363:1–24. https://doi.org/10.1016/j.jcis.2011.07.017
Durán N, Nakazato G, Seabra AB (2016) Antimicrobial activity of biogenic silver nanoparticles, and silver chloride nanoparticles: an overview and comments. Appl Microbiol Biotechnol 100:6555–6570. https://doi.org/10.1007/s00253-016-7657-7
El-Esawi MA (2017) SSR analysis of genetic diversity and structure of the germplasm of faba bean (Vicia faba L.). C R Biol 340:474–480. https://doi.org/10.1016/j.crvi.2017.09.008
El-Esawi MA, Alayafi AA (2019) Overexpression of Rice Rab7 Gene improves drought and heat tolerance and increases grain yield in rice (Oryza sativa L.). Genes. https://doi.org/10.3390/genes10010056
El-Esawi MA, Alaraidh IA, Alsahli AA et al (2018) Serratia liquefaciens KM4 improves salt stress tolerance in maize by regulating redox potential, ion homeostasis, leaf gas exchange and stress-related gene expression. Int J Mol Sci. https://doi.org/10.3390/ijms19113310
El-Esawi MA, Elkelish A, Soliman M et al (2020) Serratia marcescens BM1 enhances cadmium stress tolerance and phytoremediation potential of soybean through modulation of osmolytes, leaf gas exchange, antioxidant machinery, and stress-responsive genes expression. Antioxidants 9:43. https://doi.org/10.3390/antiox9010043
Elkeilsh A, Awad YM, Soliman MH et al (2019) Exogenous application of β-sitosterol mediated growth and yield improvement in water-stressed wheat (Triticum aestivum) involves up-regulated antioxidant system. J Plant Res. https://doi.org/10.1007/s10265-019-01143-5
Elkelish A, Alnusaire TS, Soliman MH et al (2019a) Calcium availability regulates antioxidant system, physio-biochemical activities and alleviates salinity stress mediated oxidative damage in soybean seedlings. J Appl Bot Food Qual. https://doi.org/10.5073/JABFQ.2019.092.036
Elkelish AA, Alhaithloul HAS, Qari SH et al (2019b) Pretreatment with Trichoderma harzianum alleviates waterlogging-induced growth alterations in tomato seedlings by modulating physiological, biochemical, and molecular mechanisms. Environ Exp Bot. https://doi.org/10.1016/j.envexpbot.2019.103946
Elkelish AA, MonaH Soliman, Alhaithloul HA, El-Esawi MA (2019c) Selenium protects wheat seedlings against salt stress-mediated oxidative damage by up-regulating antioxidants and osmolytes metabolism. Plant Physiol Biochem. https://doi.org/10.1016/j.plaphy.2019.02.004
Elkelish A, Qari SH, Mazrou YSA et al (2020) Exogenous ascorbic acid induced chilling tolerance in tomato plants through modulating metabolism, osmolytes, antioxidants, and transcriptional regulation of catalase and heat shock proteins. Plants 9:431. https://doi.org/10.3390/plants9040431
Elsayed MA, Othman AM, Hassan MM, Elshafei AM (2018) Optimization of silver nanoparticles biosynthesis mediated by Aspergillus niger NRC1731 through application of statistical methods: enhancement and characterization. 3 Biotech 8:132. https://doi.org/10.1007/s13205-018-1158-6
El-Temsah YS, Joner EJ (2012) Impact of Fe and Ag nanoparticles on seed germination and differences in bioavailability during exposure in aqueous suspension and soil. Environ Toxicol 27:42–49. https://doi.org/10.1002/tox.20610
Farrag M (2016) Monodisperse and polydisperse platinum nanoclusters supported over TiO2 anatase as catalysts for catalytic oxidation of styrene. J Mol Catal Chem 413:67–76. https://doi.org/10.1016/j.molcata.2015.12.011
Franca JR, De Luca MP, Ribeiro TG et al (2014) Propolis-based chitosan varnish: drug delivery, controlled release and antimicrobial activity against oral pathogen bacteria. BMC Complement Altern Med 14:478. https://doi.org/10.1186/1472-6882-14-478
Geisler-Lee J, Wang Q, Yao Y et al (2012) Phytotoxicity, accumulation and transport of silver nanoparticles by Arabidopsis thaliana. Nanotoxicology 7:323–337. https://doi.org/10.3109/17435390.2012.658094
Ghanati F, Bakhtiarian S (2013) Changes of natural compounds of Artemisia annua L. by methyl jasmonate and silver nanoparticles. Adv Environ Biol 8:2251–2259
Gopinath V, MubarakAli D, Priyadarshini S et al (2012) Biosynthesis of silver nanoparticles from Tribulus terrestris and its antimicrobial activity: a novel biological approach. Colloids Surf B Biointerfaces 96:69–74. https://doi.org/10.1016/j.colsurfb.2012.03.023
Gregory RPF (1966) A rapid assay for peroxidase activity. Biochem J 101:582–583
Gutierrez-Goncalves J, Marcucci C (2009) Antioxidant and antimicrobial activities of propolis and açai (Euterpe oleracea Mart.) extracts. Rev Fitos 4:81–86
Habib N, Ali Q, Ali S et al (2020) Use of nitric oxide and hydrogen peroxide for better yield of wheat (Triticum aestivum L.) under water deficit conditions: growth, osmoregulation, and antioxidative defense mechanism. Plants Basel Switz. https://doi.org/10.3390/plants9020285
Harris AT, Bali R (2008) On the formation and extent of uptake of silver nanoparticles by live plants. J Nanoparticle Res 10:691–695. https://doi.org/10.1007/s11051-007-9288-5
Hashim AM, Alharbi BM, Abdulmajeed AM et al (2020) Oxidative stress responses of some endemic plants to high altitudes by intensifying antioxidants and secondary metabolites content. Plants 9:869. https://doi.org/10.3390/plants9070869
Hatami M, Ghorbanpour M (2013) Effect of nanosilver on physiological performance of pelargonium plants exposed to dark storage. J Hortic Res 21:15–20. https://doi.org/10.2478/johr-2013-0003
Heidari Z, Salehzadeh A, Sadat Shandiz SA, Tajdoost S (2018) Anti-cancer and antioxidant properties of ethanolic leaf extract of Thymus vulgaris and its bio-functionalized silver nanoparticles. 3 Biotech 8:177. https://doi.org/10.1007/s13205-018-1199-x
Hetta HF (2016) Regulatory B cells: key players in hepatocellular carcinoma progression. Gastroenterol Hepatol Open Access. https://doi.org/10.15406/ghoa.2016.05.00136
Hetta HF, Mwafey IM, Batiha GE-S et al (2020) CD19 + CD24hi CD38hi regulatory B cells and memory B cells in periodontitis: association with pro-inflammatory and anti-inflammatory cytokines. Vaccines 8:340. https://doi.org/10.3390/vaccines8020340
Hossain Z, Mustafa G, Komatsu S (2015) Plant responses to nanoparticle stress. Int J Mol Sci 16:26644–26653. https://doi.org/10.3390/ijms161125980
Hu Y, Bellaloui N, Sun G et al (2014) Exogenous sodium sulfide improves morphological and physiological responses of a hybrid Populus species to nitrogen dioxide. J Plant Physiol 171:868–875. https://doi.org/10.1016/j.jplph.2013.10.018
ISTA (1985) International rules for seed testing. Rules 1985(13):299–520
Jasim B, Thomas R, Mathew J, Radhakrishnan EK (2017) Plant growth and diosgenin enhancement effect of silver nanoparticles in Fenugreek (Trigonella foenum-graecum L.). Saudi Pharm J SPJ 25:443–447. https://doi.org/10.1016/j.jsps.2016.09.012
Judy JD, Unrine JM, Bertsch PM (2011) Evidence for biomagnification of gold nanoparticles within a terrestrial food chain. Environ Sci Technol 45:776–781. https://doi.org/10.1021/es103031a
Junior V, Arruda I, Bemme L (2013) Caracterização térmica e espectroscópica de microcápsulas de quitosana incorporada de própolis. Rev Eletrônica Univar 2:161–165
Kannan RRR, Stirk WA, Van Staden J (2013) Synthesis of silver nanoparticles using the seaweed Codium capitatum P.C. Silva (Chlorophyceae). South Afr J Bot 86:1–4. https://doi.org/10.1016/j.sajb.2013.01.003
Khodakovskaya MV, de Silva K, Biris AS et al (2012) Carbon nanotubes induce growth enhancement of tobacco cells. ACS Nano 6:2128–2135. https://doi.org/10.1021/nn204643g
Krishnaraj C, Jagan EG, Ramachandran R et al (2012) Effect of biologically synthesized silver nanoparticles on Bacopa monnieri (Linn.) Wettst. plant growth metabolism. Process Biochem 47:651–658. https://doi.org/10.1016/j.procbio.2012.01.006
Lediga ME, Malatjie TS, Olivier DK et al (2018) Biosynthesis and characterisation of antimicrobial silver nanoparticles from a selection of fever-reducing medicinal plants of South Africa. South Afr J Bot 119:172–180
Lei Z, Mingyu S, Xiao W et al (2008) Antioxidant stress is promoted by nano-anatase in spinach chloroplasts under UV-B radiation. Biol Trace Elem Res 121:69–79. https://doi.org/10.1007/s12011-007-8028-0
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Logeswari P, Silambarasan S, Abraham J (2015) Synthesis of silver nanoparticles using plants extract and analysis of their antimicrobial property. J Saudi Chem Soc 19:311–317. https://doi.org/10.1016/j.jscs.2012.04.007
Lu C, Zhang C, Wen J et al (2002) Research of the effect of nanometer materials on germination and growth enhancement of Glycine max and its mechanism. Soybean Sci 21:168–171
Ma X, Geisler-Lee J, Geiser-Lee J et al (2010) Interactions between engineered nanoparticles (ENPs) and plants: phytotoxicity, uptake and accumulation. Sci Total Environ 408:3053–3061. https://doi.org/10.1016/j.scitotenv.2010.03.031
Manby B (2001) Unequal protection: the state response to violent crime on South African farms. Human Rights Watch, New York
Manke A, Wang L, Rojanasakul Y (2013) Mechanisms of nanoparticle-induced oxidative stress and toxicity. In: BioMed Res. Int. https://www.hindawi.com/journals/bmri/2013/942916/. Accessed 18 Mar 2018
McHenry EW, Graham M (1935) Observations on the estimation of ascorbic acid by titration. Biochem J 29:2013–2019
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Moustafa-Farag M, Almoneafy A, Mahmoud A et al (2020a) Melatonin and its protective role against biotic stress impacts on plants. Biomolecules 10:54. https://doi.org/10.3390/biom10010054
Moustafa-Farag M, Mohamed HI, Mahmoud A et al (2020b) Salicylic acid stimulates antioxidant defense and osmolyte metabolism to alleviate oxidative stress in watermelons under excess boron. Plants 9:724. https://doi.org/10.3390/plants9060724
Mukherjee M, Mahapatra A (2010) Effect of coinage metal nanoparticles and zwitterionic surfactant on reduction of [Co(NH3)5Cl](NO3)2 by iron(II). Colloids Surf Physicochem Eng Asp 368:112–118. https://doi.org/10.1016/j.colsurfa.2010.07.021
Nair PMG, Chung IM (2015) Physiological and molecular level studies on the toxicity of silver nanoparticles in germinating seedlings of mung bean (Vigna radiata L.). Acta Physiol Plant. https://doi.org/10.1007/s11738-014-1719-1
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Nowack B (2010) Nanosilver revisited downstream. Science 330:1054–1055. https://doi.org/10.1126/science.1198074
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Parveen A, Rao S (2015) Effect of nanosilver on seed germination and seedling growth in Pennisetum glaucum. J Clust Sci 26:693–701. https://doi.org/10.1007/s10876-014-0728-y
Pavlović N, Zdravković M, Gvozdanović-Varga J et al (2016) Heredity mode of onion (Allium cepa L.) bulb shape index. Ratar Povrt 53:85–89. https://doi.org/10.5937/ratpov53-10028
Pradhan S, Mailapalli DR (2017) Interaction of engineered nanoparticles with the agri-environment. J Agric Food Chem 65:8279–8294
Prasad R, Kumar M, Kumar V (2017) Nanotechnology: an agricultural paradigm. Springer, Berlin
Priyadarshini S, Gopinath V, Meera Priyadharsshini N et al (2013) Synthesis of anisotropic silver nanoparticles using novel strain, Bacillus flexus and its biomedical application. Colloids Surf B Biointerfaces 102:232–237. https://doi.org/10.1016/j.colsurfb.2012.08.018
Qian H, Peng X, Han X et al (2013) Comparison of the toxicity of silver nanoparticles and silver ions on the growth of terrestrial plant model Arabidopsis thaliana. J Environ Sci 25:1947–1956. https://doi.org/10.1016/S1001-0742(12)60301-5
Rai M, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 27:76–83. https://doi.org/10.1016/j.biotechadv.2008.09.002
Rashad Y, Aseel D, Hammad S, Elkelish A (2020) Rhizophagus irregularis and Rhizoctonia solani differentially elicit systemic transcriptional expression of polyphenol biosynthetic pathways genes in sunflower. Biomolecules 10:379. https://doi.org/10.3390/biom10030379
Rastogi A, Zivcak M, Sytar O et al (2017) Impact of metal and metal oxide nanoparticles on plant: a critical review. Front Chem. https://doi.org/10.3389/fchem.2017.00078
Saleem MH, Ali S, Rehman M et al (2020) Influence of phosphorus on copper phytoextraction via modulating cellular organelles in two jute (Corchorus capsularis L.) varieties grown in a copper mining soil of Hubei Province. China. Chemosphere 248:126032. https://doi.org/10.1016/j.chemosphere.2020.126032
Sharaf S, Higazy A, Hebeish A (2013) Propolis induced antibacterial activity and other technical properties of cotton textiles. Int J Biol Macromol 59:408–416. https://doi.org/10.1016/j.ijbiomac.2013.04.030
Siddiqi KS, Husen A (2016) Engineered gold nanoparticles and plant adaptation potential. Nanoscale Res Lett 11:400. https://doi.org/10.1186/s11671-016-1607-2
Sigamoney M, Shaik S, Govender P et al (2016) African leafy vegetables as bio-factories for silver nanoparticles: a case study on Amaranthus dubius C Mart. Ex Thell. South Afr J Bot 103:230–240. https://doi.org/10.1016/j.sajb.2015.08.022
Singhal G, Bhavesh R, Kasariya K et al (2011) Biosynthesis of silver nanoparticles using Ocimum sanctum (Tulsi) leaf extract and screening its antimicrobial activity. J Nanoparticle Res 13:2981–2988. https://doi.org/10.1007/s11051-010-0193-y
Soliman MH, Alayafi AAM, El Kelish AA, Abu-Elsaoud AM (2018) Acetylsalicylic acid enhance tolerance of Phaseolus vulgaris L. to chilling stress, improving photosynthesis, antioxidants and expression of cold stress responsive genes. Bot Stud. https://doi.org/10.1186/s40529-018-0222-1
Soliman M, Alhaithloul HA, Hakeem KR et al (2019) Exogenous nitric oxide mitigates nickel-induced oxidative damage in eggplant by upregulating antioxidants, osmolyte metabolism, and glyoxalase systems. Plants 8:562. https://doi.org/10.3390/plants8120562
Soliman M, Elkelish A, Souad T et al (2020a) Brassinosteroid seed priming with nitrogen supplementation improves salt tolerance in soybean. Physiol Mol Biol Plants. https://doi.org/10.1007/s12298-020-00765-7
Soliman MH, Abdulmajeed AM, Alhaithloul H et al (2020b) Saponin biopriming positively stimulates antioxidants defense, osmolytes metabolism and ionic status to confer salt stress tolerance in soybean. Acta Physiol Plant 42:114. https://doi.org/10.1007/s11738-020-03098-w
Thiruvengadam M, Rajakumar G, Chung I-M (2018) Nanotechnology: current uses and future applications in the food industry. 3 Biotech 8:74
Thuesombat P, Hannongbua S, Akasit S, Chadchawan S (2014) Effect of silver nanoparticles on rice (Oryza sativa L. cv. KDML 105) seed germination and seedling growth. Ecotoxicol Environ Saf 104:302–309. https://doi.org/10.1016/j.ecoenv.2014.03.022
Toraih EA, Alrefai HG, Hussein MH et al (2019) Overexpression of heat shock protein HSP90AA1 and translocase of the outer mitochondrial membrane TOM34 in HCV-induced hepatocellular carcinoma: a pilot study. Clin Biochem 63:10–17. https://doi.org/10.1016/j.clinbiochem.2018.12.001
Vannini C, Domingo G, Onelli E et al (2014) Phytotoxic and genotoxic effects of silver nanoparticles exposure on germinating wheat seedlings. J Plant Physiol 171:1142–1148. https://doi.org/10.1016/j.jplph.2014.05.002
Velmurugan N, Kalpana D, Cho JY, Lee YS (2018) Chemical composition and antioxidant capacity of the aqueous extract of Phellodendron amurense. J For Res 29:1041–1048. https://doi.org/10.1007/s11676-017-0532-2
Vwioko E, Adinkwu O, El-Esawi MA (2017) Comparative physiological, biochemical, and genetic responses to prolonged waterlogging stress in okra and maize given exogenous ethylene priming. Front Physiol 8:632. https://doi.org/10.3389/fphys.2017.00632
Xu J, Aileni M, Abbagani S, Zhang P (2010) A reliable and efficient method for total rna isolation from various members of spurge family (Euphorbiaceae). Phytochem Anal 21:395–398. https://doi.org/10.1002/pca.1205
Yang J, Cao W, Rui Y (2017) Interactions between nanoparticles and plants: phytotoxicity and defense mechanisms. J Plant Interact 12:158–169. https://doi.org/10.1080/17429145.2017.1310944
Yasur J, Rani PU (2013) Environmental effects of nanosilver: impact on castor seed germination, seedling growth, and plant physiology. Environ Sci Pollut Res 20:8636–8648. https://doi.org/10.1007/s11356-013-1798-3
Yin L, Colman BP, McGill BM et al (2012) Effects of Silver Nanoparticle exposure on germination and early growth of eleven wetland plants. PLOS One 7:e47674. https://doi.org/10.1371/journal.pone.0047674
Zahran AM, Zharan KM, Hetta HF (2018) Significant correlation between regulatory T cells and vitamin D status in term and preterm labor. J Reprod Immunol 129:15–22. https://doi.org/10.1016/j.jri.2018.07.004
Zamin M, Fahad S, Khattak AM et al (2019) Developing the first halophytic turfgrasses for the urban landscape from native Arabian desert grass. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-019-06218-3
Zhang T, Wang L, Chen Q, Chen C (2014) Cytotoxic potential of silver nanoparticles. Yonsei Med J 55:283–291. https://doi.org/10.3349/ymj.2014.55.2.283
Zheng L, Hong F, Lu S, Liu C (2005) Effect of nano-TiO(2) on strength of naturally aged seeds and growth of spinach. Biol Trace Elem Res 104:83–92. https://doi.org/10.1385/BTER:104:1:083
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by O. Ferrarese-Filho.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Soliman, M., Qari, S.H., Abu-Elsaoud, A. et al. Rapid green synthesis of silver nanoparticles from blue gum augment growth and performance of maize, fenugreek, and onion by modulating plants cellular antioxidant machinery and genes expression. Acta Physiol Plant 42, 148 (2020). https://doi.org/10.1007/s11738-020-03131-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-020-03131-y