Abstract
The variation of light intensity has obvious effects on leaf external morphology, internal anatomy, and physiological characteristics; it even induces changes in secondary metabolite production. The effects of different irradiance levels on biomass, gas exchange parameters, and photosynthetic pigment contents in Mahonia bodinieri (Gagnep.) Laferr. were analyzed here. Combined analyses of physiology, cytology, and HPLC were used to study the differences in leaf morphology, structure, physiological characters, and alkaloid content in response to different irradiances. The results indicated that the highest foliar biomass was observed under I 50 (50 % of full sunlight) followed by I 30 (30 % of full sunlight), the highest net photosynthetic rate, stomatal conductance, transpiration rate values were observed under I 30 followed by I 50, and lower values occurred in I 10 (10 % of full sunlight) and I 100 (full sunlight). With increased light intensity, total leaf area and the contents of chlorophyll a (Chl a), chlorophyll b (Chl b), and chlorophyll (Chl a+b) per unit leaf area were clearly reduced, whereas leaf mass per area, carotenoid content, leaf thickness, thickness of palisade and spongy parenchyma, and stomatal density were all significantly increased. Electron microscopic observation revealed that the number of grana, stroma lamellae and the number of starch grains in chloroplasts were decreased, the number of plastoglobuli was increased when irradiance levels increased. The estimated total yield of alkaloids in a single plant was higher under I 30 and I 50 than under I 10 or I 100 as a result of the higher biomass of the plants. Therefore, I 30 and I 50 were not only beneficial to increase biomass, but also suitable for the synthesis and accumulation of the major secondary metabolites (alkaloids). Our findings provide valuable data for the determination and regulation of irradiance levels during artificial cultivation of M. bodinieri.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Irradiance is one of the most essential factors affecting plant growth and physiological functions (Aleric and Kirkman 2005). In general, light-demanding plants can survive and grow only under high light, whereas shade-tolerant plants can survive in deep shade (Augspurger 1984; Kitajima 1994). Therefore, leaf acclimation to the light environment is an important factor to increase net carbon gain. In the process of acclimation to various environments, the plant can modify its morphology, anatomy, and physiological characteristics. Leaves are the major organs for photosynthesis. Significant modifications are observed in foliar external morphology, internal anatomy and physiological characteristics in response to irradiance changes (Evans and Poorter 2001). Compared with shade-tolerant species, typical sun leaves are thicker and smaller with higher stomatal density on both surfaces as well as more developed palisade tissue (Murchie and Horton 1997). Moreover, with increased irradiance, the ultrastructure of chloroplasts also changes significantly. For instance, the number of chloroplasts under high light is less than under low light conditions. In addition, sun-type chloroplasts exposed to high light intensity have less thylakoid membrane appression (e.g., lower grana). On the contrary, more appressed thylakoid membranes present in shade-type chloroplasts (Anderson et al. 2008; Yin et al. 2012). Additionally, plants grown under low light often have decreased photosynthetic enzymes activity (e.g., ribulose bisphosphate carboxylase/oxygenase) and reduced net photosynthetic rate; thus, this reduction in carbon gain may inhibit growth (Liao et al. 2005). Plants growing under high fluorescence intensity can absorb a large number of photons to maintain higher photosynthetic net rate and thereby increase biomass significantly, but long time exposure under high light conditions may damage the photosynthetic apparatus, with reduced photosynthetic capability as well as in biomass synthesis and accumulation, and probably results in chronic photo-inhibition (Hua et al. 2007; Liao et al. 2005). Furthermore, the adaptation of plant morphology, anatomy, and physiological functions to the changes in light intensity may influence the accumulation of secondary metabolites (Ma et al. 2010). For example, an increase in light intensity may promote the synthesis and accumulation of total phenolics and flavonoids in young ginger (Ghasemzadeh et al. 2010), but excessive light intensity may result in low photosynthetic capability and low flavonoid contents in Anoectochilus (Ma et al. 2010), whereas shading strongly reduces total volatile oil content in fresh leaves of basil (Ocimum basilicum L.) (Chang et al. 2008). Similarly, light conditions can also influence alkaloid component synthesis and accumulation by altering gene expression and enzyme activity in the alkaloid synthesis pathway (Liu et al. 2011; Pompelli et al. 2013). Therefore, for the cultivation of medicinal plants, it is very important to study the responses of photosynthetic physiological characteristics of plants to different irradiance conditions and the plasticity of leaf morphology and anatomy, and the relationships of these factors with the formation of secondary metabolites.
Mahonia bodinieri (Gagnep.) Laferr. belongs to genus Mahonia (Berberidaceae) and is endemic to China. The dry leaves from M. bodinieri have been widely used as the raw material “GongLaoYe” in traditional Chinese medicine (Ye 2009; Zhejiang Food and Drug Administration 2005). The major bioactive components in the raw plant material of “GongLaoYe” are alkaloids including berberine, jatrorrhizine, and palmatine (Zeng et al. 2006). M. bodinieri is a short shrub mainly distributed in the southern China including Guangdong, Guangxi, Guizhou, Hunan, Jiangxi, Zhejiang and Sichuan provinces; it exists in three types of forests: evergreen broad-leaved forests, mixed evergreen and deciduous forests, forests and coniferous forests, at an altitude of 100–1800 m (Liu and He 2010). In recent years, with the increasing demand for “GongLaoYe” on the global market, the number of plants in the wild populations is rapidly decreasing so that the yields from wild “GongLaoYe” resources do not satisfy the market demand. The technique of artificial cultivation represents an effective way to protect and utilize the wild plant resources. Therefore, understanding the effects of irradiation levels on plant growth and development, especially on the accumulation of secondary metabolites, is key to successful and economically viable cultivation of medicinal plants. Here, the physiological responses of M. bodinieri to different light conditions were studied on the basis of variation of gas exchange parameters and photosynthetic pigment contents under different irradiances combined with changes of biomass. The relationships between chloroplast ultrastructure and main secondary metabolites content were analyzed using transmission electron microscopy (TEM) and high performance liquid chromatography (HPLC). This study provides a theoretical basis and technical support for the cultivation and breeding of M. bodinieri.
Materials and methods
Plant material and growth conditions
The study was conducted in the experimental farm of Guangxi Institute of Botany in Yanshan, Guilin city, located in Guilin, China (110°17′E, 25°01′N), 150 m above sea level and under a subtropical monsoon climate. The yearly average temperature is 19.2 °C. There is a high photosynthetic photon flux density (PPFD) in daytime (maximum value is approximately 2000 µmol photons m−2 s−1 at noon in summer). Annual average rainfall is 1865.7 mm, which peaks in April–August, and the annual average relative humidity is 78 %.
Seeds of M. bodinieri were sown on March 15, 2011 in the experimental farm of Guangxi Institute of Botany. Seedlings of M. bodinieri of uniform sizes (15–17 cm in height) were selected and transplanted into pots (height: 25 cm, diameter: 25 cm) with a mixture of limestone mountain soil and peat soil (1:1, v/v; pH at 5.8) on April 15, 2012. Two weeks later, the experimental plants were treated under four different irradiances: 10 % (I 10), 30 % (I 30), 50 % (I 50), and 100 % (I 100) of full sunlight (the irradiance intensity of full sunlight in an experiment conducted on the farm was approximately 2000 ± 20 µmol photons m−2 s−1 at noon as determined by a Li-6400 portable photosynthesis system (Li-6400, LI-CoR, Lincoln, NE, USA). Light intensity control was treated according to the method of Liao et al. (2005) with modification. Each treatment included 20 pots, there were a total of 80 pots for four treatments. In addition, the positions of the pots were changed randomly every day to ensure that each individual received averagely equal irradiance under each treatment. The physiological experiment and sample collection were conducted after 6 months’ irradiance for each treatment (October 15, 2012).
Biomass and LMA
Five plants from 20 pots in each treatment were randomly collected to determine total leaf area, leaf dry biomass, and total dry biomass. These physiological parameters were measured using the method of Tang et al. (2015).
Gas exchange
The largest mature leaves (in fact leaflets) at the top of a compound leaf from the third branch counting top down from the plant trunk were selected to measure the photosynthesis in M. bodinieri with Li-6400. Data were collected from five leaves of five different plants per irradiation treatment. The selected leaves were oriented westward and horizontally. The gas exchange parameters were measured and calculated according to the method of Xia et al. (2015). All data were recorded from 9:00 to 11:00 am on a calm and cloudless day. There was no photo-inhibition occurred in this period, thus the maximum photosynthetic rates could be measured for these M. bodinieri plants.
Chemical determinations
Determination of photosynthetic pigment
The chlorophyll (Chl) and carotenoid (Car) contents were measured according to the method of Tang et al. (2015). Fresh leaf samples were first cut into pieces and weighed 0.10 g, then put into volumetric flask and adjusted to a final volume of 25 mL with extraction buffer (acetone:ethyl alcohol:distilled water = 4.5:4.5:1). Samples were kept in the dark at room temperature for 24–48 h in this buffer until the leaf pieces turned completely white. The extraction solution was then homogenized by hand shaking, 3 mL of the supernatant was sucked up into a colorimetric cup, and the absorbance was determined at 663, 646 and 470 nm. The same buffer (without leaves) was used as blank.
HPLC analysis of alkaloids
Except for ten plants used for the determination of biomass and photosynthetic parameters, five plants of the other ten were randomly collected to determine leaf alkaloid content, the remaining five plants were collected to determine the whole-plant alkaloid content in each treatment and washed clean. The leaves were dried in the oven at 105 °C for 20 min to deactivate enzymes, then quickly cooled down to 55 °C, then leaves, roots and stems were dried together in a 55 °C oven for at least 48 h. The samples which were used to determine leaf and whole-plant alkaloid content were all ground by a high-speed multi-function crusher and the powder was filtered with a 60 mesh filter. A total of 0.50 g of filtered sample, accurately weighed, was put into a 250 mL conical flask; 100 mL of hydrochloric acid–methanol (1:100, v/v) was added, and the mixture was heated by reflux for 15 min at 55 °C. After reflux, the mixture was extracted with ultrasonication for 45 min, then they were shaken and cooled down to room temperature and filtered. The residue was adjusted to 100 mL of hydrochloric acid–methanol (1:100, v/v) to repeat the above operation. Finally, the two filtrates were merged and the mixture was filtered, then the filtered fluid was dried, re-dissolved, and filtered through a 0.45 μm syringe filter prior to HPLC–DAD analysis using the method of Chan et al. (2007).
An Agilent 1100 HPLC system (Agilent Technologies, Palo Alto, USA) consisting of a vacuum degasser, a G1311A quaternary pump, a G1315A diode array detector (DAD) and a G1329A autosampler was utilized to obtain HPLC chromatograms. The system was controlled by Agilent Chemstation software (Agilent Technologies, Palo Alto, USA). Chromatography was operated on a Gemini C18 reversed-phase column (4.6 mm × 250 mm, 5 μm) maintained at 25 °C. The mobile phase was performed by using solvent A (0.05 mol L−1 KH2PO4 buffer solution, pH 3.0 adjusted with H3PO4) and B (acetonitrile) (72:28, V:V) at a flow rate of 1.0 mL min−1. The pump of the HPLC equipment was carefully washed by 10 % isopropyl alcohol for 10 min before determining the samples, and 10 min was required to equilibrate the column by mobile phase after each sample. The UV spectra were recorded at 265 nm. Identification of jatrorrhizine, palmatine and berberine was performed by comparing the retention times of chromatographic signals between detected samples and those of the three alkaloids standards under the same experimental conditions. Twenty μL of each sample was injected and HPLC analyses were performed in triplicate for statistical analysis (Fig. 1).
In order to measure calibration curves, jatrorrhizine chloride (110733–200806), berberine chloride (110713–200910) and palmatine chloride (110732–200907) (internal standards) were purchased from the National Institute for the Control of Pharmaceutical and Biological Products in Beijing, China. Standard samples of jatrorrhizine (1.0 mg), berberine (3.2 mg) and palmatine (2.3 mg) were weighed, diluted to 0.020, 0.064 or 0.046 mg/mL by adding a solution containing acetonitrile–water (1:1, V:V) as a control solution. Six concentrations of jatrorrhizine, berberine, and palmatine ranging from 0.020 to 0.700 μg for jatrorrhizine, from 0.064 to 2.240 μg for berberine, and from 0.046 to 1.610 μg for palmatine were prepared. Each concentration of the three types of alkaloids was injected in five replications. The calibration curves of jatrorrhizine, palmatine and berberine were constructed by plotting the peak area versus the actual amount (µg) of each analyte.
Leaf anatomy and ultrastructure
Measurement of stomatal density
In each treatment, 15 leaves were randomly selected from the plant that had photosynthetic parameters determined. The specimens were treated according to the method of Lu et al. (2008). The density of stomata (number of stomata per mm2) was measured and averaged within 15 randomly selected 1000 × 1000 μm2 on the sections.
Frozen sections
According to the method of Li et al. (2012), the leaf and stem of M. bodinieri was cut with cryostat microtome (Leica CM1900) carried at −22 °C, and each sample was cut to 30-μm sections. Due to some alkaloids (especially berberine) have strong autofluorescence (spontaneous fluorescence emission) under a certain excitation wavelength of ultraviolet light, the sections were photographed on the microscope (Leica DMLB) with fluorescent device (excitation wavelength: 365 nm).
Berberine was the main alkaloid compound in M. bodinieri, therefore, standard berberine was used as positive control in the experiment. Negative control section was first dehydrated by gradient alcohol, to 95 % alcohol. The section was then transported into mixed solution (tartaric acid: alcohol, V:V, 1:20) for 15 h, so that the alkaloids were completely extracted from the samples.
Semi-thin sections
The tissue of mature leaf was cut into 1 mm × 1 mm × 0.5 mm pieces. The specimens were treated according to the method of Hu et al. (2015). The specimens were cut to sections with 1-μm thickness on a microtome (Leica RM2155), the sections were stained with 0.5 % toluidine blue and observed with a Leica DMLB microscope. The number of the mesophyll chloroplasts per mm2 were counted and averaged within 15 randomly selected 1000 × 1000 μm squares on the sections of palisade and spongy parenchyma cells excluding vascular bundle and epidermis plastids.
Ultrathin sections
Based on the results obtained from the thin sections, the specimens were then cut to 70–90 nm sections with a diamond knife (Diatome, Switzerland) using a Leica EM UC6 ultramicrotome and collected on copper grids (150-mesh). Then the specimens were stained for 15–20 min using uranyl acetate and lead citrate and observed using a Philips FEI-TECHNAI12 TEM at 100 kV accelerating voltage. The chloroplast size (n ≥ 50 chloroplasts) was measured on transmission micrographs figures of palisade and spongy parenchyma cells.
Statistical analyses
The physiological data and growth variables were analyzed by Statistic Package for Social Science13.0 (SPSS13.0, Chicago, IL, USA). The yields of total alkaloids in leaves of a single plant were estimated for each treatment according to the average biomass of total leaves per plant × leaf alkaloid content, the yields of total alkaloids in a single plant were estimated for each treatment according to the average biomass per plant × alkaloid content in the whole plant.
Results
Total biomass, leaf biomass and leaf mass per area
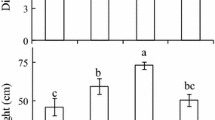
Our results indicated light conditions had a significant influence on the total biomass, leaf biomass, total leaf area and leaf mass per area (LMA) of M. bodinieri (Fig. 2). The total biomass was highest under I 30 irradiance followed by I 50 irradiance. Although the difference was not significant between I 30 and I 50 (Fig. 2a), the total biomass under both I 30 and I 50 was statistically higher than that at I 10 or I 100. In addition, total leaf area decreased with increasing light intensity, whereas LMA increased (Fig. 2b).
Gas exchange parameters
The gas exchange parameters of M. bodinieri varied significantly under different irradiances. As shown in Fig. 3, the values of net photosynthetic rate (P n), intercellular CO2 concentration (C i), stomatal conductance (G s), and transpiration rate (T r) all first increased and then decreased with increasing irradiance. The values of P n, C i, G s, and T r were always highest under I 30 followed by I 50. The lowest P n, G s, and T r values occurred under I 100, whereas the lowest C i value appeared at I 10. The limiting value of stomata (Ls) values under I 10 and I 100 were higher than those under I 30 and I 50 (Fig. 3e).
Chlorophyll content
The Chl content of M. bodinieri leaves was significantly affected by different irradiance levels. Chl a, Chl b, and Chl a+b levels per unit area decreased and Chl a/b increased with increasing light intensities (Fig. 4). Additionally, the Chl a, Chl b, Chl a+b contents were not significantly different between low light conditions (I 10 and I 30) and high light conditions (I 50 and I 100), but Chl a, Chl b, Chl a+b contents at low light intensities were always higher than those of high irradiance-treated M. bodinieri, in contrast with the levels of Car, Car/Chl and Chl a/b (Fig. 4).
Leaf structure
Cross-sections of M. bodinieri leaves showed that the leaves are composed of upper and lower epidermis, palisade and spongy parenchyma (Fig. 5). A sclerenchymatic hypodermis cell layer was below the adaxial epidermis (Fig. 5e–h). There were notable changes taking place in leaf anatomical characteristics induced by light intensity. In particular, increasing light intensity resulted in a gradual increase of the thickness of the entire lamina, palisade parenchyma, spongy parenchyma, as well as of stomatal density (Fig. 5; Table 1). The most obvious changes occurred in the outermost epidermis cells of the adaxial epidermis cell layer which gradually became smaller in size, and the ratio between protoplasm/cytoplasm and the thick secondary cell wall decreased in the second epidermis cell layer (Fig. 5e–h). At the same time, the number of chloroplasts in the palisade parenchyma decreased with increasing light intensity (Fig. 5e–h).
Light micrographs of characteristic semi-thin cross-sections from M. bodinieri leaves at various light levels. a–d Thickness changes of leaf blades; e–h thickened walls of the hypodermal cells and changes in the number of chloroplasts in palisade parenchyma; I 10 (a, e), I 30 (b, f), I 50 (c, g), I 100 (d, h). Scale bar 50 µm (a–d) or 20 µm (e–h)
The observations revealed that chloroplast numbers and structure were obviously influenced by light levels in M. bodinieri. The number of mesophyll chloroplasts per unit leaf area decreased as light irradiance increased (Table 2). The number of chloroplasts was highest under I 10 (6318 mm−2) and lowest under I 100 (4126 mm−2). This is even more striking as leaf thickness increased at high light intensities (Table 1). Chloroplasts under I 10 were large in size and presented a normal ultrastructural organization that the grana and stroma thylakoids could be obviously observed in chloroplasts (Fig. 6b). The grana stacks were tight and clear, and each chloroplast contained 1–2 giant starch grains (Fig. 6a), but only with a few plastoglobules (Fig. 6b). Chloroplasts contained well-developed thylakoids and grana stacks under I 30 (Fig. 6d–f). The number of starch grains was typically 2–3 per chloroplast (Fig. 6d, e). Although the number of electron-dense plastoglobules (PGs) remained relatively stable, electron-transparent PGs appeared in the chloroplasts (Fig. 6e, f). The width of chloroplast was significantly reduced and abnormal chloroplast structure with blurring and irregular arrangement of grana lamellae occurred under I 50 (Table 2; Fig. 6h, i). The number of electron-dense PGs was significantly increased, while starch grains completely disappeared at I 50 (Fig. 6g, h). Under full light intensity conditions the widths of chloroplasts became even smaller (Table 2), the grana completely ruptured and disappeared (Fig. 6k, l), but abundant PGs filled the chloroplasts (Fig. 6j–l). Taken together, starch grains and PGs showed inverse tendency with the former decreased (even completely disappeared) while the latter increased with increasing light intensity. Simultaneously, the structure of the stroma lamellae and grana were severely damaged under high light intensities.
Chloroplast ultrastructure in the leaves of M. bodinieri at various light levels. I 10 (a–c), I 30 (d–f), I 50 (g–i), I 100 (j–l). DP electron-dense plastoglobules, G granum, St starch grain, TP electron-transparent plastoglobules. Scale bar 2 µm (a, d, g, j), 1 µm (b, e, h, k) and 0.5 µm (c, f, i, l)
Alkaloid contents
The frozen sections combined with fluorescence microscopy were used to observe the distribution and storage sites of alkaloid in different parts (leaf and stem) of M. bodinieri (Fig. 7). Standard berberine emits yellow green fluorescence (Fig. 7e). The distribution features of the alkaloids in leaf of M. bodinieri indicated that the alkaloids were mainly present in the cell wall of epidermal cells and spongy parenchyma, but absent in palisade parenchyma (Fig. 7c). In stem of M. bodinieri, the alkaloids were mainly presented in the cell wall of xylem tissue and pith (Fig. 7b), only a small amount of them occurred in cortex, periderm and the sclerenchymatous cells’ of phloem (Fig. 7a). After berberine was completely extracted from leaf samples, no yellow–green fluorescence could be detected in the leaves (Fig. 7d). These results showed that there were plenty of alkaloids both in the leaves and the stems of M. bodinieri. In order to analyze the influence of different irradiance levels on alkaloid content of M. bodinieri, the contents of jatrorrhizine, palmatine and berberine in leaves and per plant from M. bodinieri were analyzed by HPLC in this work (Figs. 1, 8a, b). The results showed that the contents of jatrorrhizine, palmatine and berberine in leaves were higher in low or moderate light conditions. For example, although no significant difference existed in contents of berberine and palmatine between I 10 and I 30, and between I 50 and I 100, the contents of berberine and palmatine under I 10 and I 30 were all significantly higher than those under I 50 and I 100. The contents of jatrorrhizine under I 50 were significantly higher than those in other treatments of M. bodinieri leaves (Fig. 8a). Compared with the leaves, the three components in the whole plant were more likely to accumulate under high light condition, especially, the contents of jatrorrhizine and palmatine under I 100 were significantly higher compared with those in the other shade treatments (Fig. 8b), while the berberine content showed no significant variation at various light intensities (Fig. 8b). To further analyze the data, changes of total alkaloid yields in leaves and per plant under different light conditions were estimated. The results demonstrated that the yields of alkaloids both in leaves and per plant were obviously influenced by light levels of M. bodinieri (Fig. 8c, d). The jatrorrhizine yields in leaves and per plant all displayed differences under the various light levels. The highest yields of jatrorrhizine in leaves were obtained from I 50 followed by I 30 (Fig. 8c), contrarily, the highest yields of jatrorrhizine per plant were obtained from I 30 followed by I 50 (Fig. 8d), while the lowest yields both in leaves and per plant were under I 100 (Fig. 8c, d). The highest yields of palmatine and berberine both in leaves and per plant were obtained from I 30 and I 50, and although no significant difference existed in the yields between I 30 and I 50, the yields of these two component under I 30 and I 50 were significantly different from those under I 10 and I 100. Although total alkaloids yields of leaves and per plant first increased and then decreased with increasing irradiance, there were still differences between both of them under different light conditions. For instance, the highest total alkaloids yields in leaves or per plant were obtained from I 50 followed by I 30, and the lowest yields were under I 100. However, the highest yields of total alkaloids per plant were obtained from I 30 and I 50, and there were no significant differences in the yields between I 30 and I 50.
Alkaloid autofluorescence (pointed by arrow) in leaves and stems of M. bodinieri under fluorescence microscope. a, b Alkaloid autofluorescence in the stem of M. bodinieri. c Alkaloid autofluorescence in the leaf of M. bodinieri. d Negative control experiment of histochemical localization in M. bodinieri leaves after extraction of alkaloids. e Fluorescence of the berberine standard. Ec epidermal cell, P pith, Ph phloem, Pp palisade parenchyma, Sp spongy parenchyma, Xy xylem
Alkaloid contents in M. bodinieri. a Leaf alkaloid contents in M. bodinieri at various light levels (mg g−1 DW). b Alkaloid contents of whole plant in M. bodinieri at various light levels (mg g−1 DW). c Estimated yields of total alkaloids in leaves of a single plant at various light levels (g per plant leaves). d Estimated alkaloid yields in a single plant at various light levels (g per plant). The values represent the mean ± SE, and different letters mark statistical differences among shade treatments (P < 0.05)
Discussion
Light intensity influences leaf anatomy and photosynthetic activity
Irradiance was taken as one of the most important natural resources affecting the plant growth and reproduction (Zhang et al. 2005). Low light levels may reduce net carbon gain and plant growth because of limited photosynthesis of the plants. On the contrary, high light levels may cause direct physical damage (e.g., chronic photo-inhibition) to the photosynthetic apparatus (Tang et al. 2015). Therefore, plants may modify its morphology, anatomy, and physiological characteristics to cope with these light stresses (Aleric and Kirkman 2005). A typical example was that LMA, an integrative index of leaf structure, showed a positive correlation with maximum net photosynthetic rate in different Acer species (Hanba et al. 2002; Wyka et al. 2012). Some reports have suggested that leaf physiological adaptation to external environmental changes is due to changes of LMA, and LMA has been used to judge the strength of photosynthetic capacity (Le Roux et al. 2001). However, in M. bodinieri, although the irradiance level caused a sustained increase in LMA, the total biomass and P n first increased and then decreased (Figs. 2a, 3a). The reason may be directly related to the changes of leaf anatomy caused by high irradiance (Tomás et al. 2013). Previous studies have revealed that, the LMA usually has a positive relationship with photosynthetic capacity, but in some cases, the changes of LMA display a negative relationship with photosynthetic capacity under different irradiance, which may be partially caused by the greater biomass investment in support tissues and cell wall thickening, thus resulted in stronger CO2 diffusion limitations and reduction of photosynthesis (Niinemets 1999; Niinemets et al. 2007; Tomás et al. 2013). Increase in LMA was influenced by the thickness of palisade and spongy parenchyma (Marques et al. 1999) and cell wall thickening (Takashima et al. 2004). In addition, changes in LMA may be related with the cell sizes and the air space (intercellular cavities) in a leaf which affect the resistance to CO2 diffusion within a leaf and P n (Björkman 1981). Indeed, foliar thickness, the ratio of palisade and spongy tissue thickness, as well as palisade and spongy tissue thickness increased in M. bodinieri with increasing irradiance and reached their maximum values under I 100. Similarly, Vuleta et al. (2011) report that the sun-leaves of Iris pumila showed significantly higher LMA values and a greater stomatal density than shade-leaves. Both, high LMA and stomatal density, in conjunction with a thicker cell wall may cause a decrease in stomatal conductivity, leading to increased water use efficiency (Hanba et al. 2002; Vuleta et al. 2011). These changes could protect the photosynthetic tissue under high irradiance and drought conditions (Hanba et al. 2002; Ivancich et al. 2012).
C i/C a is an important parameter closely related to photosynthetic capacity and G s. Farquhar and Sharkey (1982) considered that the variation values of P n in different light conditions could be affected by stomatal or non-stomatal factors. If the increase of stomatal limitation value (Ls) resulted in both P n and C i decline, a decline in P n would be mainly attributed to stomatal factors. However, if intercellular CO2 concentration has an inversely changing trend to P n accompanied with the decline of Ls, suggesting that the decline of P n should be caused by non-stomatal factors under various light levels. In our work, C i, P n, and G s first increased and then decreased with increasing irradiance. Of them, low Ls values under I 30 and I 50 irradiance with high stomatal opening facilitated an increase in G s and C i so that P n was significantly enhanced. In contrast, high Ls values caused an apparent decrease of G s and C i and further resulted in a significant decrease of P n under low (I 10) and high (I 100) light conditions. These characteristics of coordinated variation further demonstrate that the photosynthetic capability of M. bodinieri leaves is mainly regulated by stomata through controlling the intercellular CO2 concentration under different irradiance conditions.
As previously reported, the fluctuation in Chl a/Chl b ratios could be taken as an index of the changes in the ratio of PSII to PSI, because Chl a is mainly concentrated around PSII, Chl a and Chl b are abundant in light-harvesting complexes (LHCs) in thylakoids (Panda et al. 2006; Kitajima and Hogan 2003). In our research, compared with high or moderate light levels, the Chl a/b ratio was significantly lower under low light conditions in M. bodinieri, which might suggest that the ratio of PSII to PSI was higher when plants were exposed to lower light level (Walters and Horton 1994). The reduction in the Chl a/b ratio was mainly due to the increase in Chl b content under shade conditions, which might promote to capture more light to enhance the photosynthesis efficiency and contribute to the enhancement of the light capture for photosynthesis (Murchie and Horton 1997). Contrarily, a higher Chl a/b ratio under high irradiance was due to low Chl b that might contribute to the enhancement of the photoprotection of the PSII through reducing light absorption (Walters 2005; Feng 2008). In addition, decreases of the Chl a, Chl b and Chl a+b contents were observed under I 100, indicating that excessive irradiance induced damage to pigments (Shao et al. 2014). This finding was consistent with the response of Tetrastigma hemsleyanum to light (Dai et al. 2009). Therefore, the P n decline in high light conditions might be caused by the declined photosynthetic activity of mesophyll cells. In our study, the numbers of chloroplasts, granum and stroma thylakoids were high under I 10 treatment, while the number of chloroplasts was significantly decreased at I 100. Moreover, the grana structure was completely broken and disappeared (Fig. 6). The overlap degree of the thylakoids in the chloroplast (i.e. the ratio of unstacked stroma lamellae to stacked granal membranes) is affected by the content of the light-harvesting pigment and PSII complexes (mainly located in stacked grana) (Tang et al. 2015; Yin et al. 2012). This indicates that excessive light irradiance not only inhibited chloroplast biogenesis and homeostasis, but also caused grana rupture, and influenced the proportion of Chl a/b. Therefore, under high light conditions the chloroplasts ultrastructural damage could indirectly result in P n decline in M. Bodinieri.
Light intensity influences the accumulation of secondary metabolites
Irradiance is one of the major essential factors affecting plant secondary metabolite synthesis and accumulation (Coelho et al. 2007), whereas different secondary metabolites have apparent differences in their response to different irradiance levels. For instance, low light intensity significantly increases the accumulation of glycyrrhizic acid and liquiritin (Hou et al. 2010). I 60 was found to be suitable for the accumulation of flavonoids including quercetin, luteolin, and apigenin, whereas most phenolic acids such as ferulic acid, gallic acid, tannic acid, vanillic acid, and caffeic acid have been observed under full irradiance. Furthermore, caffeic acid has only been detected in ginger grown under full irradiance (Ghasemzadeh and Ghasemzadeh 2011). Similarly, Foeniculum vulgare grown in high irradiance conditions usually has highest content of essential fennel oil (Xiao et al. 2007). Generally, the appropriate light environment is not only beneficial to plant growth, development, and synthesis of secondary metabolites, but it also enhances dry substance accumulation and the formation of active compounds to the largest extent (Salmore and Hunter 2001). The total flavonoid content [mg g−1 DW (dry weight)] of Anoectochilus formosanus under I 30 is lower than that of it under I 60, but the total flavonoid content per plant is higher under I 30 than that under I 60 due to the higher biomass under I 30 (Ma et al. 2010). With increased irradiance levels, the contents of berberine, jatrorrhizine and palmatine in amur corktree seedlings significantly increase and reach their highest values in full sunlight conditions, whereas I 75 gives the highest biomass production; therefore, the total alkaloid yields from a single plant are highest under that irradiance (Li et al. 2009). In this work, the contents of jatrorrhizine, palmatine and berberine in leaves were preferentially accumulated under low or moderate light intensity conditions. However, on whole plant basis, the contents of jatrorrhizine, palmatine were more likely to be accumulated under high light condition, while the content of berberine was not significantly different among the various light levels. The leaf biomass and the total biomass of whole plant in M. bodinieri under I 30 and I 50 were apparently higher than under other irradiances, the jatrorrhizine, palmatine and berberine yields and the total alkaloid yields both in leaves and per plant under I 30 and I 50 were higher than those in the other treatments. Under nitrogen nutrition-rich environments, if the plants growth was limited by adverse environments (e.g., shade or cloudy weather), the plants would show a shift in allocation from carbon-based compounds to nitrogen-based compounds such as alkaloids (Ralphs et al. 1998). Therefore, the carbon/nutrient balance theory could be applied to explain the mechanism whereby irradiance affects alkaloid synthesis and accumulation (Bryant et al. 1983). In this present study, under high light intensity the photosynthetic efficiency was significantly lower and starch synthesis was also significantly reduced, but the estimated amounts of jatrorrhizine, palmatine, and to a lesser extent berberine per plant were increased. These phenomena indicate that the regulation of carbohydrates and alkaloids with changes of irradiance levels are coordinated with the carbon/nutrient balance theoretical hypothesis. Previous research revealed that alkaloids mainly accumulated in the parenchyma cell of the phloem, followed by xylem, and alkaloids were mainly distributed in the cell walls of the xylem tissue and a few parenchyma cells in Rauvolfia serpentina root (Li et al. 2010). Our research discovered that most alkaloids accumulated in the epidermal and spongy parenchyma in the leaf of M. bodinieri, and most of them accumulated in the xylem tissue and the pith, only small part of them were present in the sclerenchymatous phloem cells of the stem of M. bodinieri. So we concluded that the alkaloids were mainly located to the cell walls in the leaves and stems (including the roots) of M. bodinieri. Although an integrated view of the mechanism whereby irradiance regulates and controls alkaloid synthesis is lacking, there has been some progress on the mechanism of action of related enzymes and genes in subcellular localization. Pompelli et al. (2013) demonstrated that caffeine synthase (CS), which catalyzes the final two steps of caffeine biosynthesis, is located in the chloroplasts. Lysine decarboxylase and 17-oxosparteine synthase, which catalyzes the first two steps of quinolizidine alkaloid are localized in the chloroplast stroma in Lupinus polyphyllus (Wink and Hartmann 1982). However, berberine, jatrorrhizine and palmatine are isoquinoline alkaloids, berberine is the typical isoquinoline alkaloids, the information of biosynthesis regulated enzyme has been clearly confirmed (Amann et al. 1986). It was demonstrated that berberine biosynthesis key regulatory enzyme [berberine bridge enzyme (BBE) and (S)-tetrahydroprotoberberine oxidase (STOX)] are exclusively located in a vesicle, and these vesicles frequently located as aggregates in small vacuoles in some species, for instance, in leaf tissue of Annona reticulata and in cell culture of Thalictrum glaucum, Berberis aggregata, Chasmanthera dependens (Amann et al. 1986; Bock et al. 2002). Therefore, although the synthesis and accumulation sites of different types of alkaloids may occur in cytoplasm vesicles of different cells, finally most of them will be transported to cell wall for storage. In addition, alkaloid components have a variety of biological activities, and regulatory mechanism whereby irradiance affects alkaloid component synthesis in plants is worth of in-depth study in each species.
Light intensity alters the accumulation of starch grains and the formation of plastoglobules (PGs) in chloroplasts
Thylakoids are the sites of photosynthetic light reactions, and the internal storage materials are also closely related to plant photosynthetic capacity (Dyall et al. 2004). With increased light intensity, not only did the chloroplast structure change significantly, but also starch accumulation was reduced and the number of PGs containing lipids and other substances such as Car and Chl breakdown products increased rapidly. With irradiance levels ≥I 50, the starch grains almost completely disappeared from the chloroplasts, and instead they were filled with abundant PGs. It has been demonstrated that decline in starch grains and increase in PG number at high irradiance are associated with reduced chloroplast function and senescence (Biswal 1995). Our research revealed that high irradiance causes impairment of thylakoid membrane structure and results in decreased contents of Chl a, Chl b, and Chl a+b and decreased photosynthetic rate, but the contents of Car significantly increase. Therefore, excessively high irradiance would result in chloroplast structural damage and reduced photosynthetic capacity, causing a decrease in starch synthesis. As for PGs, an integrated view about the physiological functions of the PG is lacking. For several years, the PGs were taken as a deposition site for the plastids. Recently, the PGs were proved to have some specific functions including Chl degradation (Lundquist et al. 2012) and even storage of some Car enzymes (Shumskaya et al. 2012). During the process of Chl breakdown, PGs synthesized in the chloroplast may carry Chl to the chloroplast envelope where the first step of Chl degradation could be performed by some enzymes (Matile et al. 1996; Guiamét et al. 1999). During senescence and chloroplast nutrients remobilization, chlorophylls are decoupled from chlorophyll–protein complexes in the photosystem and result in occurring phototoxicity (Christ and Hörtensteiner 2014). Reduced photosynthetic pigment content is beneficial to reduce chloroplast absorbance of excess light energy, thereby avoiding photo-inhibition and potentially helping to limit photo-damage to protect the chloroplast from light breakdown (Biswal 1995). Similar phenomenon (decreased chlorophyll content, complete degradation of grana and increase in electron-dense PGs) can be observed upon dehydration of poikilochlorophyllous desiccation tolerant plants such as Xerophyta to protect the plastids against photo-oxidative damage (Solymosi et al. 2013). In contrast, increased content of Cars may fulfill another protective function by accepting excessive excited states of chlorophyll and releasing it in the form of heat energy, stabilizing the thylakoid membranes and avoiding peroxidative damage (Havaux et al. 2007). Therefore, Chl breakdown and Car synthesis may be regarded as processes of detoxification at high irradiance (Havaux et al. 2007; Matile et al. 1996; Solymosi et al. 2013). Indeed, under high irradiance, the contents of Chl a, Chl b, and Chl a+b were apparently reduced, but the content of Car was significantly increased in the chloroplasts of M. bodinieri during this process; and although the photosynthetic efficiency was lower, photosynthesis still occurred. This phenomenon implies that excessive irradiance would lead to a decline of photosynthetic capability in M. bodinieri, but the plants may protect chloroplasts from high irradiance damage through active defense mechanisms to maintain a certain photosynthetic capacity. Among them, the processes of Chl degradation and Car accumulation are effective ways to avoid light damage under high irradiance. In this process, the abundant PGs in chloroplasts may play a very important role in Chl degradation and Car synthesis. The mechanism whereby PGs are induced by light stress in the process of Chl degradation in M. bodinieri is worth of further study.
Conclusion
In our research, light intensity significantly influences the photosynthetic capacity and morphological structure of M. bodinieri. Suitable light irradiance is necessary to maintain chloroplast biogenesis, physiological functions, and biomass accumulation. M. bodinieri can adapt to different irradiances. Under excessively high or low irradiance, plants may alter their foliar morphological structure, photosynthetic pigment content, and photosynthetic capacity in a positive way to adapt to the different light environments. Especially, PGs play an important role in light detoxification via Chl degradation and Car synthesis under high light. According to variations of biomass and composition, I 30 and I 50 may be chosen as the optimal growth environment to obtain the highest yields of total alkaloids and to improve the economic value of medicinal plants. These results can provide useful reference for the rational utilization, introduction, and cultivation of Mahonia resources.
Author contribution statement
Carried out the experiments: DK YL MW MB RZ HT. Conceived, designed the experiments and wrote the manuscript: HW DK YL. Analyzed the data: DK YL. Contributed reagents/materials/analysis tools: HW. All authors read and approved the manuscript.
References
Aleric KM, Kirkman KL (2005) Growth and photosynthetic responses of the federally endangered shrub, Linder amelissifolia (Lauraceae), to varied light environments. Am J Bot 92:682–689
Amann M, Wanner G, Zenk MH (1986) Intracellular compartmentation of two enzymes of berberine biosynthesis in plant cell cultures. Planta 167:310–320
Anderson JM, Chow WS, De Las Rivas J (2008) Dynamic flexibility in the structure and function of photosystem II in higher plant thylakoid membranes: the grana enigma. Photosynth Res 98:575–587
Augspurger CK (1984) Light requirements of neotropical tree seedlings: a comparative study of growth and survival. J Ecol 72:777–795
Biswal B (1995) Carotenoid catabolism during leaf senescence and its control by light. J Photochem Photobiol B 30:3–13
Björkman O (1981) Responses to different quantum flux densities. In: Lange OL, Nobel PS, Osmond CB, Ziegler H (eds) Encyclopedia of plant physiology, new series, vol 12A. Springer, Berlin, pp 57–107
Bock A, Wanner G, Zenk MH (2002) Immunocytological localization of two enzymes involved in berberine biosynthesis. Planta 216:57–63
Bryant JP, Chapin FS, Klein DR (1983) Carbon/nutrient balance in boreal plants in relation to vertebrate herbivory. Oikos 40:357–368
Chan CO, Chu CC, Mok Kam-wah W, Chau FT (2007) Analysis of berberine and total alkaloid content in Cortex Phellodendri by near infrared spectroscopy (NIRS) compared with high-performance liquid chromatography coupled with ultra-visible spectrometric detection. Anal Chim Acta 592:121–131
Chang X, Alderson PG, Wright CJ (2008) Solar irradiance level alters the growth of basil (Ocimum basilicum L.) and its content of volatile oils. Environ Exp Bot 63:216–223
Christ B, Hörtensteiner S (2014) Mechanism and significance of chlorophyll breakdown. J Plant Growth Regul 33:4–20
Coelho GC, Rachwal MFG, Dedecek RA, Curcio GR, Nietsche K, Schenkel EP (2007) Effect of light intensity on methylxanthine contents of Ilex paraguariensis A. St. Hil. Biochem Syst Ecol 2:75–80
Dai YJ, Shen ZG, Li Y, Wang LL, Hannaway D, Lu HF (2009) Effects of shade treatments on the photosynthetic capacity, chlorophyll fluorescence, and chlorophyll content of Tetrastigma hemsleyanum Diels et Gilg. Environ Exp Bot 65:177–182
Dyall SD, Brown MT, Johnson PJ (2004) Ancient invasions: from endosymbionts to organelles. Science 304:253–257
Evans JR, Poorter H (2001) Photosynthetic acclimation of plants to growth irradiance: the relative importance of specific leaf area and nitrogen in maximizing carbon gain. Plant Cell Environ 24:755–767
Farquhar GD, Sharkey TD (1982) Stomatal conductance and photosynthesis. Annu Rev Plant Physiol 33:317–345
Feng YL (2008) Photosynthesis, nitrogen allocation and specific leaf area in invasive Eupatorium adenophorum and native Eupatorium japonicum grown at different irradiances. Physiol Plant 133:318–326
Ghasemzadeh A, Ghasemzadeh N (2011) Effects of shading on synthesis and accumulation of polyphenolic compounds in ginger (Zingiber officinale Roscoe) varieties. J Med Plants Res 11:2435–2442
Ghasemzadeh A, Jaafar HZE, Rahmat A, Wahab PEM, Halim MRA (2010) Effect of different light intensities on total phenolics and flavonoids synthesis and anti-oxidant activities in young ginger varieties (Zingiber officinale Roscoe). Int J Mol Sci 11:3885–3897
Guiamét JJ, Pichersky E, Noodén LD (1999) Mass exodus from senescing soybean chloroplasts. Plant Cell Physiol 40:986–992
Hanba YT, Kogami H, Terashima I (2002) The effect of growth irradiance on leaf anatomy and photosynthesis in Acer species differing in light demand. Plant Cell Environ 25:1021–1030
Havaux M, Dall’Osto L, Bassi R (2007) Zeaxanthin has enhanced antioxidant capacity with respect to all other xanthophylls in Arabidopsis leaves and functions independent of binding to PSII antennae. Plant Physiol 145:1506–1520
Hou JL, Li WD, Zheng QY, Wang WQ, Xiao B, Xing D (2010) Effect of low light intensity on growth and accumulation of secondary metabolites in roots of Glycyrrhiza uralensis Fisch. Biochem Syst Ecol 38:160–168
Hu ML, Li YQ, Bai M, Wang YL, Wu H (2015) Variations in volatile oil yields and compositions of Magnolia zenii Cheng flower buds at different growth stages. Trees 29:1649–1660
Hua YB, Sun GY, Wang XC (2007) Induction characteristics and response of photosynthetic quantum conversion to changes in irradiance in mulberry plants. J Plant Physiol 164:959–968
Ivancich HS, Lencinas MV, Pastur GJ, Esteban RM, Hemandez L, Lindstrom L (2012) Foliar anatomical and morphological variation in Nothofagus pumilio seedlings under controlled irradiance and soil moisture levels. Tree Physiol 32:554–564
Kitajima K (1994) Relative importance of photosynthetic traits and allocation patterns as correlates of seedling shade tolerance of 13 tropical trees. Oecologia 98:419–428
Kitajima K, Hogan KP (2003) Increases of chlorophyll a/b ratios during acclimation of tropical woody seedlings to nitrogen limitation and high light. Plant Cell Environ 26:857–865
Le Roux X, Walcroft AS, Daudet FA, Sinoquet H, Chaves MM, Rodrigues A, Osorio L (2001) Photosynthetic light acclimation in peach leaves: importance of changes in mass: area ratio, nitrogen concentration and leaf nitrogen partitioning. Tree Physiol 21:377–386
Li X, Wang Y, Yan XF (2009) Effect of light intensity on the contents of three main alkaloids in amur corktree seedlings. Acta Ecol Sin 4:1656–1659
Li YP, Long JX, Cao FX, Dong XJ (2010) Histochemical localization of alkaloid accumulation in root of Rauvolfia serpentina. J Cent South Univ For Technol 30:157–161 (in Chinese)
Li ZQ, Tang TX, Liang SJ, Ning XP, Bai M, Wu H (2012) The synthesis and storage sites of phenolic compounds in the root and rhizome of Echinacea purpurea. Am J Plant Sci 3:551–558
Liao JX, Ge Y, Huang CC, Zhang J, Liu QX, Chang J (2005) Effects of irradiance on photosynthetic characteristics and growth of Mosla chinensis and M. scabra. Photosynthetica 43:1–4
Liu AL, He SZ (2010) Study on species and geographic distributions of the medicinal plants resources of Mahonia. Res Pract Chin Med 24:20–24 (in Chinese)
Liu Y, Zhao DM, Zu YG, Tang ZH, Zhang ZH, Jiang Y, Shi DY (2011) Effects of low light on terpenoid indole alkaloid accumulation and related biosynthetic pathway gene expression in leaves of Catharanthus roseus seedlings. Bot Stud 52:191–196
Lu HF, Jiang B, Shen ZG, Shen JB, Peng QF, Chen CG (2008) Comparative leaf anatomy, FTIR discrimination and biogeographical analysis of Camellia section Tuberculata (Theaceae) with a discussion of its taxonomic treatments. Plant Syst Evol 274:223
Lundquist PK, Poliakov A, Bhuiyan NH, Zybailov B, Sun Q, Wijk KJ (2012) The functional network of the Arabidopsis plastoglobule proteome based on quantitative proteomics and genome-wide coexpression analysis. Plant Physiol 15:1172–1192
Ma ZQ, Li SS, Zhang MJ (2010) Light intensity affects growth, photosynthetic capability, and total flavonoid accumulation of Anoectochilus plants. HortScience 45:863–867
Marques AR, Garcia QS, Fernandes GW (1999) Effects of sun and shade on leaf structure and sclerophylly of Sebastiania Myrtilloides (Euphorbiaceae) from Serra do CipÓ, Minas Gerais, Brazil. Bol Bot Univ São Paulo 18:21–27
Matile P, Hörtensteiner S, Thomas H, Kräutler B (1996) Chlorophyll breakdown in senescent leaves. Plant Physiol 112:1403–1409
Murchie EH, Horton P (1997) Acclimation of photosunthesis to irradiance and spectral quality in British plant species: chlorophyll content, photosynthetic capacity and habitat preference. Plant Cell Environ 20:438–448
Niinemets Ü (1999) Components of leaf dry mass per area thickness and density alter leaf photosynthetic capacity in reverse directions in woody plants. New Phytol 144:35–47
Niinemets Ü, Portsmuth A, Tena D, Tobias M, Martesanz S, Valladares F (2007) Do we underestimate the importance of leaf size in plant economics? Disproportional scaling of support costs within the spectrum of leaf physiognomy. Ann Bot 100:283–303
Panda D, Rao DN, Sharma SG, Strasser RJ, Sarkar RK (2006) Submergence effects on rice genotypes during seedling stage: probing of submergence driven changes of photosystem 2 by chlorophyll a fluorescence induction O-J-I-P transients. Photosynthetica 44:69–75
Pompelli MF, Pompelli GM, Oliveira AFM, Antunes W (2013) The effect of light and nitrogen availability on the caffeine, theophylline and allantoin contents in the leaves of Coffea arabica L. AIMS Environ Sci 1:1–11
Ralphs MH, Manners GD, Gardner DR (1998) Influence of light and photosynthesis on alkaloid concentration in larkspur. J Chem Ecol 1:167–179
Salmore AK, Hunter MD (2001) Environmental and genotypic influences on isoquinoline alkaloid content in Sanguinaria canadensis. J Chem Ecol 27:1729–1747
Shao QS, Wang HZ, Guo HP, Zhou A, Huang YQ, Sun YL, Li MY (2014) Effects of shade treatments on photosynthetic characteristics, chloroplast ultrastructure, and physiology of Anoectochilus roxburghii. PLoS One 2:1–10
Shumskaya M, Bradbury LMT, Monaco RR, Wurtzel ET (2012) Plastid localization of the key carotenoid enzyme phytoene synthase is altered by isozyme, allelic variation, and activity. Plant Cell 24:3725–3741
Solymosi K, Tuba Z, Böddi B (2013) Desiccoplast-etioplast-chloroplast transformation under rehydration of desiccated poikilochlorophyllous Xerophyta humilis leaves in the dark and upon subsequent illumination. J Plant Physiol 170:583–590
Takashima T, Hikosaka K, Hirose T (2004) Photosynthesis or persistence: nitrogen allocation in leaves of evergreen and deciduous Quercus species. Plant Cell Environ 27:1047–1054
Tang H, Yuan HY, Yu WW, Song LL, Wu JS (2015) Growth, photosynthetic and physiological responses of Torreya grandis seedlings to varied light environments. Trees 29:1011–1022
Tomás M, Flexas J, Copolovici L, Galmés J, Hallik L, Medrano H, Carbó MR, Tosens T, Vislap V, Niinemets Ü (2013) Importance of leaf anatomy in determining mesophyll diffusion conductance to CO2 across species: quantitative limitations and scaling up by models. J Exp Bot 64:2269–2281
Vuleta A, Jovanović SM, Tucić B (2011) Light intensity influences variations in the structural and physiological traits in the leaves of Iris pumila L. Arch Biol Sci Belgrade 63:1099–1110
Walters RG (2005) Towards an understanding of photosynthetic acclimation. J Exp Bot 56:435–447
Walters RG, Horton P (1994) Acclimation of Arabidopsis thaliana to the light environment: changes in composition of the photosynthetic apparatus. Planta 195:248–256
Wink M, Hartmann T (1982) Localisation of the enzymes of quinolizidine alkaloid biosynthesis in leaf chloroplasts of Lupinus polyphyllus. Plant Physiol 70:74–77
Wyka TP, Oleksy J, Zytkowiak R, Karolewski P, Jagodziński AM, Reich PB (2012) Responses of leaf structure and photosynthetic properties to intra-canopy light gradients: a common garden test with four broadleaf deciduous angiosperm and seven evergreen conifer tree species. Oecologia 170:11–24
Xia JB, Zhang SY, Guo J, Rong QQ, Zhang GC (2015) Critical effects of gas exchange parameters in Tamarix chinensis Lour on soil water and its relevant environmental factors on a shell ridge island in China’s Yellow River Delta. Ecol Eng 76:36–46
Xiao YH, He JM, Wang YM (2007) Effect of light intensity on plant growth, contents and components of essential oil in fennel (Foeniculum vulgare Mil.l). Plant Physiol Commun 43:551–555 (in Chinese)
Ye SJ (2009) Herb verification and authentication of Chinese holly leaf and gonglao leaf. J Zhejiang Coll Tradit Chin Med 33:431–432 (in Chinese)
Yin L, Fristedt R, Herdean A, Solymosi K, Bertrand M, Andersson MX, Mamedov F, Vener AV, Schoefs B, Spetea C (2012) Photosystem II function and dynamics in three widely used Arabidopsis thaliana accessions. PLoS One 7:e46206
Zeng XY, Dong YL, Sheng GY, Dong XC, Sun XH, Fu JM (2006) Isolation and structure determination of anti-influenza component from Mahonia bealei. J Ethnopharmacol 8:317–319
Zhang SB, Hu H, Zhou ZK, Xu K, Yan N (2005) Photosynthesis in relation to reproductive success of Cypripedium flavum. Ann Bot 96:43–49
Zhejiang Food and Drug Administration (2005) Traditional Chinese Medicine processing specification of Zhejiang Province. Zhejiang Science and Technology Press, Zhejiang, p 358
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Project No. 31470293) to HW. The PhD Start-up Fund of Natural Science Foundation of Guangdong Province, China (S2012040007655) to MB. The Guilin city scientific and technological achievements transformation and application project (20100103-6), the Guangxi Scientific Research and Technological Development Plan (No.GuiKeZhuan1346004-29) and Guangxi Natural Science Foundation Program (2011GXNSFB018096) to DK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by M Garstka.
De-Xin Kong and Yan-Qun Li contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kong, DX., Li, YQ., Wang, ML. et al. Effects of light intensity on leaf photosynthetic characteristics, chloroplast structure, and alkaloid content of Mahonia bodinieri (Gagnep.) Laferr.. Acta Physiol Plant 38, 120 (2016). https://doi.org/10.1007/s11738-016-2147-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-016-2147-1