Abstract
Key message
Shading could improve plant growth in Torreya grandis seedling, and 75 % shade is likely the optimum light irradiance level for its growth.
Abstract
Light is a critical factor that affects the survival and early growth of tree seedlings. Torreya grandis, an economically important subtropical plant, is a shade-preferring species; however, the optimum light intensity for the growth of this species was still unclear. To determine the optimum light intensity, we examined the growth, chlorophyll fluorescence, gas exchange, and chloroplast ultrastructure of T. grandis seedlings growing under four levels of shade (i.e., 0, 50, 75, and 90 %). The results showed that T. grandis attained the greatest Pn and biomass when cultivated with 75 % shade. Seedlings grown under 75 % shade exhibited a 155 % increase in the height increment, a 440 % increase in the diameter increment, a 42.2 % increase in biomass, and a 102 % increase in the photosynthetic rate compared with seedlings grown in full sun. Moreover, 75 % shaded plants had the lowest antioxidant enzyme activities, malondialdehyde content and ion leakage. Full sunlight and 50 % shade significantly reduced the growth of T. grandis which was associated with a decrease in the maximal photochemical efficiency, photosynthetic rate, chlorophyll content and biomass compared with those under 75 % shade. Compared with the 75 % shaded plants, seedlings grown under 90 % shade had a reduced photosynthetic rate, which was accompanied by increased malondialdehyde content, relative electrolyte conductivity and antioxidant enzymes activities, suggesting that seedlings under the 90 % shade had the lower energy utilizing capacity. Higher antioxidant enzyme activities might be an efficient adaptation to protection against oxidative stress under low light conditions. Therefore, our results indicate that 75 % shade is likely the optimum light irradiance level for T. grandis seedling growth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Torreya is an endangered and primitive member of the yew family (Taxaceae) and consists of only six species with a restricted worldwide distribution. Among these, Torreya jackii, T. nucifera, T. californica and T. taxifolia are now included in the IUCN (International Union for Conservation of Nature) Red List of Threatened Species (http://www.incnredlist.org/), while T. fargesii and T. grandis have been listed as national second-grade key protected wild plants (Yu 1999). The conservation and recovery of these species have received increasing attention in recent years. Torreya grandis cv. Merrilli, which is one of the most important cultivated plant species in China, has been used for food and traditional Chinese medicine for more than 1000 years (Li et al. 2004; Cheng et al. 2007). Torreya seeds possess anti-oxidative and acute anti-inflammatory properties (Chen et al. 2006); the endophytic fungi isolated from T. grandis are used in anti-tumor and anti-fungal medicine (Huang et al. 2001). T. grandis has become an important species in China (especially in Zhejiang province) because of its ornamental, medicinal and edible values. The demand for T. grandis seeds is increasing and, consequently, this species has recently been cultivated in various locations in China. Since 2000, the growing area of T. grandis has rapidly expanded, increasing approximately 11.5-fold from 2000 to 2012 (He et al. 2013). Our preliminary experiments on the survival and early growth of these tree seedlings indicated that they may be strongly affected by light intensity. In general, full sunlight easily injured T. grandis seedlings, leading to poor growth performance. Therefore, it is necessary to investigate the effects of light intensity on the growth of T. grandis seedlings.
Light is one of the major environmental factors influencing growth and distribution of plant species (Larcher 1995). The study of photosynthetic and morphological responses of plants to different light intensities, which can reveal the tolerance of a species to light conditions, has been useful in agriculture, ecology, reforestation, and horticulture (Valladares et al. 2002; Aleric and Kirkman 2005). This approach can also be useful in assessing optimal habitat conditions for the conservation of rare species found in only a few populations or in extremely varied conditions (Smith et al. 1993; Aleric and Kirkman 2005).
While low light levels can limit photosynthesis, resulting in poor growth, an excess of light can also be detrimental to growth. Plants grown in low light often experience decreased Rubisco activity and reduced CO2 assimilation rates (Stitt and Schulze 1994), leading to a reduction in growth and development. Under profuse light radiation, the photosynthetic apparatus absorbs excessive light energy, which can result in the inactivation and/or impairment of the chlorophyll-containing reaction centers of the chloroplasts (Bertamini et al. 2006). This can damage the photosynthetic apparatus through the process of photoinhibition (Dai et al. 2009). Chronic photoinhibition can significantly decrease plant productivity (Huner et al. 1998). One of the important biochemical changes that occur in plants subjected to high or low irradiance is the production of ROS. It is now widely accepted that harmful reactive oxygen species (ROS) that are produced upon illumination are involved in photoinhibition (Asada 1999). The production of ROS in plant cells is enhanced by high light, which limits CO2 fixation. To cope with ROS, plants are equipped with a complex antioxidant system that includes enzymatic and non-enzymatic mechanisms that protect cells from the toxic effects of ROS (Allen 1995; Alscher et al. 2002). The non-enzymatic mechanisms involve low molecular weight antioxidants (i.e., carotenoid (Car), ascorbate, glutathione (GSH), and α-tocopherol). The enzymatic mechanisms include superoxide dismutase (SOD), which catalyzes the reaction of superoxide to hydrogen peroxide (H2O2), and catalase (CAT) and ascorbate peroxidase (APX), which function to detoxify the H2O2 produced (Asada 1999; Logan et al. 2006; Marchese et al. 2008). Glutathione peroxidase (GSH-Px) also catalyzes the formation of GSSG (i.e., oxidized glutathione, which is formed by the oxidation of GSH), and catalyzes the reduction of poisonous peroxide into non-toxic hydroxyl compounds, while simultaneously promoting the decomposition of H2O2 (Kuwabara and Katoh 1999; Ben Ahmed et al. 2009).
T. grandis is mostly planted on hills and mountain slopes in south China. It has been reported that T. grandis seedlings are shade-preferring (Cheng et al. 2007). In actual cultivation, T. grandis seedlings are usually grown under shade. However, we are still in shortage of the physiological knowledge of this management. Our aims were to determine the optimal light conditions for this species when grown for agricultural purposes, and to understand acclimation under shade conditions to provide information for improved propagation and cultivation. In the present study, the growth and photosynthetic characteristics (i.e., chlorophyll fluorescence and gas exchange) were investigated in T. grandis seedlings grown under various shade levels. Two questions were addressed during this study: (1) what photosynthetic adjustments do the T. grandis seedlings make to different light environments? (2) Do morphology, growth, and biomass differ for T. grandis seedlings grown in varied light conditions? It is anticipated that this information will contribute to more detailed management prescriptions and potentially to the conservation and cultivation of other Torreya species (including endangered species).
Materials and methods
Plant material and culture conditions
The experiment was conducted outdoors at Zhejiang A & F University (30°23′N, 119°72′E) in China. Two-year-old uniform and healthy T. grandis seedlings were transplanted into 1-gallon pots containing a substrate mixture of pine bark: peat: soil (4:4:2, v/v/v, 40 kg m−3 of organic manure). The loam substrate had a pH of 6.4, an organic matter content of 112 g kg−1, an alkali-hydrolyzable nitrogen content of 714 mg kg−1, available phosphorus content of 86.1 mg kg−1 and available potassium content of 512 mg kg−1. No fertilizer was applied because the soil media were considered to be sufficiently fertile. All plants were kept well irrigated and exposure to full light conditions during watering and measurements was brief and considered to have a negligible effect on the results. Materials for the measurements of chlorophyll fluorescence, pigment, gas exchange, antioxidant enzyme activities, cellular damage and chloroplast ultrastructure were collected from the penultimate current-year leaves.
Shade treatments
Uniform seedlings (n = 100) were divided into four treatments. The experiment was set up as a completely randomized design with five replicates per treatment and five plants per replicate. The seedlings were grown under full sun (0 % shade) or under shelters with black shade nets commonly used in agriculture (resembling square tents) that provided three levels of shade (50, 75, or 90 % shade). The plants were subjected to the four irradiance levels for 60 days, beginning on June 10, 2013. The light intensity, which was measured in the shade treatments using a Digital Lux Meter (TES-1339R, Taiwan, China) on a sunny, cloudless day (July 4, 2013), indicated that the four treatments provided 0, 50, 75, and 90 % shade. Specifically, the 50, 75, and 90 % shade net reduced the incident light level to 51.8, 74.8, and 90.3 %, respectively, of the photosynthetically active radiation (PAR). At the beginning of shade treatments, fully expanded (not fully developed) penultimate leaves from current-year growth were labeled with plastic tags and used for the measurements of morphology, gas exchange and pigment content.
Growth analysis
The seedling height and stem diameter were measured prior to imposing the shade treatments. Sixty days after treatments, the seedlings were collected and the height and stem diameters were measured. Then, the root, leaf and stem were separated and the dry mass was determined after they have been dried in oven at a temperature near 80 °C until constant weight was achieved. The stem, leaf and root mass ratios were estimated by the dry mass of each organ divided by the total dry mass of the whole plant (the sum of all organs).
Chlorophyll fluorescence
The maximum quantum efficiency of PSII photochemistry (Fv/Fm) was determined in the morning (08:00-11:00 h) on the penultimate current-year leaves using a pulse modulation fluorometer (PAM-2500, Walz, Effeltrich, Germany). After 30 min of dark adaptation (based on our previous experiment), the minimum fluorescence (Fo) was determined using a measuring light of approximately 0.5 μmol photon m−2 s−1, and the maximum fluorescence (Fm) was determined using a 0.8 s saturating flash of 10,000 μmol photon m−2 s−1. The Fv/Fm value was calculated as (Fm − Fo)/Fm (Maxwell and Johnson 2000). Chlorophyll fluorescence was recorded on five randomly selected plants per treatment.
Gas exchange measurements
The youngest fully developed leaves from 5 to 6 randomly selected seedlings were chosen for net photosynthesis measurements, which were made on attached leaves using a LI-6400 XT portable photosynthesis system (LI-COR Inc., Lincoln, NE, USA; software version: OPEN 6.1.2) equipped with a 6400-22 opaque leaf chamber, using a 6400-18 RGB LED light source. Measurements were conducted on sunny days from 8:30 to 11:00 AM at an air concentration of 21 % O2, 400 µmol mol−1 CO2, 1200 μmol m−2 s−1 PAR white light, 50 % relative humidity and a temperature of 29–32 °C. The leaf area was traced on white paper; then, the leaf area was calculated by dividing the paper weight of the traced area by the paper weight per unit area. The leaf area was used when calculating the photosynthetic parameters.
Antioxidant enzyme activities
Leaf tissue [0.3 g fresh weight (FW)] was ground using a mortar and pestle containing 8.0 ml of grinding media consisting of 50 mM phosphate buffer solution (pH 7.8) and 1 % polyethylene pyrrole (PVP) at 4 °C. Following centrifugation at 10,000 rpm for 15 min at 4 °C, the supernatants were used for SOD, APX, and GSH-Px assays. Total SOD activity was determined spectrophotometrically at 525 nm (Favaretto et al. 2011). One unit of SOD was defined as the amount needed for 50 % inhibition of nitroblue tetrazolium (NBT) reduction. The APX activity was assayed as a decrease in absorbance at 290 nm, which was resulted from ascorbate oxidation (Ushimaru et al. 1997). The GSH-Px activity was measured as the decrease in the GSH, which is reflected as a change in the absorbance at 412 nm (Sun et al. 2009).
Cellular damage
Lipid peroxidation was measured in terms of the malondialdehyde (MDA) content (Hodges et al. 1999). To determine cell membrane stability and integrity, ion leakage was measured using a simplified method (Nayyar 2003). Leaves were thoroughly washed, cut into small discs and placed in vials filled with 10 ml of deionized water. After incubation at 25 °C for 12 h in dark conditions, the electrical conductivity (initial EC) in the bathing solution was determined using a conductivity meter (Model DJS-1C; Shanghai Analytical Instrument Co., Shanghai, China). Then, the samples were heated at 100 °C for 20 min and the conductivity (final EC) in the bathing solution was determined. Membrane permeability was calculated as follows:
Pigment analysis
The penultimate current-year leaves (0.1 g) at the same position were taken from seedlings in each treatment. Leaf tissues were quickly cut into small pieces (0.2 cm strips) in a dry-ice-cooled mortar in the dark. Then, pieces of tissue were placed in glass vials with 8 mL of 95 % (v/v) ethanol (100 %, Sinopharm Chemical Reagent Company, Shanghai, China) in the dark for 24 h at 25 °C until they were blanched (no green color in the leaf tissue). Absorbance of the supernatant was measured with a spectrophotometer (UV-2550, Shimadzu, Kyoto, Japan) at 649, 664 and 470 nm after centrifugation of the mixture on standing. The total chlorophyll (Chlt), chlorophyll a (Chla), chlorophyll b (Chlb), and total Carotenoid (Car) contents were determined according to Lichtenthaler (1987).
Chloroplast ultrastructure
Tissue samples for the analysis of chloroplast ultrastructure were taken from a leaf of three separate plants in each treatment. The tissue samples were immediately fixed in 2.5 % (v/v) glutaraldehyde (0.1 mol L−1 phosphate buffer, pH 7.0) for at least 4 h once cut from the plants. The samples were then immersed in 1 % (v/v) osmium tetroxide for post-fixation. The specimens were dehydrated using a graded series of ethanol and embedded in epoxy resin for ultrathin sectioning for examination with a transmission electron microscope (H7650, HITACHI, Tokyo, Japan). For the analysis, 4–6 slices of leaf adaxial surfaces of seedlings in each treatment were taken.
Statistical analysis
An analysis of variance (ANOVA, GLM procedure) and Duncan’s new multiple range tests (DMRT) were performed using SAS 9.2 (SAS Institute, Cary, NC, USA). The data are presented as mean ± standard deviation (SD). Differences at P < 0.05 were considered significant.
Results
Leaf morphology and growth
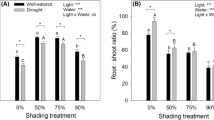
The leaf color of seedlings grown under shaded conditions was dark green, while those growing in full sun were yellowish green (Fig. 1). The leaf length (LL) and leaf width (LW) in the non-shade control plants were smaller than those of the leaves of shaded plants. Moreover, the leaf area (LA) increased with the increasing level of shade (Fig. 1). Over the experimental period, the height increment increased by 69.5, 155.2, and 49.5 % in the 50, 75, and 90 % shade treatment, respectively, compared with the open-grown (full sunlight) seedlings (Fig. 2A). The stem diameter increment increased by approximately 250.5, 440.2, and 175.3 % in the 50, 75, and 90 % shade treatments, respectively, compared with the control plants growing in full sunlight (Fig. 2B).
Changes in leaf morphology, leaf length (LL), leaf width (LW) and leaf area (LA) of T. grandis seedlings under various shade treatments. Data are presented as the mean ± standard deviation (SD). Different letters indicate statistical differences (P < 0.05) between four different shade levels (at the same line) according to Duncan’s new multiple range test; n = 5
Influence of different shade levels on the height and diameter increment of T. grandis seedlings over the experimental period from June 10th to August 8th. Data are presented as the mean ± standard deviation (SD). Different letters indicate statistical differences (P < 0.05) between four different shade level according to Duncan’s new multiple range test; n = 5
Biomass and allocation
Shading increased the biomass allocated to the leaves and reduced that allocated to the roots; this shift being the greatest under 75 % shade (Fig. 3). The shade treatments resulted in a significant increase in total seedling biomass accumulation compared with seedlings grown in full sunlight. The greatest biomass accumulation was measured under 75 % shade.
Proportional dry mass allocation to the leaf, stem and root, and total biomass accumulation of T. grandis seedlings grown under different shade levels. Data are presented as the mean ± standard deviation (SD). Different letters indicate statistical differences (P < 0.05) between four different shade level according to Duncan’s new multiple range test; n = 5
Chlorophyll fluorescence
The chlorophyll fluorescence parameters of leaves of T. grandis seedlings grown at 75 and 90 % shade were similar. The photochemical efficiency of photosystem II (Fv/Fm) was significantly higher in the 75 and 90 % shade treatment. The leaves of seedlings grown at 0 and 50 % shade exhibited Fv/Fm ratios significantly less than 0.75; the Fv/Fm ratios were reduced by 48.5 and 9.9 % in the 0 and 50 % shade treatments, respectively, compared with plants subjected to 75 % shade (Fig. 4).
The maximal photochemical efficiency (Fv/Fm) of T. grandis seedlings under various shade treatments. Data are presented as the mean ± standard deviation (SD). Different letters indicate statistical differences (P < 0.05) between four different shade levels according to Duncan’s new multiple range test; n = 5
Gas exchange measurements
The seedlings grown in full sun had significantly lower photosynthetic rate (Pn) and transpiration (Tr) values than shade-grown seedlings (Fig. 5). There was a significant increase in Pn from 1.17 to 2.36 μmol CO2 m−2 s−1 over the range of shading intensities from 0 to 75 % shade. At shade levels greater than 75 %, the Pn decreased. T. grandis seedlings grown under 75 % shade exhibited the highest Pn and Tr among the shade treatments.
Influence of different shade levels on the net photosynthetic rate (Pn) and transpiration (Tr) of Torreya grandis seedlings. Data are presented as the mean ± standard deviation (SD). Different letters indicate statistical differences (P < 0.05) between four different shade levels according to Duncan’s new multiple range test; n = 5
Leaf mass per unit area (LMA)
Although deep shade (90 % shade) had little effect on leaf mass per unit area (LMA) during this study, higher LMA values were recorded in the 50 and 75 % shade treatments compared with full sunlight (Fig. 6).
Antioxidant enzyme activities
A marked decrease was observed in the SOD activity in plants growing under 50 and 75 % shade compared with the control plants growing under full sunlight (Fig. 7). Similarly, the APX and GSH-Px activity showed a large decrease in response to shade. Interestingly, plants growing under 75 % shade also had lower levels of antioxidant enzyme activities than plants grown under the 90 % shade, indicating 75 % as the optimal condition tested.
Influence of different shade levels on superoxide dismutase (SOD), ascorbate peroxidase (APX), and glutathione peroxidase (GSH-Px) activities of T. grandis seedlings. Data are presented as the mean ± standard deviation (SD). Different letters indicate statistical differences (P < 0.05) between four different shade levels according to Duncan’s new multiple range test; n = 5
Cellular damage
There was significantly lower MDA accumulation and less electrolyte leakage in the shaded treatments compared with the control. Seedlings grown under 75 % shade exhibited the lowest MDA content and relative electrolyte conductivity (REC) among the shade treatments, although this trend was not always significant (Fig. 8).
Influence of different shade levels on malondialdehyde (MDA) content and relative electrolyte conductivity (REC) of T. grandis seedlings. Data are presented as the mean ± standard deviation (SD). Different letters indicate statistical differences (P < 0.05) between four different shade levels according to Duncan’s new multiple range test; n = 5
Photosynthetic pigments
Seedlings in the shade treatments had significantly higher Chla and Chlb than those grown in full sunlight. The total chlorophyll (Chl) concentration per unit fresh weight increased with increasing shade (Table 1). There were no significant differences in Chla/b ratio and Car content among different shading treatments. However, the Car/Chl ratio significantly decreased with increasing shading levels (Table 1).
Chloroplast ultrastructure
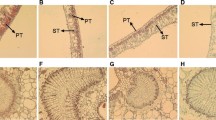
The number of grana and grana lamellae increased with a reduction in light irradiance (Fig. 9; Table 2). The plants grown in 75 and 90 % shade had grana that contained more thylakoids than plants grown under full sunlight. Additionally, the number of osmiophilic globules was highest in 0 % shade, and was lowest in 75 % shade. However, the largest and the smallest size of osmiophilic globule were observed in the 75 and 90 % shade, respectively.
Discussion
Sunlight is one of the major environmental factors influencing photosynthesis, growth and reproduction of understory plants (Zhang et al. 2005). Insufficient light levels may stress plants by limiting photosynthesis, resulting in reduced net carbon gain and plant growth. Conversely, high light levels may damage the photosynthetic apparatus (Larcher 1995). Therefore, plants have developed various strategies to cope with these stresses. Differences in leaf morphology and physiology have been well documented for species adapted to sun or shade environment (Valladares et al. 2002; Aleric and Kirkman 2005). In this study, we have illustrated the physiological mechanisms of T. grandis in response to various irradiance levels.
Shading is necessary for the growth of T. grandis seedlings. Longer and wider leaves were observed from T. grandis grown under shade treatments compared with plants grown under full sunlight (Fig. 1). It has been also reported that the length and width of European yew (Taxus baccata L.) increased with increasing shade (Perrin and Mitchell 2013). Larger leaves may be a response to low photosynthetic photon flux density (PPFD). For example, larger leaves in shade would improve light interception and absorption for photosynthesis when light levels are low (Delagrange et al. 2006; Peri et al. 2007; Valladares and Niinemets 2008). Compared with the T. grandis seedlings grown under full sunlight, significant increases in height and stem diameter increment were observed in plants grown under shading (Fig. 2), indicating that T. grandis seedlings have an optimal light intensity between 50 and 90 % shade. Maximum height and stem diameter increment were achieved at 75 % shade, suggesting that 75 % shade should be the optimum light irradiance level for the growth of T. grandis seedlings.
The plasticity in allocation of biomass to different organs may be crucial to the success of a seedling to establish itself in a new environment. Higher biomass allocation to leaves at the expense of roots occurred with an increase in the level of shading (from 0 to 75 % shade, Fig. 3). This is a common response among forest species in several ecosystems, for example, the Atlantic Forest species Cecropia glaziovii, Cedrela fissilis, and Bathysa australis (Duz et al. 2004). Additionally, full sunlight reduced the total seedling biomass (Fig. 3D), mainly as a result of a reduction in leaf biomass, presumably because of chronic photoinhibition which decreased photochemical efficiency and which required repair of photoinactivated PS II. Under low light conditions, European yew (Taxus baccata L.) seedlings have been shown to allocate proportionately less biomass to roots (Perrin and Mitchell 2013). In the present study, the result implies that full sunlight had a greater inhibitory effect on shoot growth (leaf plus stem biomass) than root growth. On the other hand, the total biomass of plants grown under 90 % shade was lower than those grown under 75 % shade (Fig. 3D), which suggests that a 90 % shade level would result in decreased T. grandis seedling growth.
Chlorophyll fluorescence always is the focus in studies of photosynthetic regulation and plant responses to the environment due to its sensitivity, convenience and non-destructive characteristics (Dai et al. 2009). Photoinhibition of PSII can easily be detected in vivo by a decrease in the ‘dark-adapted’ ratio of variable to maximum chlorophyll a fluorescence (Fv/Fm) (Krause and Weis 1991). It has been reported that lower Fv/Fm was observed in sun-grown foliage than in shade-grown foliage of Pacific yew (Demmig-Adams and Adams III 1992a). Fv/Fm was also lower in foliage grown under full sun than that grown under 5–10 % of full sun (Mitchell 1998). Generally, plants subjected to high-irradiance stress typically have lower Fv/Fm values than non-stressed plants (Björkman and Demmig 1987; Baker 2008). In the present study, Fv/Fm was significantly reduced by full sunlight and 50 % shade (Fig. 4), indicating the occurrence of chronic photoinhibition under such circumstances. Indeed, it was consistent with our gas exchange results (Fig. 5). Shading significantly increased the Pn and Tr values (compared with leaves subjected to full sunlight), indicating that shading is necessary for the growth of T. grandis seedlings. Our results show that the leaves of plants grown under 75 % shade had significantly the highest Pn and Tr than those grown in full sunlight.
Plants had lower LMA values under the 0 and 90 % shade treatments compared with the other treatments (Fig. 6). Several tropical and temperate studies suggested that a higher LMA was correlated with leaf toughness, leaf chemical defenses and a longer leaf life span (Reich et al. 1991; Wright and Cannon 2001). Higher LMA values are important for evergreen species that need to produce leaves that are sufficiently tough to survive longer time (Hikosaka 2005). Moreover, Pearcy and Sims (1994) proposed that the higher LMA of sun leaves, which is related to higher photosynthetic capacities, may confer some protection against photoinhibition. Similarly, we suggest that the higher LMA may protect leaves against photoinhibition in the 50 and 75 % shade treatments.
The main antioxidant enzymes of chloroplasts are SOD and APX (Mehlhorn et al. 1996; Alscher et al. 2002). Shaded plants exhibited lower activities of SOD, APX, and GSH-Px compared with plants grown under full sunlight (Fig. 7). Therefore, our results suggest that lower PPFD levels induced a different response of T. grandis seedlings to oxidative stress because the need for antioxidant defenses is reduced under low light intensities. This is consistent with our findings that shade treatments induced lower MDA contents and REC (Fig. 8) and with previous results where shaded plants showed lower activities of SOD, APX, and GSH-Px, and lower MDA contents and ion leakage levels compared with those of non-shaded plants (Adriano et al. 2004). In contrast, plants grown under full sunlight had higher values of SOD, APX and GSH-Px in response to oxidative stress caused by the production of ROS. In fact, plants grown in full sunlight showed clear signs of photo-oxidative damage (based on the significant increases in MDA and REC relative to the shade-treated plants). In addition, our results indicated higher values of SOD, APX, GSH-Px, MDA, and REC in plants grown under 90 % shade compared with plants grown under 75 % shade, which may partly be explained by the lower energy utilizing capacity, as the 90 % shade plants showed lower Pn than the 75 % shade plants. Thus, a higher ROS generation rate and high capacity of protection against oxidative stress could be seen as consequences of efficient photosynthesis under very low light conditions (90 % shade).
Under high irradiance, the transfer of absorbed energy becomes particularly important because excess energy can lead to the formation of ROS, a prime cause of photoinhibition (Critchley 1998). Compared with shaded leaves, sunlit leaves have a decreased ability to absorb incident radiation because of a decreased light-harvesting capacity (i.e., a lower Chl concentration; Adams and Barker 1998). In addition, sunlit leaves should have an increased capacity to dissipate excess excitation energy, e.g., through Car, which protects the photosynthetic membrane from photo-oxidation by effectively scavenging singlet oxygen and quenching the triplet state of chlorophyll (Demmig-Adams 1990). Plants grown under shaded conditions are known to optimize their effectiveness of light absorption by increasing pigment density (Wittmann et al. 2001). The marked increase in leaf Chl content in leaves under 90 % shade demonstrated the ability of plants to maximize the light-harvesting capacity under low-light growth conditions (Lei et al. 1996). Plants grown in shade increased their light-use efficiency by preferentially investing resources to capture light at the expense of photosynthetic capacity (Anderson and Osmond 1987). Our results were consistent with this finding; the Pn under the 90 % shade treatment was significantly lower than the Pn measured under 75 % shade (Fig. 5). The combination of a strong reduction in the Chla, Chlb, and total Chl content and an increased Car/Chl in plants grown in full sunlight might dissipate excess light energy by Car (Table 1). It has also been reported that the higher Car/Chl ratio in plants under high-sunlight conditions compared with shade plants resulted from the combination of a reduction in the total chlorophyll content and no significant changes in the carotenoid content (Demmig-Adams and Adams III 1992b).
In our study, the numbers of grana and grana lamellae increased with increasing shade levels (Fig. 9). The overlap degree of the grana layer in the chloroplast, an important shade adaptation mechanism designed to capture more light energy, increased the content of the light-harvesting pigment and PSII complexes (mainly located in stacked grana), which could effectively collect more light to enhance the absorption and conversion of light energy (Ai et al. 2004; Lu et al. 2013). Osmiophilic globules usually are plastoglobuli which formed predominantly in developing and senescing chloroplasts (Lichtenthaler, 2013). The number and size of osmiophilic globules can also be used as an indicator of chloroplast development and senescence (Shao et al. 2014). Compared with leaves subjected to full sunlight, chloroplasts in leaves of 75 and 90 % shade contained significant fewer osmiophilic globules (Table 2). Additionally, the largest and the smallest osmiophilic globules were observed in leaves of 75 and 90 % shade. These results indicated that the plants grown at 75 and 90 % had remarkably less content of osmiophilic globules than plants grown under full sunlight. Thus, we tentatively suggested that 75 and 90 % shade was beneficial, whereas 0 and 50 % shade accelerated the senescence of chloroplasts.
Conclusion
T. grandis seedlings grown in full sunlight had low photosynthesis rate and Fv/Fm, LMA, Chl concentration, which was accompanied by increased lipid peroxidation. Plants grown under 75 % shade had the highest Fv/Fm and Pn values, and the lowest antioxidant enzyme activities and MDA content and REC. Compared with plants grown under 75 % shade, plants subjected to 90 % shade had lower photosynthetic rate and Gs, which was accompanied by an increase in MDA, REC. Thus, 75 % shade is suggested as the optimum light irradiance level for T. grandis cultivation.
References
Adams WW III, Barker DH (1998) Seasonal changes in xanthophyll cycle-dependent energy dissipation in Yucca glauca Nuttall. Plant Cell Environ 21(5):501–511
Adriano S, Bartolomeo D, Cristos X, Andrea M (2004) Effects of different irradiance levels on some antioxidant enzymes and on malondialdehyde content during rewatering in olive tree. Plant Sci 166(2):293–302
Ai XZ, Guo YK, Ma XZ, Xing YX (2004) Photosynthetic characteristics and ultrastructure of chloroplast of cucumber under low light intensity in solar greenhouse. Scientia Agricultura Sinica 37(2):268–273 (in Chinese)
Aleric KM, Kirkman LK (2005) Growth and photosynthetic responses of the federally endangered shrub, Lindera melissifolia (Lauraceae), to varied light environments. Am J Bot 92(4):682–689
Allen RD (1995) Dissection of oxidative stress tolerance using transgenic plants. Plant Physiol 107(4):1049–1054
Alscher RG, Erturk N, Heath LS (2002) Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot 53(372):1331–1341
Anderson JM, Osmond CB (1987) Shade-sun responses: compromises between acclimation and photoinhibition. In: Kyle DJ, Osmond CB, Arntzen CJ (eds) photoinhibition. Elsevier Science Publishers, Amsterdam, pp 1–38
Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50:601–639
Baker NR (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol 59:89–113
Ben Ahmed C, Ben Rouina B, Sensoy S, Boukhriss M, Abdullah FB (2009) Saline water irrigation effects on antioxidant defense system and proline accumulation in leaves and roots of field-grown olive. J Agr Food Chem 57(24):11484–11490
Bertamini M, Muthuchelian K, Rubinigg M, Zorer R, Velasco R, Nedunchezhian N (2006) Low-night temperature increased the photoinhibition of photosynthesis in grapevine (Vitis vinifera L. cv. Riesling) leaves. Environ Exp Bot 57(1):25–31
Björkman O, Demmig B (1987) Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta 170:489–504
Chen BQ, Cui XY, Zhao X, Zhang YH, Piao HS, Kim JH, Yun YP (2006) Antioxidative and acute anti-inflammatory effects of Torreya grandis. Fitoterapia 77(4):262–267
Cheng XJ, Li ZJ, Yu WW, Dai WS, Fu QG (2007) Distribution and ecological characteristics of Torreya grandis in China. J Zhejiang For Coll 24(4):383–388 (in Chinese)
Critchley C (1998) Photoinhibition. In: Raghavendra AS (ed) Photosynthesis: A comprehensive Treatise. Cambridge University Press, Cambridge, pp 264–272
Dai Y, Shen Z, Liu Y, Wang L, Hannaway D, Lu H (2009) Effects of shade treatments on the photosynthetic capacity, chlorophyll fluorescence, and chlorophyll content of Tetrastigma hemsleyanum Diels et Gilg. Environ Exp Bot 65(2–3):177–182
Delagrange S, Montpied P, Dreyer E et al (2006) Does shade improve light interception efficiency? A comparison among seedlings from shade-tolerant and -intolerant temperate deciduous tree species. New Phytol 172(2):293–304
Demmig-Adams B (1990) Carotenoids and photoprotection in plants: a role for the xanthophyll zeaxanthin. BBA-Bioenergetics 1020(1):1–24
Demmig-Adams B, Adams WW III (1992a) Photoprotection and other responses of plants to high light stress. Annu Rev Plant Physiol Plant Mol Biol 43:599–626
Demmig-Adams B, Adams WW III (1992b) Carotenoid composition in sun and shade leaves of plants with different life forms. Plant, Cell Environ 15(4):411–419
Duz SR, Siminnski A, Santos M, Paulilo MTS (2004) Crescimento inicial de três espécies arbóreas da Floresta Atlântica em resposta à variacão na quantidade de luz. Rev Bras Botânica 27:587–596
Favaretto VF, Martinez CA, Soriani HH, Furriel RPM (2011) Differential responses of antioxidant enzymes in pioneer and late-successional tropical tree species grown under sun and shade conditions. Enivron Exp Bot 70(1):20–28
He DT, Chu KJ, Ren JJ, Yao CF, Dai WS (2013) Chinese Torreya industry and its culture in Shengzhou. J Zhejiang Forest Sci Technol 33(5):113–115 (in Chinese)
Hikosaka K (2005) Leaf canopy as a dynamic system: ecophysiology and optimality in leaf turnover. Ann Bot 95(3):521–533
Hodges DM, DeLong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207(4):604–611
Huang Y, Wang J, Li G, Zheng Z, Su W (2001) Antitumor and antifungal activities in endophytic fungi isolated from pharmaceutical plants Taxus mairei, Cephalataxus fortunei and Torreya grandis. FEMS Immunol Med Mic 31(2):163–167
Huner NPA, Öquist G, Sarhan F (1998) Energy balance and acclimation to light and cold. Trends Plant Sci 3(6):224–230
Krause GH, Weis E (1991) Chlorophyll fluorescence and photosynthesis: the basics. Ann Rev Plant Physiol Plant Mol Biol 42:313–349
Kuwabara T, Katoh Y (1999) Involvement of the binuclear copper site in the proteolytic activity of polyphenol oxidase. Plant Cell Physiol 40(10):1029–1035
Larcher W (1995) Physiological plant ecology, 3rd edn. Springer, Berlin, pp 74–89:253–264
Lei TT, Tabuchi R, Kitao M, Koike T (1996) Functional relationship between chlorophyll content, leaf reflectance, and light-capturing efficiency of Japanese forest species. Physiol Plantarum 96:411–418
Li ZJ, Cheng XJ, Dai WS, Jing BH, Wang AG (2004) History and status and development of Torreya grandis in Zhejiang Province. J Zhejiang Forest Coll 21(4):471–474 (in Chinese)
Lichtenthaler HK (1987) Chlorophylls and carotenoids - pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Lichtenthaler HK (2013) Plastoglobuli, thylakoids, chloroplast structure and development of plastids. In: Biswal B, Krupinska K, Biswal UC (eds) Plastid development in leaves during growth and senescence advances in photosynthesis and respiration. Springer, Berlin, pp 337–361
Logan BA, Kornyeyev D, Hardison J, Holaday AS (2006) The role of antioxidant enzymes in photoprotection. Photosynth Res 88:119–132
Lu JH, Li YF, Wang X, Ren L, Feng YM, Zhao XL, Zhang CL (2013) Impact of shading on growth, development and physiological characteristics of Trollius chinensis Bunge. Scientia Agricultura Sinica 46(9):1772–1780 (in Chinese)
Marchese JA, Mattana RS, Ming LC, Broetto F, Vendramini PF, Moraes RM (2008) Irradiance stress responses of gas exchange and antioxidant enzyme contents in pariparoba [Pothomorphe umbellata (L.) Miq.] plants. Photosynthetica 46(4):501–505
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence-a practical guide. J Exp Bot 51(345):659–668
Mehlhorn H, Lelandais M, Korth HG, Foyer CH (1996) Ascorbate is the natural substrate for plant peroxidases. FEBS Lett 378(3):203–206
Mitchell AK (1998) Acclimation of Pacific yew (Taxus brevifolia) foliage to sun and shade. Tree Physiol 18:749–757
Nayyar H (2003) Accumulation of osmolytes and osmotic adjustment in water-stressed wheat (Triticum aestivum) and maize (Zea mays) as affected by calcium and its antagonists. Environ Exp Bot 50:253–264
Pearcy RW, Sims DA (1994) Photosynthetic acclimation to changing light environments: scaling from the leaf to the whole plant. In: Caldwell MM, Pearcy RW (eds) Exploitation of environmental heterogeneity by plants: ecophysiological processes above- and below-ground. Academic Press, San Diego, pp 145–174
Peri PL, Lucas RJ, Moot DJ (2007) Dry matter production, morphology and nutritive value of Dactylis glomerata growing under different light regimes. Agroforestry Syst 70(1):63–79
Perrin PM, Mitchell FJG (2013) Effects of shade on growth, biomass allocation and leaf morphology in European yew (Taxus baccata L.). Eur J Forest Res 132(2):211–218
Reich PB, Walters MB, Ellsworth DS (1991) Leaf lifespan as a determination of leaf structure and function among 23 Amazonian tree species. Oecologia 86:16–24
Shao QS, Wang HZ, Guo HP, Zhou AC, Huang YQ, Sun YL, Li MY (2014) Effects of shade treatments on photosynthetic characteristics, chloroplast ultrastructure, and physiology of Anoectochilus roxburghii. PLoS One 9(2):e85996
Smith M, Wu Y, Green O (1993) Effect of light and water-stress on photosynthesis and biomass production in Boltonia decurrens (Asteraceae), a threatened species. Am J Bot 80(8):859–864
Stitt M, Schulze D (1994) Does Rubisco control the rate of photosynthesis and plant growth? An exercise in molecular ecophysiology. Plant Cell Environ 17(5):465–487
Sun DQ, Li AW, Li J, Li DG, Li YX, Gong MZ (2009) Changes of lipid peroxidation in carbon disulfide-treated rat nerve tissues and serum. Chem Biol Interact 179(2–3):110–117
Ushimaru T, Maki Y, Sano S, Koshiba K, Asada K, Tsuji H (1997) Induction of enzymes involved in the ascorbate-dependent antioxidative system, namely, ascorbate peroxidase, monodehydroascorbate reductase and dehydroascorbate reductase, after exposure to air of rice (Oriza sativa) seedlings germinated under water. Plant Cell Physiol 38(5):541–549
Valladares F, Niinemets Ü (2008) Shade tolerance, a key plant feature of complex nature and consequences. Annu Rev Ecol Evol S 39(1):237–257
Valladares F, Chico J, Aranda I, Balaguer L, Dizengremel P, Manrique E, Dreyer E (2002) The greater seedling high-light tolerance of Quercus robur over Fagus sylvatica is linked to a greater physiological plasticity. Trees 16:395–403
Wittmann C, Aschan G, Pfanz H (2001) Leaf and twig photosynthesis of young beech Fagus sylvatica and aspen Populus tremula trees grown under different light regime. Basic Appl Ecol 2(2):145–154
Wright IJ, Cannon K (2001) Relationships between leaf lifespan and structural defences in a low-nutrient, sclerophyll flora. Funct Ecol 15:351–359
Yu YF (1999) A milestone of wild plants protection in China-the list of wild plants protected by the nation (the first batch). Plant Mag 5:3–11
Zhang SB, Hu H, Zhou ZK, Xu K, Yan N (2005) Photosynthesis in relation to reproductive success of Cypripedium flavum. Ann Bot 96:43–49
Author contribution statement
Designing the work: J.S.W.; running the experiments: H.T., Y.-Y. Hu., W.-W. Yu., L.-L.S.; data analysis and statistics: H.T. and Y.-Y. Hu; article writing and revising: H.T., Y.-Y. Hu., W.-W. Yu., L.-L.S., J.-S.W.
Acknowledgments
This work was funded by the Fruit Innovation Team Project of Zhejiang Province (2009R50033-7), the Zhejiang Provincial Natural Science Foundation of China (LZ12C16001), the Major Project of National Spark Plan of China (2012GA700001), the Launching Funds for Zhejiang A&F University (2013FR063), and the open project funds for forestry discipline in Zhejiang province (KF201312).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by W. Bilger.
H. Tang and Y.-Y. Hu contribute equally to this study.
Rights and permissions
About this article
Cite this article
Tang, H., Hu, YY., Yu, WW. et al. Growth, photosynthetic and physiological responses of Torreya grandis seedlings to varied light environments. Trees 29, 1011–1022 (2015). https://doi.org/10.1007/s00468-015-1180-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-015-1180-9