Abstract
Nanotechnology can be effectively used in agriculture to improve crop production. Biosynthesized nanoparticles can enhance the growth and yield of several crops. In this work, we examine the effect of green synthesized silver nanoparticles from Pongamia pinnata (L.) Pierre leaf extract on the germination and growth of Black gram (Vigna mungo (L.) Hepper). Upon treatment with biosynthesized silver nanoparticles, we found a 22% increase in seed germination and a 36% enhancement in seedling growth. Further, pot experiments revealed a significant increase in root length (33%), pod weight (21%) and grain weight (26%). A significant increase in phenolics (20%) and carbohydrates (17%) was also observed. Assessment of the antioxidative enzymes revealed a significant increase in catalase activity (9%), thus indicating a reduced oxidative stress and associated damage. The results suggest that biocompatibility of the synthesized nanoparticles and efficient ROS scavenging mechanisms together play an important role in exhibiting an overall positive effect on germination, growth and development of Black gram (Vigna mungo (L.) Hepper). The evidence also suggests that Pongamia pinnata (L.) Pierre may be a good source for the biosynthesis of nanoparticles and use as a plant growth enhancer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent times, advancements in nanotechnology has resulted in its application in several fields such as agriculture, chemical synthesis, manufacturing, medicine and drug delivery (Saratale et al. 2018). Enhanced application in agriculture is mainly because nanoparticles (NPs) easily penetrate different parts of the plants and interact with multitude of living cells and tissues (Salem and Fouda 2020). NPs are small particles having a size range of 1–100 nm. Both metallic and non-metallic NPs have been synthesized from different materials (Iravani et al. 2014). Such engineered NPs have been shown to have a high potential for increasing crop yields and also protecting crops (Basavegowda and Baek 2021).
Metallic NPs have been synthesized using two different approaches viz., the top-down approach (includes mechanical milling, sputtering, laser ablation, electro-explosion and chemical etching) and the bottom-up approach (which includes sol–gel process, spray pyrolysis, aerosol process, laser pyrolysis, chemical vapour deposition and atomic condensation) (Iravani et al. 2014). Irrespective of the approach used, toxic chemicals and expensive equipment are used, rendering them disadvantageous. Several chemicals such as sodium borohydride, hydrazine and dimethylformamide have been used as reducing agents in chemical synthesis methods (Iravani et al. 2014). On the other hand, the green synthesis methods being currently followed do not use toxic chemicals and are environmentally friendly as they do not produce any harmful by-products (Singh et al. 2018). This is because the stabilizing and reducing agents used are either microbes (fungi, bacteria and algae) (Ghosh et al. 2021) or plant sources (such as roots, leaves, fruit, flowers and seeds) which are non-toxic, cost-effective and eco-friendly (Jadoun et al. 2021). Compared to microbial synthesis, plant-mediated synthesis has a higher yield of synthesized NPs (Rafique et al. 2017). A recent comprehensive review discusses the methods used for the biological synthesis of metallic NPs and their applications (Kumar and Seth 2021; Krishnasamy and Obbineni 2022).
Chemically and biologically synthesized NPs have played a significant role in the field of agriculture (seed priming, nano-fertilizer, pesticides etc.) and the effect of these synthesized NPs on plant growth and nutrition has been examined. The growth of the seedlings of Vigna radiata L. and the soil microbial community was improved by titanium oxide NPs synthesized from Aspergillus flavus (Raliya et al. 2015). The growth of the maize plant was improved by zinc oxide NPs synthesized from the bacteria, Bacillus subtilis (Sabir et al. 2020). Plant growth has also been boosted by foliar spraying of biosynthesized sulphur NPs (Salem et al. 2016a, b; Ragab and Saad-Allah 2020; Najafi et al. 2020) and green synthesized magnesium oxide NPs (Jhansi et al. 2017; Vijai Anand et al. 2020). The growth of Phaseolus vulgaris was enhanced using chemically synthesized silver NPs (Das et al. 2018). Using silver NPs, increased Arbuscular Mycorrhizal Fungal colonization and enhanced growth of pea was obtained (Rahman et al. 2023). In rice seedlings, silver NPs mediated stress was relieved by endogenous nitric oxide accumulation and ethylene played a very crucial role in the regulation (Tripathi et al. 2023).
Among metallic NPs, silver nanoparticles (AgNPs) have gained prominence and find several applications in crop improvement and protection. In the case of biological synthesis of silver nanoparticles (AgNPs), silver nitrate is reduced by proteins present in the extracts, thus causing a change in the secondary structure of proteins and the creation of silver nuclei. Later, the silver nuclei expand as a result of further reduction of silver ions and their aggregation at the nuclei. Flavonoids have been reported to be responsible for the green synthesis of AgNPs (Mustapha et al. 2022). Synthesized AgNPs have been used as an antimicrobial agent, in food packaging, wastewater treatment and biomedical applications (Vanlalveni et al. 2021). Biosynthesized AgNPs have been shown to have potential use as fertilizer, and pesticides, to manage diseases and abiotic stress tolerance (Sangeetha et al. 2021; Al-khattaf 2021; Alabdallah and Hasan 2021). Phyto-functionalized AgNPs were able to protect the silk worms from Flacherie and Sappe microbial diseases (Surendra et al. 2023). Biologically synthesized AgNPs were shown to increase physiological growth parameters in water hyacinth plants (Rani et al. 2016). Seed germination and seedling growth were increased using biosynthesized AgNPs from seaweed extracts (Roy and Ananthraman 2017). AgNPs synthesized using agro-industrial by-products improved seedling development in watermelon seed, with no significant variations in nutritional content between the treated and untreated groups (Acharya et al. 2020). AgNPs synthesized from the plant extract of Capparis spinosa have increased the germination and seedling growth of the wheat plant under salt stress (Ismail and Abou-Zeid 2018). Biosynthesized AgNPs from Malva parviflora showed significant differences in carbohydrate and proline content thereby enhancing growth of hydroponically cultivated Brassica oleracea (Oraibi et al. 2022). Biologically synthesized AgNPs have been shown to enhance the growth of soybean seedlings by regulating several proteins involved in degradation as well as ATP generation (Mustafa et al. 2020).
Pongamia pinnata (L.) Pierre, commonly known as Pongam Tree has been profoundly used in agriculture as green leaf manure for a long time. The leaves and branches of the plant are collected and incorporated into the soil well before the cultivation of a crop. The oil cake from the seed has been found to contain nitrogen (4–6%) and sulphur (0.2%) and is used as organic manure. The plant is also known for its medicinal properties and has been used for relieving headaches as well as wound healing (Bhandirge et al. 2015; Balasooriya et al. 2021). Pongam seeds have been used as a source for the production of lignocelluloses and ethanol (Radhakumari et al. 2017). The methanolic extract of leaves has exhibited antimicrobial activity, wound healing in rats (Dwivedi et al. 2017), and pesticidal activity (Tran et al. 2017). Previously, Pongamia pinnata (L.) Pierre extracts have been used for the synthesis of silver (Beg et al. 2017; Kishanji et al. 2020), gold (Khatua et al. 2020) and zinc (Malaikozhundan et al. 2017; Malaikozhundan and Vinodhini 2018) NPs. AgNPs synthesized from an aqueous seed extract of pongam have shown the ability to bind human serum albumin (Beg et al. 2017). The dried leaves of pongam were used to synthesize AgNPs and exhibited antibacterial activity (Raut et al. 2010). Biosynthesized gold NPs from an aqueous extract of pongam leaves showed antifungal activity (Khatua et al. 2020). Zinc NPs synthesized from pongam leaf extract showed pesticidal (Malaikozhundan and Vinodhini 2018), antibacterial, and antifungal activity (Malaikozhundan et al. 2017).
Although, biosynthesized AgNPs have been used for several biological applications, there has been only one recent study on yield improvement in a pulse crop using microbe mediated synthesis of AgNPs (Sambangi and Gopalakrishnan 2023). There has been no study on yield improvement in pulse crops using plant mediated synthesis of AgNPs. In addition, there has been no report of yield improvement using Pongamia pinnata (L.) Pierre extract synthesized AgNPs. In this work, we for the first time report the use of green synthesized AgNPs from Pongamia pinnata (L.) Pierre extract to assess the seed germination, growth and yield of Black gram (Vigna mungo (L.) Hepper) by priming and treatment.
Methods
Plant Extract Preparation and Silver Nanoparticle (AgNP) Biosynthesis
Fresh and disease-free Pongamia pinnata (L.) Pierre leaves were picked from the VIT farm. The leaves were washed several times with de-ionized water to remove any debris and dust particles from the surface. Subsequently, the samples were shade dried for 2 weeks. The dried leaf samples were then crushed into a fine powder using a mechanical grinder. 10 gms of the powder was mixed with 100 mL of double distilled water in a beaker and the beaker was placed in a water bath at 60 °C for 20 min. After 20 min, the beaker was cooled down to room temperature, and the solution was filtered using Whatman No. 1 filter paper (GE life sciences) and stored at 4 °C for subsequent use. Different concentrations of silver nitrate (AgNO3) (0.5 mM, 1 mM, 1.5 mM, 2 mM and 2.5 mM) were mixed with the plant extract in a ratio of 1:10 (v/v) and placed on a hot plate stirrer with a constant speed of 500 rpm and temperature of 60 °C for 3 h. At every 15 min, 2 mL of the sample was taken, and absorbance was measured using a UV–Vis spectrophotometer (Shimadzu UV-1280). After 3 h the sample was cooled to room temperature and stored at 4 °C. The biosynthesized Pongamia pinnata (L.) Pierre AgNPs (PpAgNPs) were further characterized.
Characterization of AgNPs
To characterize and assess the stability of PpAgNPs, UV–Vis spectrophotometer (Shimadzu UV-1280) was used. The absorbance of the solution was measured in the wavelength range from 300 to 700 nm. Colour change in the final product after mixing AgNO3 at different concentrations with the plant extract was noted. The size distribution and zeta potential of PpAgNPs was analyzed using dynamic light scattering (DLS) (Horiba Scientific SZ100 Nanopartica). The functional groups present in the PpAgNPs sample were determined using Fourier transform infrared (FTIR) spectroscopy (Shimadzu IR-Affinity-1). For FTIR, samples were prepared by first centrifuging at 10,000 rpm for 10 min. The supernatant was discarded and to the pellet, ethanol was added. This was centrifuged again at 10,000 rpm for 10 min. The process was repeated 2 times. Then, the pellet was dried overnight at 90 °C in a hot air oven. 1 mg of sample was mixed with potassium bromide (KBr) and used for analysis in transmittance mode. The crystalline nature was analyzed by taking 10 mg of dried PpAgNPs in a powder X-ray diffractometer (XRD) (Bruker, D8 Advance). The morphological characteristics such as shape and size of PpAgNPs were obtained by diluting the sample in double distilled water (1:10) and imaging in a 120 keV transmission electron microscope (TEM) (FEI Tecnai G2 Spirit BioTWIN) with a 4kx4k CCD camera (Gatan US4000).
Seed Germination Test
Black gram (Vigna mungo (L.) Hepper) cv Vamban 6 seeds were surface sterilized by immersing in 4% sodium hypochlorite (NaOCl) solution for 15 min. The solution was stirred for 30 s every 5 min. The seeds were then washed with distilled water to remove any residual sodium hypochlorite from the seed surface. After that, the seeds were soaked for 3 h in Milli-Q water (control) and various concentrations of PpAgNPs (treatment). Seed germination test was performed by placing the seeds on top of two sheets of blotting paper in a Petri plate. Nine seeds were placed in each plate. The blotting paper was soaked with 10 mL of Milli-Q water in the case of control plate and 10 mL of PpAgNPs in different concentration in the treatment plates. The Petri plates were covered with parafilm to avoid moisture loss by evaporation. Subsequently, the plates were placed at room temperature and maintained under a normal light/dark cycle. Germination was recorded every day. After 10 days, the germination percentage (GP) and vigour index were calculated using the formula given below –
where \({N}_{g}\) is the number of germinated seeds and \(N\) is the total number of seeds.
where \(RL\) is the root length and \(SL\) is the shoot length (Abdul-Baki and Anderson 1973).
The root and shoot lengths of the seedlings were measured and recorded. The experiment was repeated 3 times in duplicate.
Pot Experiment
This experiment was conducted at the greenhouse of the School of Agriculture, Vellore Institute of Technology, Vellore, India. A total of 9 PVC (polyvinyl chloride) pots (30 cm in diameter and 25 cm in height) were used to set up triplicates with three different conditions viz., control (no fertilizer), liquid fertilizer (19:19:19 N:P:K) and PpAgNPs in a completely randomized design. Each pot was filled with 8000 gms of air-dried and sieved (with a 250-micron mesh sieve) red soil (pH 7.2). Black gram (Vigna mungo (L.) Hepper) cv Vamban 6 seeds were surface sterilized using 4% sodium hypochlorite for 10 min and subsequently washed with distilled water for 10 min to remove any residue. Seeds were primed with distilled water (group A), 19:19:19 N:P:K liquid fertilizer (group B), and 25 mg/L PpAgNPs (group C). Eight seeds per pot were sown in each group. After germination, thinning was done, and only two plants per pot (seedlings with equal height across different conditions) were retained. Soil water content was maintained by watering 1 lit of tap water 3 times a week. On the 20th, 35th and 50th day after sowing (DAS), 10 mL of treatment was given by foliar application for the different groups i.e., only deionized water for group A, liquid fertilizer (19:19:19 N:P:K) for group B and 50 mg/L of PpAgNPs for group C. The concentration of PpAgNPs for treatment by foliar application was chosen based on a previous reported study (Das et al. 2018).
Physiological Parameters
Several physiological parameters viz., root length and shoot length, fresh weight and dry weight, as well as flower count and pod count, were measured and recorded. On the 70th DAS, two plants per group were uprooted and washed with tap water to remove any soil on the root surface. The length of the roots and shoots was measured using a measuring scale and recorded. The plant samples were spread on a blotting paper to remove any water molecules on the surface. The weight of each plant sample was measured on a weighing balance and recorded as the fresh weight of the sample. The sample was then placed in a hot air oven at 70 °C for 48 h. The weight of the sample was again measured and recorded as the dry weight of the sample (Khalid et al. 2017). On the 35th, 45th and 55th DAS, the number of flowers per plant was counted. The number of pods per plant was also counted after physiological maturity (black mature pods), and yield was evaluated by measuring the overall grain weight.
Biochemical Parameters
After maturity, the total phenol, carbohydrate and protein content in the leaves was estimated. For total phenol, the leaf samples were prepared by following a previously published method (Karthik et al. 2020) and concentration of phenol was estimated by Folin–Ciocalteau reagent (Martins et al. 2021). For total carbohydrate and protein content, a previously published method by (Mathur et al. 2015) was followed. Estimation of total carbohydrate was done by phenol–sulphuric acid method (Chow and Landhäusser 2004) and total protein by Lowry method (LOWRY et al. 1951). The photosynthetic pigments, Chlorophyll a and b were also estimated by following the method described in (Karthik et al. 2020). All the experiments were carried out two times with duplicates.
Oxidative Stress Markers
The amount of hydrogen peroxide (H2O2) in the leaf sample was quantified using a spectrophotometer as done previously (Velikova et al. 2000; Yadav et al. 2022). Briefly, 5 mL of pre-chilled 0.1% trichloroacetic acid (TCA) was added to 500 mg of leaf and the mixture homogenized using a pre-cooled mortar and pestle. The homogenate was centrifuged at 4 °C at 11,500 rpm for 15 min. The supernatant (0.5 mL) was mixed with 0.5 mL of 10 mM potassium phosphate buffer (pH 7) and 1 mL of 1 M potassium iodide (KI). The solution was placed in the dark for 1 h and the absorbance was recorded at 390 nm. The H2O2 concentration was calculated by applying the formula -
where \(FW\) denotes the fresh weight of the leaf gmL−1 and \(V\) denotes volume of extract (mL).
Lipid peroxidation was assessed to evaluate the cell membrane damage. The assessment was done by measuring the concentration of malondialdehyde (MDA), which is one of the final products of peroxidation. This was assessed indirectly by estimating the concentration of thiobarbituric acid reactive substance (TBARS) (Du and Bramlage 1992; Agnihotri and Seth 2020; Gupta and Seth 2021). Briefly, 5 mL of pre-chilled 80% ethanol was added to 0.2 g of leaf and homogenized using a pre-chilled mortar and pestle. The homogenate was then centrifuged at 4 °C, 8200 rpm for 10 min. The supernatant (1 mL) was added to 4 mL of 20% TCA with 0.67% of 2-thiobituric acid (TBA) and placed in a water bath for 30 min at 90 °C and subsequently cooled by placing on ice. The solution was centrifuged once again and the resulting supernatant was collected. Absorbance was recorded at three different wavelengths (440, 532 and 600 nm) and the concentration of MDA was calculated using the formula -
where \(FW\) denotes the fresh weight of the leaf.
Antioxidant Activity
To determine the antioxidant activities, enzymes such as catalase (CAT), superoxide dismutase (SOD), ascorbate peroxidase (APX) and peroxidase (POD) were estimated using the plant leaf extract (Singh et al. 2017). Leaf samples were collected on 37th DAS and stored immediately at − 80 °C. The leaf sample (100 mg) was homogenized in 1 mL of ice cold 100 mM phosphate buffer (pH 7) containing 0.1 mM ethylenediaminetetraacetic acid (EDTA), 0.2% Triton X-100, 1 mM phenylmethylsulfonyl fluoride (PMSF) and 2% polyvinylpyrrolidone (PVP). The solution was centrifuged at 4 °C at 12,000 rpm for 20 min. The supernatant obtained was used for further analyses. To evaluate the CAT activity, we followed the method by (Dutta Gupta and Datta 2004). Briefly, a solution containing 100 µL of enzyme extract and 1.8 mL of 50 mM phosphate buffer (pH 7) was prepared and absorbance measured at 240 nm. To this solution, 100 µL of 0.6% H2O2 was added and absorbance was measured every 30 s for 3 min. A blank without the enzyme extract was also prepared. H2O2 breakdown at 1 µmol/min was termed as one unit of enzyme activity which was estimated using ε = 0.036 mM−1 cm−1 as the extinction-coefficient of H2O2. SOD activity was estimated by inhibiting the reduction of nitro blue tetrazolium (NBT) as reported by (Beyer and Fridovich 1987). In short, a solution containing 100 µL of extract, 22.5 µM NBT, 0.1 mM EDTA, 2 µM riboflavin, 13 mM L-methionine was prepared and absorbance noted at 560 nm. The solution was then exposed to light and after 5 min, another measurement was noted. A blank was prepared without the enzyme extract. The enzyme concentration sufficient for 50% inhibition in NBT degradation was considered equivalent to one unit of SOD activity. APX activity was estimated using the method reported by (Nakano and Asada 1981). A reaction mixture containing 1 mL of 50 mM phosphate buffer (pH 7), 200 µL of 5 mM ascorbate and 200 µL of 1 mM EDTA was prepared. To this mixture, 200 µL of enzyme extract and 200 µL of 1 mM H2O2 were added. The decrease in ascorbate concentration was noted by recording the absorbance at 290 nm for 3 min. One unit of enzyme activity was calculated as the degradation of ascorbate at a rate of 1 mol/min. To compute the activity, the extinction coefficient of ascorbate at 290 nm was set at ε = 2.8 mM−1 cm−1. POD activity was evaluated by monitoring the conversion of pyrogallol compound to purpurogallin in the presence of H2O2 (Kar and Mishra 1976). Briefly, 10 µL of enzyme extract was added to 5 mL of pyrogallol solution prepared in 25 mM of phosphate buffer (pH 6.8) containing 50 µL of 3% H2O2. The shift in absorbance at 420 nm was examined for 4 min. One unit of enzyme activity was specified as formation of 1 mol purpurogallin per min, which was estimated using ε = 2.47 mM−1 cm−1 as the extinction-coefficient.
Statistical Analysis
Statistical analyses of all the data were done using JMP software (SAS Institute, 1989). The means of different groups were compared by performing the Student’s t-test and the level of significance was set at p < 0.05.
Results
Biosynthesis of AgNPs
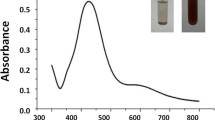
The aqueous extract of Pongamia pinnata (L.) Pierre leaves is red in colour and AgNO3 is colourless. Upon mixing and following the protocol for AgNPs synthesis, the colour of the solution changed to dark brown (Fig. 1A Inset), corroborating well with previously published observations (Roy and Ananthraman 2017). The colour change is due to a reduction of Ag+ to Ag0 wherein the plant extract acts as a reducing and stabilizing agent.
A: UV–Vis Spectrum of PpAgNPs with different concentration of AgNO3 and visual observation of colour change upon biosynthesis of AgNPs (Inset). B: Dynamic light scattering particle size analysis of PpAgNPs (1.5 mM). C: Zeta potential analysis of PpAgNPs (1.5 mM). D: FT-IR spectrum of AgNPs and pongam leaves aqueous extract. E: X-ray Diffraction data of PpAgNPs (1.5 mM). F: Transmission electron microscopy image of PpAgNPs (1.5 mM)
Characterization of AgNPs
The product obtained after biosynthesis was analyzed using UV–Visible spectrophotometer. The maximum absorbance was observed at 410 nm with a broadening peak indicating the polydisperse nature of the synthesized particles (Fig. 1A). Based on previous studies, it is evident that different characterization peaks between 410 and 480 nm typically indicate AgNP synthesis (Gogoi et al. 2015; Taha et al. 2019) and the various wavelengths can be attributed to the size and shape of the AgNPs (Hussain et al. 2018).
The DLS particle size distribution of PpAgNPs shows the polydisperse nature of the particles with a size ranging from 25 to 200 nm and an average particle size of 54.3 nm (Fig. 1B). The larger size particles seem to appear due to the agglomeration of the PpAgNPs. The zeta potential value of PpAgNPs was -30.8 mV indicating a stable synthesized NP (Fig. 1C). A zeta potential of minimum ± 30 mV is required for a physically stable suspension stabilized by electrostatic repulsion (Casula et al. 2021).
FTIR spectrum of PpAgNPs (Fig. 1D) showed different peaks at 3250.05, 2935.66, 1587.68, 1271.09, 1066.64 and 1043.49 cm−1. The peak at 3250.05 cm−1 and 2935.66 cm−1 could be due to O–H group in alcohols and carboxylic acids, respectively. A peak at 1587.68 cm−1 may be attributed to N–O in nitro compounds. The band observed at 1271.09 is due to C–O stretching of aromatic esters. The peak observed at 1066.64 cm−1 and 1043.49 cm−1 may be recognized as stretching of S=O in the sulfoxide group. There is a shift in these absorption peaks after the biosynthesis of AgNPs indicating the binding of molecules containing these groups to the AgNPs thereby stabilizing the nanoparticles. Such a shift has been previously observed (Masum et al. 2019) and also Pongamia pinnata (L.) Pierre leaf extract has been reported to contain flavonoids in abundance (Degani et al. 2022) corroborating well with the observations. Reports also indicate that Ag+ ions possibly bind to flavonoid, alkaloid, terpenoid, aldehyde and amide compounds thus resulting in bioreduction and formation of capped AgNPs (Behravan et al. 2019).
Figure 1E shows the XRD pattern of PpAgNPs. The pattern shows three peaks at 2θ values 38°, 45°, and 65° with corresponding miller index values 111, 200, and 220, respectively. This indicates that the AgNPs are in a cubic crystalline phase in agreement with previously published results (JCPDS card No. 040783, 870,598, 870,720 and 893,722). The size of the particle can be estimated using the Scherrer formula –
where, \(D\) is the crystalline size, \(\lambda\) is the wavelength of X-ray used, \(\beta\) is the width at half-maximal light (maximum intensity peak) and \(\theta\) is the Bragg’s angle. Using the formula, the size was estimated to be about 15 nm.
The shape of nanoparticles was mostly spherical as visualized in the TEM image (Fig. 1F). Spherical AgNPs have also been observed previously (Santhosh et al. 2019).
Seed Germination Test
The effect of different concentrations of PpAgNPs on seed germination and seedling growth was examined. The germination percentage (GP) of PpAgNPs at 0.5 mM, 1 mM and 1.5 mM AgNO3 was found to increase significantly by 13%, 20% and 21%, respectively. In comparison, there was only a 3% increase in GP for filtered plant extract with respect to the control (Table 1). The GP was found to increase with increasing concentrations of PpAgNPs (upto a concentration of 1.5 mM) but at higher concentrations (greater than 1.5 mM) the GP started to decrease and was even lower than the control condition at 2.5 mM concentration of AgNPs. The GP was found to be highest (98.76%) at 1.5 mM concentration of AgNPs, and this was significantly different when compared to the control group.
After the 10th day, the root and shoot length of the seedlings were measured and the seedling vigour index (VI) was calculated. Table 1 summarizes the parameters, root length, shoot length and vigour index of the seedlings for the control and treatment conditions. The seedlings treated with PpAgNPs (1 mM AgNO3) showed a 19% increase in root length whereas that with 1.5 mM AgNO3 showed a 23% increase in shoot length. The seedlings treated with PpAgNPs (1.5 mM AgNO3) showed a 35% increase in VI.
Biosynthesized AgNPs have previously been shown to promote seed germination in several plant species (Gupta et al. 2018). This has mainly been attributed to the ability of biosynthesized AgNPs to penetrate the seed coat and stimulate the growth of the embryo (Al-Huqail et al. 2018). In addition, some studies have shown that biosynthesized AgNPs have influenced the activities of several enzymes, thereby promoting seed germination (Roy and Ananthraman 2018).
Pot Study Experiment
Physiological Parameters
As the PpAgNPs (1.5 mM AgNO3) showed the highest VI, pot study experiments were conducted using these synthesized AgNPs to assess the effect on overall growth and yield. After the 70th day, the plants were uprooted, and several growth parameters, viz. root length, shoot length, fresh weight, dry weight, flower count, pod count, the number of nodules, pod weight and grain weight, were estimated. Figure 2A shows the uprooted plants. Plants treated with PpAgNPs had a root length and shoot length of 68.53 ± 1.75 cm and 38.7 ± 1.85 cm. The root length was found to be 33% higher and shoot length 20% lower when compared to plants treated with the liquid fertilizer. The root length showed a 77% increase and shoot length a 78% increase as compared to plants in the control group (Fig. 2B and C). The plants treated with PpAgNPs showed similar fresh and dry weights when compared to the plants treated with liquid fertilizer (Fig. 2D and E). The plants treated with PpAgNPs showed a sixfold increase in fresh weight and threefold increase in dry weight as compared to the control plants.
A: Morphological differences in Black gram (Vigna mungo (L.) Hepper) cv Vamban 6 plants grown under a. (control)—treated with water, b. liquid fertilizer (19:19:19) and c. PpAgNPs (1.5 mM AgNO3). B: Root length, C: Shoot length, D: Fresh weight and E: Dry weight examination of black gram plant treated with liquid fertilizer (19:19:19) and 50 mg/L PpAgNPs (1.5 mM) in pot study. * and ** indicate p < 0.05 and p < 0.01 respectively
The number of flowers was counted on the 35th, 45th and 55th day after sowing whereas the number of nodules and pods per plant were examined after the 70th day i.e., when the plants were uprooted. Plants treated with PpAgNPs had the greatest number of flowers, nodules and pods per plant when compared to the other treatment groups (Fig. 3A–C). Black gram (Vigna mungo (L.) Hepper) cv Vamban 6 plants treated with PpAgNPs yielded pods and grains with a total weight of 78.14 ± 0.84 gms and 75.6 ± 0.57 gms, respectively. This was found to be significantly greater than the plants treated with liquid fertilizer (by onefold) as well as the control group (by twofold) (Fig. 3D and E).
Differences in growth and yield attributing characters in Black gram (Vigna mungo (L.) Hepper) cv Vamban 6 plants treated with water (control), liquid fertilizer (19:19:19), and 50 mg/L PpAgNPs (1.5 mM AgNO3) in pot study. A: flowers per plant, B: nodules per plant, C: pods per plant, D: pod weight and E: grain weight. * and ** indicate p < 0.05 and p < 0.01 respectively
Biosynthesized AgNPs have previously been shown to promote seedling and plant growth (Sadak 2019). Studies have shown that the biosynthesized AgNPs influence the activity of several enzymes which are involved in plant growth and metabolism (Iqbal et al. 2019; Tripathi et al. 2020; Azeez et al. 2022).
Biochemical Parameters
The effect of treatment of PpAgNPs on the concentration of biochemical parameters such as total phenol, carbohydrate, protein, chlorophyll a, chlorophyll b and carotenoid was assessed. The total phenol content was high in PpAgNPs treated group (0.66 ± 0.019 mg/ml) and was found to be 20% higher when compared to liquid fertilizer group and 81% higher when compared to the control group (Fig. 4A). The carbohydrate and protein content in the AgNPs treated sample was 0.69 ± 0.016 mg/ml and 0.82 ± 0.022 mg/ml respectively. The carbohydrate and protein content were 86% and 81% higher than the control group whereas they were 17% and 10% higher than the liquid fertilizer treated group (Fig. 4B and C). The content of photosynthetic pigments such as chlorophyll a and chlorophyll b were higher in AgNPs treated sample (1.06 ± 0.02 µg/ml and 2.69 ± 0.08 µg/ml). Chlorophyll a was found to be 18% higher in PpAgNPs treated sample as compared to liquid fertilizer treated sample and 91% higher as compared to control samples (Fig. 4D). Chlorophyll b was 32% higher in PpAgNPs treated samples with respect to the control samples (Fig. 4E). Carotenoid content in PpAgNPs group was 0.06 ± 0.006 µg/ml and this was 15% higher when compared to the liquid fertilizer group (Fig. 4F).
Concentration of biomolecules in Black gram (Vigna mungo (L.) Hepper) cv Vamban 6 plants treated with liquid fertilizer (19:19:19) vs 50 mg/L PpAgNPs (1.5 mM) in pot study. A: Phenol, B: Total carbohydrate, C: Total protein, D: Chlorophyll a, E: Chlorophyll b and F: Carotenoid. * and ** indicate p < 0.05 and p < 0.01 respectively
Oxidative Stress Markers
The concentration of oxidative stress markers was assessed upon treatment. There was a 34% decrease in hydrogen peroxide (H2O2) and a 60% decrease in malondialdehyde (MDA) in PpAgNPs treated sample with respect to the control group. The decrease in concentration of H2O2 and MDA in PpAgNPs treated sample was 27% and 33% in comparison to the liquid fertilizer treated group (Fig. 5A and B).
Differences in concentration of oxidative stress markers and antioxidant activity in Black gram (Vigna mungo (L.) Hepper) cv Vamban 6 plants treated with liquid fertilizer (19:19:19) vs 50 mg/L PpAgNP (1.5 mM) in pot study. A: Concentration of H2O2, B: Concentration of MDA, C: CAT enzyme activity, D: SOD enzyme activity, E: APX enzyme activity and F: POD enzyme activity. * and ** indicate p < 0.05 and p < 0.01, respectively
Antioxidant Activity
In tandem with the assessment of oxidative stress markers, the activity of enzymes involved in relieving oxidative stress was also assessed. There was a onefold increase in catalase (CAT) enzyme activity in PpAgNPs treated group compared to both liquid fertilizer and control group (Fig. 5C). Superoxide dismutase (SOD), ascorbate peroxidase (APX) and peroxidase (POD) activities were also onefold higher in PpAgNPs treated group but the difference was not significant (Fig. 5D–F).
Discussion
Plants have several biomolecules viz. phenols, polysaccharides, flavonoids, terpenes and alkaloids (Teoh 2016; Erb and Kliebenstein 2020) and many of them have a growth stimulatory effect (Erb and Kliebenstein 2020; Pang et al. 2021). Hence, plant parts can be used to biosythesize metallic nanoparticles. In this study, we synthesized spherical AgNPs with an average particle size of 54 nm using Pongamia pinnata (L.) Pierre leaf extract. Further to that, the biosynthesized PpAgNPs (1.5 mM AgNO3) were used for nano-priming of black gram (Vigna mungo (L.) Hepper) seeds. We observed a 21% enhancement in seed germination when compared to hydro primed seeds. In addition, seedling growth as estimated using the vigour index improved by 35%. This effect could be attributed to the loosening of seed coat by OH− radicals (Feizi et al. 2013), subsequent formation of nanopores on the seed coat for increased water uptake (Hussain et al. 2016) and elevation of the starch-hydrolyzing enzyme activity (Singh et al. 2020). In addition, increased germination may be because of breaking of seed dormancy where ROS play a crucial role by interacting with gibberellic acid and abscisic acid, the two plant hormones which are linked to germination and dormancy (Bailly 2019). H2O2 is also known to break the bonds within polysaccharides in seed endosperm (Guha et al. 2018). Hence, biosynthesized AgNPs could induce production of optimum ROS levels (Mittler 2017) thereby breaking seed dormancy and enhancing seed germination. Enhanced seed germination and seedling growth using biosynthesized AgNPs has also been seen earlier in chick pea (Sambangi and Gopalakrishnan 2023).
Pot experiments revealed a significant increase in several morphological and physiological parameters. Notably there was an increase in root length (77%), shoot length (78%), fresh weight (sixfold) and dry weight (threefold) when compared to the plants treated with only water. There was also an increase in plant photosynthetic pigments such as chlorophyll a (91%), chlorophyll b (32%) and carotenoid (twofold). An elevation in biomolecules such as phenols (81%), carbohydrate (86%) and protein (81%) were observed. An important measure of plant health is the chlorophyll content (Pavlović et al. 2014) and this can be directly correlated to the photosynthetic rate (Fleischer 1935). Increased chlorophyll content in PpAgNPs-treated samples can be attributed to an enhanced nutrient absorption thereby leading to production of soluble proteins as well as sugars due to an accelerated CO2 fixation (Young 1991). As a result, plants treated with PpAgNPs show higher biomass as evident from the fresh and dry weights. Such an effect has been observed previously in AgNPs-treated seedlings of Brassica juncea (Sharma et al. 2012), Oryza sativa (Gupta et al. 2018), Phaseolus vulgaris (Verma et al. 2020), and Withania coagulans (Tripathi et al. 2021). Carbohydrates act as important source of energy and are involved in synthesis of several organic compounds in plants. Additionally, they are involved in signalling and are key molecules that connect metabolism in plants to their growth and development (Trouvelot et al. 2014). Evidence also suggests that sugars can act as antioxidants and have a role in ROS scavenging (Keunen et al. 2013).
To understand the underlying mechanism of growth enhancement, the study assessed the production of ROS and subsequent ROS scavenging by antioxidative enzymes. The present study showed a lower H2O2 levels and also decreased lipid peroxidation. Plants use several antioxidative enzymes to safely dissipate ROS. These include catalase (CAT), superoxide dismutase (SOD), ascorbate peroxidase (APX) and peroxidase (POD). Changes in the activity of these enzymes may represent plant’s ability to efficiently scavenge ROS. In this study, we observed a significant increase in CAT (20%), SOD (20%) and POD activity (11%) as well as an increase in APX activity (7%). The increase in antioxidative enzyme activities indicates a fine balance between the ROS levels which have decreased and ROS scavenging activity thus promoting plant growth and development. A similar trend has been observed earlier in tobacco (Cvjetko et al. 2018) and rice (Gupta et al. 2018) and has been attributed to lower toxicity and stable nature of the AgNPs.
Phenols are secondary metabolites that protect the plants from stress, bacterial and fungal pathogens (Dakora and Phillips 1996; Lattanzio et al. 2006). Plant phenolics have also been implicated in attracting symbiotic microbes and pollinators (Ndakidemi and Dakora 2003; Bhattacharya et al. 2010). Several studies have shown the increase in phenolics upon treatment of AgNPs. In this study, elevated levels of phenols (by 81%) have been observed when treated with PpAgNPs. This could explain the increase in root nodule formation in PpAgNPs treated plants as well as improved efficiency in ROS scavenging.
Several studies have shown the negative impact of AgNPs on plant growth and development (Yin et al. 2011; Vannini et al. 2014; Tripathi et al. 2017; Matras et al. 2022; Iannelli et al. 2022). This may be related to the nature of particles synthesized, methods used for synthesis and concentrations of nanoparticles applied. In this study, we see an effect of PpAgNPs on germination, growth and development of Black gram (Vigna mungo (L.) Hepper). This is probably due to the enhanced stability, capping and biocompatibility of the synthesized AgNPs as suggested earlier (Leela and Vivekanandan 2008; Moulton et al. 2010). An increase in germination, root length, vigour index and physiological as well as biochemical parameters indicate an overall positive effect. However, we also see a decrease in shoot length with respect to the liquid fertilizer treated samples which may be because of an interplay between the plant hormones auxin and cytokinin (Ongaro and Leyser 2008; Kurepa et al. 2019). Increase in root nodule formation may be because of phenolic chemo-attractants (Bhattacharya et al. 2010). Gene expression studies will provide further insights into ROS scavenging and plant regulatory signalling networks upon treatment of PpAgNPs. These are all aspects that warrant further investigation and will be of particular interest for future studies.
Conclusion
In summary, our investigation successfully demonstrated the synthesis of AgNPs using the aqueous leaf extract of Pongamia pinnata (L.) Pierre. Characterized by a spherical morphology and an average particle size of 54 nm, these nanoparticles exhibited remarkable effects on the germination, growth, and development of black gram (Vigna mungo (L.) Hepper) seeds. Germination tests revealed a substantial 21% increase in germination when black gram seeds were treated with PpAgNPs (1.5 mM AgNO3). Furthermore, seedling growth, evaluated through the vigour index, exhibited a noteworthy 35% enhancement, highlighting the positive impact of PpAgNPs. Pot studies with black gram plants demonstrated enhanced growth and yield post-seed priming and treatment, surpassing the effects of 19:19:19 (N:P:K) liquid fertilizer. This enhancement manifested in increased root length, pod weight, and grain weight, underscoring the potential of PpAgNPs in promoting plant growth. Biochemical analyses provided insights into the molecular changes induced by PpAgNPs treatment. Notably, there was a significant increase in total phenol and carbohydrate concentrations, indicative of the stimulatory effects on plant metabolism. A decrease in ROS levels were observed, the rise in catalase activity suggested an effective antioxidative response, maintaining a delicate balance conducive to plant growth. However, further research is needed to investigate the specific mechanisms involved in growth promotion. A deeper understanding into hormonal interplay and assessment of hydrolytic enzyme activity in the seed as well as plant treated with PpAgNPs will be of interest. In addition, investigations into the expression levels of genes involved in efficient ROS scavenging as well as primary and secondary metabolite synthesis will reveal the exact mechanism of growth promotion.
The biocompatibility and efficient ROS scavenging observed in this study collectively contribute to an overall positive effect of PpAgNPs on the germination, growth, and development of black gram. This implies that Pongamia pinnata (L.) Pierre holds promise as a reliable source for the biosynthesis of AgNPs, capable of inducing growth stimulatory effects in diverse plant species. As we conclude, the findings encourage further exploration into the potential applications of PpAgNPs in agriculture and plant science. Assessment of the long-term impact of PpAgNPs on plant growth and soil health will be crucial for deeming its use as a sustainable and eco-friendly approach to enhance crop productivity.
References
Abdul-Baki AA, Anderson JD (1973) Vigor determination in Soybean Seed by multiple criteria1. Crop Sci 13:630–633. https://doi.org/10.2135/CROPSCI1973.0011183X001300060013X
Acharya P, Jayaprakasha GK, Crosby KM et al (2020) Nanoparticle-mediated seed priming improves germination, growth, yield, and quality of watermelons (Citrullus lanatus) at multi-locations in Texas. Sci Rep 10:1–16. https://doi.org/10.1038/s41598-020-61696-7
Agnihotri A, Seth CS (2020) Does jasmonic acid regulate photosynthesis, clastogenecity, and phytochelatins in Brassica juncea L. in response to Pb-subcellular distribution? Chemosphere 243:125361
Alabdallah NM, Hasan MM (2021) Plant-based green synthesis of silver nanoparticles and its effective role in abiotic stress tolerance in crop plants. Saudi J Biol Sci 28:5631–5639. https://doi.org/10.1016/J.SJBS.2021.05.081
Al-Huqail AA, Hatata MM, Al-Huqail AA, Ibrahim MM (2018) Preparation, characterization of silver phyto nanoparticles and their impact on growth potential of Lupinus termis L. seedlings. Saudi J Biol Sci 25:313–319. https://doi.org/10.1016/J.SJBS.2017.08.013
Al-khattaf FS (2021) Gold and silver nanoparticles: Green synthesis, microbes, mechanism, factors, plant disease management and environmental risks. Saudi J Biol Sci 28:3624–3631. https://doi.org/10.1016/J.SJBS.2021.03.078
Azeez L, Adebisi SA, Adetoro RO et al (2022) Foliar application of silver nanoparticles differentially intervenes remediation statuses and oxidative stress indicators in Abelmoschus esculentus planted on gold-mined soil. Int J Phytoremediation 24:384–393. https://doi.org/10.1080/15226514.2021.1949578
Bailly C (2019) The signalling role of ROS in the regulation of seed germination and dormancy. Biochem J 476:3019–3032. https://doi.org/10.1042/BCJ20190159
Balasooriya D, Karunarathna C, Uluwaduge I (2021) Wound healing potential of bark paste of Pongamia pinnata along with hirudotherapy: a case report. J Ayurveda Integr Med 12:384. https://doi.org/10.1016/J.JAIM.2021.01.014
Basavegowda N, Baek KH (2021) Current and future perspectives on the use of nanofertilizers for sustainable agriculture: the case of phosphorus nanofertilizer. 3 Biotech 117(11):1–21. https://doi.org/10.1007/S13205-021-02907-4
Beg M, Maji A, Mandal AK et al (2017) Green synthesis of silver nanoparticles using Pongamia pinnata seed: Characterization, antibacterial property, and spectroscopic investigation of interaction with human serum albumin. J Mol Recognit 30:e2565. https://doi.org/10.1002/jmr.2565
Behravan M, Hossein Panahi A, Naghizadeh A et al (2019) Facile green synthesis of silver nanoparticles using Berberis vulgaris leaf and root aqueous extract and its antibacterial activity. Int J Biol Macromol 124:148–154. https://doi.org/10.1016/J.IJBIOMAC.2018.11.101
Beyer WF, Fridovich I (1987) Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem 161:559–566. https://doi.org/10.1016/0003-2697(87)90489-1
Bhandirge SK, Tripathi AS, Bhandirge RK et al (2015) Evaluation of wound healing activity of ethanolic extract of Pongamia pinnata Bark. Drug Res (stuttg) 65:296–299. https://doi.org/10.1055/S-0034-1384537
Bhattacharya A, Sood P, Citovsky V (2010) The roles of plant phenolics in defence and communication during Agrobacterium and Rhizobium infection. Mol Plant Pathol 11:705–719. https://doi.org/10.1111/J.1364-3703.2010.00625.X
Casula L, Lai F, Pini E et al (2021) Pulmonary delivery of curcumin and beclomethasone dipropionate in a multicomponent nanosuspension for the treatment of bronchial asthma. Pharmaceutics 13:1300. https://doi.org/10.3390/PHARMACEUTICS13081300/S1
Chow PS, Landhäusser SM (2004) A method for routine measurements of total sugar and starch content in woody plant tissues. Tree Physiol 24:1129–1136. https://doi.org/10.1093/treephys/24.10.1129
Cvjetko P, Zovko M, Štefanić PP et al (2018) Phytotoxic effects of silver nanoparticles in tobacco plants. Environ Sci Pollut Res 25:5590–5602. https://doi.org/10.1007/S11356-017-0928-8/METRICS
Dakora FD, Phillips DA (1996) Diverse functions of isoflavonoids in legumes transcend anti-microbial definitions of phytoalexins. Physiol Mol Plant Pathol 49:1–20. https://doi.org/10.1006/PMPP.1996.0035
Das P, Barua S, Sarkar S et al (2018) Plant extract–mediated green silver nanoparticles: efficacy as soil conditioner and plant growth promoter. J Hazard Mater 346:62–72. https://doi.org/10.1016/j.jhazmat.2017.12.020
Degani E, Prasad MVR, Paradkar A et al (2022) A critical review of Pongamia pinnata multiple applications: from land remediation and carbon sequestration to socioeconomic benefits. J Environ Manage 324:116297. https://doi.org/10.1016/J.JENVMAN.2022.116297
Du Z, Bramlage WJ (1992) Modified thiobarbituric acid assay for measuring lipid oxidation in sugar-rich plant tissue extracts. J Agric Food Chem 40:1566–1570. https://doi.org/10.1021/JF00021A018/ASSET/JF00021A018.FP.PNG_V03
Dutta Gupta S, Datta S (2004) Antioxidant enzyme activities during in vitro morphogenesis of gladiolus and the effect of application of antioxidants on plant regeneration. Biol Plant 47:179–183. https://doi.org/10.1023/B:BIOP.0000022248.62869.C7/METRICS
Dwivedi D, Dwivedi M, Malviya S, Singh V (2017) Evaluation of wound healing, anti-microbial and antioxidant potential of Pongamia pinnata in wistar rats. J Tradit Complement Med 7:79–85. https://doi.org/10.1016/j.jtcme.2015.12.002
Erb M, Kliebenstein DJ (2020) Plant secondary metabolites as defenses, regulators, and primary metabolites: the blurred functional trichotomy. Plant Physiol 184:39–52. https://doi.org/10.1104/PP.20.00433
Feizi H, Moghaddam PR, Shahtahmassebi N, Fotovat A (2013) Assessment of concentrations of nano and bulk iron oxide particles on early growth of wheat (Triticum aestivum L.). Annu Res Rev Biol 3:752–761
Fleischer WE (1935) The relation between chlorophyll content and rate of photosynthesis. J Gen Physiol 18:573. https://doi.org/10.1085/JGP.18.4.573
Ghosh S, Ahmad R, Banerjee K et al (2021) Mechanistic aspects of microbe-mediated nanoparticle synthesis. Front Microbiol 12:867. https://doi.org/10.3389/FMICB.2021.638068/BIBTEX
Gogoi N, Babu PJ, Mahanta C, Bora U (2015) Green synthesis and characterization of silver nanoparticles using alcoholic flower extract of Nyctanthes arbortristis and in vitro investigation of their antibacterial and cytotoxic activities. Mater Sci Eng C 46:463–469. https://doi.org/10.1016/J.MSEC.2014.10.069
Guha T, Ravikumar KVG, Mukherjee A et al (2018) Nanopriming with zero valent iron (nZVI) enhances germination and growth in aromatic rice cultivar (Oryza sativa cv. Gobindabhog L.). Plant Physiol Biochem 127:403–413. https://doi.org/10.1016/J.PLAPHY.2018.04.014
Gupta S, Seth CS (2021) Salicylic acid alleviates chromium (VI) toxicity by restricting its uptake, improving photosynthesis and augmenting antioxidant defense in Solanum lycopersicum L. Physiol Mol Biol Plants 27:2651–2664. https://doi.org/10.1007/S12298-021-01088-X
Gupta SD, Agarwal A, Pradhan S (2018) Phytostimulatory effect of silver nanoparticles (AgNPs) on rice seedling growth: an insight from antioxidative enzyme activities and gene expression patterns. Ecotoxicol Environ Saf 161:624–633. https://doi.org/10.1016/J.ECOENV.2018.06.023
Hussain S, Khan F, Hussain HA, Nie L (2016) Physiological and biochemical mechanisms of seed priming-induced chilling tolerance in rice cultivars. Front Plant Sci 7:178194. https://doi.org/10.3389/FPLS.2016.00116/BIBTEX
Hussain M, Raja NI, Iqbal M et al (2018) Seed germination and biochemical profile of Citrus reticulata (Kinnow) exposed to green synthesised silver nanoparticles. IET Nanobiotechnol 12:688–693. https://doi.org/10.1049/iet-nbt.2017.0303
Iannelli MA, Bellini A, Venditti I et al (2022) Differential phytotoxic effect of silver nitrate (AgNO3) and bifunctionalized silver nanoparticles (AgNPs-Cit-L-Cys) on Lemna plants (duckweeds). Aquat Toxicol 250:106260. https://doi.org/10.1016/J.AQUATOX.2022.106260
Iqbal M, Raja NI, Mashwani ZUR et al (2019) Assessment of AgNPs exposure on physiological and biochemical changes and antioxidative defence system in wheat (Triticum aestivum L.) under heat stress. IET Nanobiotechnol 13:230–236. https://doi.org/10.1049/IET-NBT.2018.5041
Iravani S, Korbekandi H, Mirmohammadi SV, Zolfaghari B (2014) Synthesis of silver nanoparticles: chemical, physical and biological methods. Res Pharm Sci 9:385
Ismail G, Abou-Zeid H (2018) The role of priming with biosynthesized silver nanoparticles in the response of Triticum aestivum L. to salt stress. Egypt J Bot. https://doi.org/10.21608/ejbo.2017.1873.1128
Jadoun S, Arif R, Jangid NK, Meena RK (2021) Green synthesis of nanoparticles using plant extracts: a review. Environ Chem Lett 19:355–374
Jhansi K, Jayarambabu N, Reddy KP et al (2017) Biosynthesis of MgO nanoparticles using mushroom extract: effect on peanut (Arachis hypogaea L.) seed germination. 3 Biotech 7:1–11. https://doi.org/10.1007/s13205-017-0894-3
Kar M, Mishra D (1976) Catalase, peroxidase, and polyphenoloxidase activities during rice leaf senescence. Plant Physiol 57:315–319. https://doi.org/10.1104/pp.57.2.315
Karthik T, Sarkar G, Babu S et al (2020) Preparation and evaluation of liquid fertilizer from Turbinaria ornata and Ulva reticulata. Biocatal Agric Biotechnol 28:101712. https://doi.org/10.1016/j.bcab.2020.101712
Keunen E, Peshev D, Vangronsveld J et al (2013) Plant sugars are crucial players in the oxidative challenge during abiotic stress: extending the traditional concept. Plant Cell Environ 36:1242–1255. https://doi.org/10.1111/PCE.12061
Khalid S, Shahid M, Dumat C et al (2017) Influence of groundwater and wastewater irrigation on lead accumulation in soil and vegetables: Implications for health risk assessment and phytoremediation. Int J Phytoremediation 19:1037–1046. https://doi.org/10.1080/15226514.2017.1319330
Khatua A, Priyadarshini E, Rajamani P et al (2020) Phytosynthesis, characterization and fungicidal potential of emerging gold nanoparticles using Pongamia pinnata leave extract: a novel approach in nanoparticle synthesis. J Clust Sci 31:125–131. https://doi.org/10.1007/s10876-019-01624-6
Kishanji M, Mamatha G, Madhuri D et al (2020) Preparation and characterization of cellulose/in situ generated silver nanoparticle composite films prepared using Pongamia pinnata leaf extract as a reducing and stabilizing agent. Inorg Nano-Metal Chem. https://doi.org/10.1080/247015562020182286951:1207-1213
Krishnasamy R, Obbineni JM (2022) Methods for green synthesis of metallic nanoparticles using plant extracts and their biological applications—a review. J Biomimetics Biomater Biomed Eng 56:75–151. https://doi.org/10.4028/P-8BF786
Kumar D, Seth CS (2021) Green-synthesis, characterization, and applications of nanoparticles (NPs) a mini review. Int J Plant Environ 7:91–95. https://doi.org/10.18811/ijpen.v7i01.11
Kurepa J, Shull TE, Smalle JA (2019) Antagonistic activity of auxin and cytokinin in shoot and root organs. Plant Direct. https://doi.org/10.1002/PLD3.121
Lattanzio V, Lattanzio VMT, Cardinali A (2006) Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. Phytochem Adv Res 661:23–67
Leela A, Vivekanandan M (2008) Tapping the unexploited plant resources for the synthesis of silver nanoparticles. African J Biotechnol 7:3162–3165
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Malaikozhundan B, Vinodhini J (2018) Nanopesticidal effects of Pongamia pinnata leaf extract coated zinc oxide nanoparticle against the Pulse beetle, Callosobruchus maculatus. Mater Today Commun 14:106–115. https://doi.org/10.1016/j.mtcomm.2017.12.015
Malaikozhundan B, Vaseeharan B, Vijayakumar S et al (2017) Biological therapeutics of Pongamia pinnata coated zinc oxide nanoparticles against clinically important pathogenic bacteria, fungi and MCF-7 breast cancer cells. Microb Pathog 104:268–277. https://doi.org/10.1016/j.micpath.2017.01.029
Martins GR, Monteiro AF, do Amaral FRL, da Silva AS (2021) A validated Folin-Ciocalteu method for total phenolics quantification of condensed tannin-rich açaí (Euterpe oleracea Mart.) seeds extract. J Food Sci Technol 58:4693–4702. https://doi.org/10.1007/S13197-020-04959-5/METRICS
Masum MI, Siddiqa MM, Ali KA et al (2019) Biogenic synthesis of silver nanoparticles using phyllanthus emblica fruit extract and its inhibitory action against the pathogen acidovorax oryzae strain rs-2 of rice bacterial brown stripe. Front Microbiol. https://doi.org/10.3389/FMICB.2019.00820
Mathur C, Rai S, Sase N et al (2015) Enteromorpha intestinalis derived seaweed liquid fertilizers as prospective biostimulant for glycine max. Brazilian Arch Biol Technol 58:813
Matras E, Gorczyca A, Pociecha E et al (2022) Phytotoxicity of silver nanoparticles with different surface properties on monocots and dicots model plants. J Soil Sci Plant Nutr 22:1647–1664. https://doi.org/10.1007/S42729-022-00760-9/FIGURES/5
Mittler R (2017) ROS are Good. Trends Plant Sci 22:11–19. https://doi.org/10.1016/j.tplants.2016.08.002
Moulton MC, Braydich-Stolle LK, Nadagouda MN et al (2010) Synthesis, characterization and biocompatibility of “green” synthesized silver nanoparticles using tea polyphenols. Nanoscale 2:763–770. https://doi.org/10.1039/C0NR00046A
Mustafa G, Hasan M, Yamaguchi H et al (2020) A comparative proteomic analysis of engineered and bio synthesized silver nanoparticles on soybean seedlings. J Proteomics 224:103833. https://doi.org/10.1016/J.JPROT.2020.103833
Mustapha T, Misni N, Ithnin NR et al (2022) A Review on Plants and Microorganisms Mediated Synthesis of Silver Nanoparticles, Role of Plants Metabolites and Applications. Int J Environ Res Public Health 19:674
Najafi S, Razavi SM, Khoshkam M, Asadi A (2020) Effects of green synthesis of sulfur nanoparticles from Cinnamomum zeylanicum barks on physiological and biochemical factors of Lettuce (Lactuca sativa). Physiol Mol Biol Plants 26:1055–1066. https://doi.org/10.1007/s12298-020-00793-3
Nakano Y, Asada K (1981) Hydrogen peroxide is Scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880. https://doi.org/10.1093/OXFORDJOURNALS.PCP.A076232
Ndakidemi PA, Dakora FD (2003) Legume seed flavonoids and nitrogenous metabolites as signals and protectants in early seedling development. Funct Plant Biol 30:729–745. https://doi.org/10.1071/FP03042
Ongaro V, Leyser O (2008) Hormonal control of shoot branching. J Exp Bot 59:67–74. https://doi.org/10.1093/JXB/ERM134
Oraibi AG, Yahia HN, Alobaidi KH (2022) Green biosynthesis of silver nanoparticles using Malva parviflora extract for improving a new nutrition formula of a hydroponic system. Scientifica (cairo). https://doi.org/10.1155/2022/4894642
Pang Z, Chen J, Wang T et al (2021) Linking plant secondary metabolites and plant microbiomes: a review. Front Plant Sci 12:300. https://doi.org/10.3389/FPLS.2021.621276/BIBTEX
Pavlović D, Nikolić B, Đurović S et al (2014) Chlorophyll as a measure of plant health: agroecological aspects. Pestic Phytomed 29:21–34
Radhakumari M, Taha M, Shahsavari E et al (2017) Pongamia pinnata seed residue—a low cost inedible resource for on-site/in-house lignocellulases and sustainable ethanol production. Renew Energy 103:682–687. https://doi.org/10.1016/j.renene.2016.10.082
Rafique M, Sadaf I, Rafique MS, Tahir MB (2017) A review on green synthesis of silver nanoparticles and their applications. Artif Cells Nanomed Biotechnol 45:1272–1291. https://doi.org/10.1080/21691401.2016.1241792
Ragab GA, Saad-Allah KM (2020) Green synthesis of sulfur nanoparticles using Ocimum basilicum leaves and its prospective effect on manganese-stressed Helianthus annuus (L.) seedlings. Ecotoxicol Environ Saf 191:110242. https://doi.org/10.1016/J.ECOENV.2020.110242
Rahman MS, Chakraborty A, Kibria A, Hossain MJ (2023) Effects of silver nanoparticles on seed germination and growth performance of pea (Pisum sativum). Plant Nano Biol 5:100042. https://doi.org/10.1016/J.PLANA.2023.100042
Raliya R, Biswas P, Tarafdar JC (2015) TiO2 nanoparticle biosynthesis and its physiological effect on mung bean (Vigna radiata L.). Biotechnol Rep 5:22–26. https://doi.org/10.1016/J.BTRE.2014.10.009
Rani PU, Yasur J, Loke KS, Dutta D (2016) Effect of synthetic and biosynthesized silver nanoparticles on growth, physiology and oxidative stress of water hyacinth: Eichhornia crassipes (Mart) Solms. Acta Physiol Plant 38:1–9. https://doi.org/10.1007/S11738-016-2074-1/METRICS
Raut RW, Kolekar NS, Lakkakula JR et al (2010) Extracellular synthesis of silver nanoparticles using dried leaves of Pongamia pinnata (L.) pierre. Nano-Micro Lett 2:106–113. https://doi.org/10.1007/bf03353627
Roy S, Ananthraman P (2017) Green synthesis of silver nanoparticles by Sargassum cinctum J. Agardh and their potential for seed germination. Int J Trend Sci Res Dev 1:1216–1225
Roy S, Ananthraman P (2018) Biosynthesis of Silver Nanoparticle by Amphiroa anceps (Lamarck) Decaisne and its biomedical and ecological implications. J Nanomed Nanotechnol. https://doi.org/10.4172/2157-7439.1000492
Sabir S, Zahoor MA, Waseem M et al (2020) Biosynthesis of ZnO nanoparticles using Bacillus subtilis: characterization and nutritive significance for promoting plant growth in Zea mays L. Dose Response 18:1559325820958911. https://doi.org/10.1177/1559325820958911
Sadak MS (2019) Impact of silver nanoparticles on plant growth, some biochemical aspects, and yield of fenugreek plant (Trigonella foenum-graecum). Bull Natl Res Cent. https://doi.org/10.1186/S42269-019-0077-Y
Salem SS, Fouda A (2020) Green synthesis of metallic nanoparticles and their prospective biotechnological applications: an overview. Biol Trace Elem Res 199:344–370. https://doi.org/10.1007/S12011-020-02138-3
Salem NM, Albanna LS, Abdeen AO et al (2016a) Sulfur nanoparticles improves root and shoot growth of tomato. J Agric Sci 8:179. https://doi.org/10.5539/jas.v8n4p179
Salem NM, Albanna LS, Awwad AM (2016b) Green synthesis of sulfur nanoparticles using Punica granatum peels and the effects on the growth of tomato by foliar spray applications. Environ Nanotechnol, Monit Manag 6:83–87. https://doi.org/10.1016/J.ENMM.2016.06.006
Sambangi P, Gopalakrishnan S (2023) Streptomyces-mediated synthesis of silver nanoparticles for enhanced growth, yield, and grain nutrients in chickpea. Biocatal Agric Biotechnol 47:102567. https://doi.org/10.1016/J.BCAB.2022.102567
Sangeetha J, Hospet R, Thangadurai D et al (2021) Nanopesticides, nanoherbicides, and nanofertilizers: the greener aspects of agrochemical synthesis using nanotools and nanoprocesses toward sustainable agriculture. Handbook of nanomaterials and nanocomposites for energy and environmental applications. Springer International Publishing, Cham, pp 1–15
Santhosh AS, Sandeep S, Kumara Swamy N (2019) Green synthesis of nano silver from euphorbia geniculata leaf extract: Investigations on catalytic degradation of methyl orange dye and optical sensing of Hg2+. Surfaces and Interfaces 14:50–54. https://doi.org/10.1016/J.SURFIN.2018.11.004
Saratale RG, Karuppusamy I, Saratale GD et al (2018) A comprehensive review on green nanomaterials using biological systems: recent perception and their future applications. Colloids Surfaces B Biointerfaces 170:20–35. https://doi.org/10.1016/J.COLSURFB.2018.05.045
Sharma P, Bhatt D, Zaidi MGH et al (2012) Silver nanoparticle-mediated enhancement in growth and antioxidant status of Brassica juncea. Appl Biochem Biotechnol 167:2225–2233. https://doi.org/10.1007/S12010-012-9759-8/METRICS
Singh D, Agnihotri A, Seth CS (2017) Interactive effects of EDTA and oxalic acid on chromium uptake, translocation and photosynthetic attributes in Indian mustard (Brassica juncea L. var. Varuna). Curr Sci 112:2034–2042
Singh J, Dutta T, Kim KH et al (2018) “Green” synthesis of metals and their oxide nanoparticles: applications for environmental remediation. J Nanobiotechnol 16:1–24
Singh Y, Kaushal S, Sodhi RS (2020) Biogenic synthesis of silver nanoparticles using cyanobacterium Leptolyngbya sp. WUC 59 cell-free extract and their effects on bacterial growth and seed germination. Nanoscale Adv 2:3972–3982. https://doi.org/10.1039/D0NA00357C
Surendra DM, Chamaraja NA, Yallappa S et al (2023) Efficacy of phytochemical-functionalized silver nanoparticles to control Flacherie and Sappe silkworm diseases in Bombyx mori L. larvae. Plant Nano Biol 5:100048. https://doi.org/10.1016/J.PLANA.2023.100048
Taha ZK, Hawar SN, Sulaiman GM (2019) Extracellular biosynthesis of silver nanoparticles from Penicillium italicum and its antioxidant, antimicrobial and cytotoxicity activities. Biotechnol Lett 41:899–914. https://doi.org/10.1007/S10529-019-02699-X/METRICS
Teoh ES (2016) Secondary metabolites of plants. Med Orchid Asia. https://doi.org/10.1007/978-3-319-24274-3_5
Tran DH, Takagp M, Ueno T (2017) Efficacy of the extract from pongam leaves (Pongamia pinnata L.) against Spodoptera exigua (Hübner) and Spodoptera litura fabricius (Lepidoptera: Noctuidae). J Fac Agric Kyushu Univ 62:439–443. https://doi.org/10.5109/1854018
Tripathi DK, Singh S, Singh S et al (2017) Nitric oxide alleviates silver nanoparticles (AgNps)-induced phytotoxicity in Pisum sativum seedlings. Plant Physiol Biochem PPB 110:167–177. https://doi.org/10.1016/J.PLAPHY.2016.06.015
Tripathi D, Rai KK, Pandey-Rai S (2020) Impact of green synthesized WcAgNPs on in-vitro plant regeneration and withanolides production by inducing key biosynthetic genes in Withania coagulans. Plant Cell Rep 40:283–299. https://doi.org/10.1007/S00299-020-02630-Z
Tripathi D, Rai KK, Pandey-Rai S (2021) Impact of green synthesized WcAgNPs on in-vitro plant regeneration and withanolides production by inducing key biosynthetic genes in Withania coagulans. Plant Cell Rep 40:283–299. https://doi.org/10.1007/S00299-020-02630-Z/METRICS
Tripathi DK, Kandhol N, Rai P et al (2023) Ethylene renders silver nanoparticles stress tolerance in rice seedlings by regulating endogenous nitric oxide accumulation. Plant Cell Physiol 63:1954–1967. https://doi.org/10.1093/PCP/PCAC159
Trouvelot S, Héloir MC, Poinssot B et al (2014) Carbohydrates in plant immunity and plant protection: roles and potential application as foliar sprays. Front Plant Sci. https://doi.org/10.3389/FPLS.2014.00592
Vanlalveni C, Lallianrawna S, Biswas A et al (2021) Green synthesis of silver nanoparticles using plant extracts and their antimicrobial activities: a review of recent literature. RSC Adv 11:2804–2837. https://doi.org/10.1039/D0RA09941D
Vannini C, Domingo G, Onelli E et al (2014) Phytotoxic and genotoxic effects of silver nanoparticles exposure on germinating wheat seedlings. J Plant Physiol 171:1142–1148. https://doi.org/10.1016/J.JPLPH.2014.05.002
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci 151:59–66. https://doi.org/10.1016/S0168-9452(99)00197-1
Verma DK, Patel S, Kushwah KS (2020) Green biosynthesis of silver nanoparticles and impact on growth, chlorophyll, yield and phytotoxicity of Phaseolus vulgaris L. Vegetos 33:648–657. https://doi.org/10.1007/S42535-020-00150-5/METRICS
Vijai Anand K, Anugraga AR, Kannan M et al (2020) Bio-engineered magnesium oxide nanoparticles as nano-priming agent for enhancing seed germination and seedling vigour of green gram (Vigna radiata L.). Mater Lett 271:127792. https://doi.org/10.1016/J.MATLET.2020.127792
Yadav M, Gupta P, Seth CS (2022) Foliar application of α-lipoic acid attenuates cadmium toxicity on photosynthetic pigments and nitrogen metabolism in Solanum lycopersicum L. Acta Physiol Plant 44:1–10. https://doi.org/10.1007/S11738-022-03445-Z/METRICS
Yin L, Cheng Y, Espinasse B et al (2011) More than the ions: the effects of silver nanoparticles on Lolium multiflorum. Environ Sci Technol 45:2360–2367. https://doi.org/10.1021/ES103995X
Young AJ (1991) The photoprotective role of carotenoids in higher plants. Physiol Plant 83:702–708. https://doi.org/10.1111/J.1399-3054.1991.TB02490.X
Acknowledgements
Jagan M. Obbineni acknowledges funding support from the Science and Engineering Research Board (SERB), Department of Science and Technology, Govt. of India under the Startup Research Grant (Project No. SRG/2020/001690) and Teachers Associateship for Research Excellence Grant (Project No. TAR/2022/000312) and VIT under the VIT Seed Grant. The authors would like to thank the School of Advanced Sciences, VIT for access to the powder X-ray diffractometer and Fourier transform infrared spectrophotometer. The authors would also like to thank School of Biosciences and Technology, VIT for access to the particle size analyzer.
Author information
Authors and Affiliations
Contributions
JMO and RK conceptualized and designed the study. Experiments were performed and results were analyzed by RK. Samples were imaged on TEM by RN. RK prepared the draft manuscript. JMO edited and prepared the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors have no competing interests to declare.
Additional information
Handling Editor: Durgesh Kumar Tripathi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Krishnasamy, R., Natesh, R. & Obbineni, J.M. Efficient ROS Scavenging Improves the Growth and Yield in Black Gram (Vigna mungo (L.) Hepper) after Seed Priming and Treatment using Biosynthesized Silver Nanoparticles with Pongamia pinnata (L.) Pierre Leaf Extract. J Plant Growth Regul 43, 2422–2438 (2024). https://doi.org/10.1007/s00344-024-11276-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-024-11276-0