Abstract
A comparative anatomical and FTIR study on the leaves of 18 disputed species of section Tuberculata (Camellia, Theaceae) combined with their biogeographical distribution have been conducted in order to investigate interspecific variations which are useful in species taxonomic treatment and systematic evolution. All Tuberculata species have bifacial and hypostomatic leaves, contain sclereids and crystals. Multivariate analysis of leaf FTIR data shows a visualization of the degree of affinity among the species in this section, which is consistent with the distribution of synonymous Tuberculata species indicated in the biogeographical maps. Our study indicates that the combination of characters based on leaf comparative anatomy, FTIR analysis and biogeographical distribution are useful in species revision. The results determine 11 species in section Tuberculata and provide the evolutionary trends in Tuberculata, which originated in three-river region of Guizhou province and spread to Guizhou, Guangxi, Yunnan, Sichuan, Chongqing, Hunan and probably Hubei provinces of China.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Section Tuberculata Chang, only distributed in China (Chang and Ren 1991; Ming and Zhong 1993), represents a good example of a taxonomic group in the genus Camellia with controversial circumscription. This section was previously classified in section Heterogenea Sealy (Sealy 1958; Keng 1962), but more recent classifications recognized it as a separate section Tuberculata (Chang 1981; Chang and Ren 1991; Ming and Zhong 1993; Ming 1999) (see Table 1).
The taxonomic history of section Tuberculata began in 1939 when Chien (1939) first discovered a new species in Yunnan province of China and named it Camellia tuberculata Chien. Subsequently, new species were described by Keng (1962), Chang (1981), Chang and Ren (1991), Lin and Lu (1984), Xu (1985), Xu et al. (1987), Li and Yang (1987) and Ming and Zhong (1993).
Sealy (1958) who first divided Camellia into 12 sections classified Tuberculata in the section Heterogenea. After comprehensively studying the genus Camellia, Chang (1981) subdivided Camellia into four subgenera (Protocamellia, Camellia, Thea and Metacamellia) and 19 sections. In his classification, the section Tuberculata was transferred from Heterogenea and recognized as section Tuberculata belonging to subgenus Camellia. Ming (1999), based on Sealy (1958) and Chang’s (1981, 1998) work, produced a new classification. In his classification, Ming (1999) divided Camellia into two subgenera (Camellia, Thea) and 14 sections. He agreed with Chang (1981), and Chang and Ren (1991) on the setup of section Tuberculata, but significantly reduced the number of species in the section, and transferred C. ilicifolia Y. K. Li ex Chang from the section Pseudocamellia in Chang and Ren’s (1991) classification into section Tuberculata.
The taxonomic treatment of a few species in section Tuberculata is still ambiguous. For example, the number of species recognized in the section varies significantly, estimated from one by Sealy (1958), 17 by Chang and Ren (1991) to six by Ming and Zhong (1993), indicating problems with species circumscription and ranking. In addition, taxonomic treatments of the section based on the traditional morphological taxonomy were significantly discordant by differently selected diagnostic characters (Chang 1981; Chang and Ren 1991; Ming and Zhong 1993; Ming 1999). Therefore, it is necessary to seek other sources of information for reexamining the classification of the section Tuberculata.
Characteristics of leaf comparative anatomy including leaf epidermis and leaf transverse section of higher plants have been proved to be of great use in correctly identifying the species and understanding relationships among extant taxa (Stace 1966; Brittan 1970; Baranova 1972, 1987, 1992; Lubke and Phipps 1973; Upchurch 1984; Kong 2001; Kocsis et al. 2004; Yang and Lin 2005). In section Tuberculata, however, information was available on the description of leaf anatomic features only for one species Camellia tuberculata Chien examined by Ao et al. (2002).
Fourier transform infrared spectroscopy (FTIR) is a rapid, simple, high-resolution analytical method which is based on the vibrations of functional groups and highly polar bonds of the components analyzed (Naumann et al. 1991; Kim et al. 2004). Thus, FTIR can provide biochemical profiles of the conducted tissue and organ. Studies have demonstrated that FTIR was robust not only in identifying closely related microbial strains (Naumann et al. 1991; Goodacre et al. 1998; Wenning et al. 2002) but also discriminating flowering plants (Lu et al. 2004; Kim et al. 2004). Lu et al. (2004) reported that FTIR spectra of leaves were of taxonomic value at the level of species and sections in the genera Hypericum and Triadenum. Kim et al. (2004) determined that FTIR was an excellent method for identifying species boundaries and determining phylogenetic relationships between flowering plants based on multivariate analysis. Thus, a detailed analysis of leaf FTIR spectra of 18 Tuberculata species can be regarded as a significant method to identify the disputed species in the section.
The present study shows a comparative analysis of 18 disputed taxa of section Tuberculata with the aims (1) to assess leaf anatomical, biochemical characters and biogeographical distribution, (2) to provide a new taxonomic treatment of the section, and (3) to discuss evolutionary trends of Tuberculata on the basis of comparative leaf anatomy, FTIR analysis and biogeographical distribution.
Materials and methods
Plant materials
This study was based on the examination of living collections and herbarium materials. Living materials were collected in the International Camellia Species Garden of Jinhua city (ICSG, 29°7′N, 119°35′S, 40 m in altitude) and vouchers were deposited in the Chemistry and Life Science College of Zhejiang Normal University (ZJNU). All living plants have similar habitat in this garden which reduces the majority of environmental/geographical effects on leaf anatomy and chemical composition. Samples for comparative leaf anatomy and biochemical analysis were taken from the third mature leaves fully exposed to sunlight and horizontally arranged on the 1-year-old branches of the plants grown in the garden. Biogeographical analysis was based on SYS (Department of Biology, Sun Yat-Sen University), KUN (Kunming Institute of Botany, Chinese Academy of Sciences), PE (Institute of Botany, Chinese Academy of Sciences), GZBI (Guizhou Institute of Biology, China) and a small number of SCBI (South China Institute of Botany, Chinese Academy of Sciences) specimens. All species examined were the following:
Camellia tuberculata Chien: Jinhua (ICSG), Peng Q. F. 05112301, 02, 03 (ZJNU); SICHUAN, Fengjie, Wu W. K. 0212 (KUN), Yang X. J. 3280 (PE); Nanchuan, Wang F. Z. 10901 (PE); Weiyuan, Hu X. Q. and Zhong M. F. 794 (SYS); Leshan, Wang S. J. 213 (SYS), Dai L. Y. 651 (PE); CHONGQING, Beipei, Qu G. L. 6621 (SYS); GUIZHOU, Chishui, Bijie Team 1328 (PE). Camellia lipingensis Chang: Jinhua (ICSG), Jiang B. and Peng Q. F. 06062301, 02, 03 (ZJNU); GUIZHOU, Liping, Zeng F. A. 81046 (SYS). C. rhytidocarpa Chang et Liang: Jinhua (ICSG), Jiang B. 06111201, 02, 03 (ZJNU); GUANGXI, Jinxiu, Dayaoshan Exp. 810579 (IBG); Longsheng, Tan H. F. 700908 (SYS); Rongshui, Chen S. Q. 16727 (KUN). HUNAN, Qianyang, Li Z. T. 500 (PE); Chengbu, Qi L. L. 83001 (SYS). C. rhytidophylla Y. K. Li et M. Z. Yang: Jinhua (ICSG), Jiang B. 06111201, 02, 03 (ZJNU); GUIZHOU, Kaiyang, Yang M. Z. 1545 (GZBI). C. leyeensis Chang: Jinhua (ICSG), Jiang B. 06111201, 02, 03 (ZJNU); GUANGXI, Leye, Zhong, Y. C. 890806 (SYS). C. anlungensis Chang: Jinhua (ICSG), Peng Q. F. 05112301, 02, 03 (ZJNU); GUIZHOU, Wangmo, Guizhou Team 1757 (GZBI); Ceheng, Cao Z. Y 1227 (PE); Anlong, Guizhou Team 5952 (PE); GUANGXI, Longlin, Zhong, Y. C. 80835A (KUN). C. rubituberculata Chang: Jinhua (ICSG), Jiang B. and Peng Q. F. 06062301, 02, 03 (ZJNU); GUIZHOU, Qinglong, Li M. J. and Lu Q. M. 155710 (SYS). C. acutiperulata Chang et Ye: Jinhua (ICSG), Jiang B. and Peng Q. F. 06062301, 02, 03 (ZJNU); GUANGXI, Longlin, Zhong Y. C. 80835 (KUN). C. acuticalyx Chang: Jinhua (ICSG), Jiang B. and Peng Q. F. 06062302, 03, 04 (ZJNU); GUANGXI, Nanlin, Liang S. Y. 7904270 (SYS). C. atuberculata Chang: Jinhua (ICSG), Jiang B. and Peng Q. F. 06090401, 02, 03 (ZJNU); GUIZHOU, Chishui, Guizhou Agr. Col. For. Flo. Div. 75309 (SYS); Li Y. K. 78028, 81092 (GZBI). C. neriiflolia Chang: Jinhua (ICSG), Jiang B. and Peng Q. F. 06090401, 02, 03 (ZJNU); GUIZHOU, Chishui, Zeng F. A. 81091(SYS). C. obovatifolia Chang: Jinhua (ICSG), Jiang B. and Peng Q. F. 06062305, 01, 02 (ZJNU); YUNNAN, Wenshan, Nanjing Unv. Exp. 15192 (PE). C. rubimuricata Chang et Z. R. Xu: Jinhua (ICSG), Jiang B. and Peng Q. F. 06090401, 02, 03 (ZJNU); GUIZHOU, Libo, Xu Z. R. L2074 (SYS). C. parvimuricata Chang: Jinhua (ICSG), Jiang B. and Peng Q. F. 06090402, 03, 04 (ZJNU); SICHUAN, Nanchuan, Li G. F. 63590 (PE); HUBEI, Lichuan, Dai L. Y. 752 (PE); GUIZHOU, Jiangkou, China and American Team Exp. 913 (PE); Shijian, Ning S. B. 623 (SCBI); HUNAN, Qianyang, Li Z. T. 1101, 1726 (PE). C. hupehensis Chang: Jinhua (ICSG), Peng Q. F. 05112301, 02, 03 (ZJNU); West Hubei, Yang B. L. 002 (SYS). C. zengii Chang: Jinhua (ICSG), Jiang B. and Peng Q. F. 06061201, 02, 03 (ZJNU); GUIZHOU, Liping, Zeng F. A. 8017 (SYS). C. pyxidiacea Xu et al.: Jinhua (ICSG), Jiang B. and Peng Q. F. 05112302, 03, 04 (ZJNU); YUNNAN, Luoping, Xu Z. R. SJ5225 (SYS); Zhang W. J. 92027 (KUN). GUIZHOU, Yixing, Dun C. Y. 2675 (KUN). C. ilicifolia Y. K. Li ex H. T. Chang: Jinhua (ICSG), Shen J. B. and Peng Q. F. 06110501, 02, 03 (ZJNU); GUIZHOU, Chishui, Li Y. K. 74357 (SYS).
Biogeographical distribution
Distribution maps for each species were scored by a grid delimited by latitude and longitude. Grid diversity represented the number of species per grid (Pearson and Juliano 1993), which provided a rapid assessment of species-rich areas.
Anatomical protocol
Approximately 1 cm2 of leaf tissue was removed from the middle section of the leaf and placed in a glass tube; 40% sodium hypochlorite solution was added, in sufficient quantity to cover the material, and the tubes were then heated to 37°C. After approximately 30 min, the epidermal layers were teased apart from each other, washed in water, stained in safranin-alcian green and mounted in neutral balsam.
For the anatomical analysis, mature leaves were fixed in FAA (5 parts formalin:5 parts acetic acid:90 parts 70% ethyl alcohol) immediately after detachment. After being fixed for at least 48 h, plant materials were transferred to 50% ethyl alcohol, dehydrated with ascending alcohol series and embedded in paraffin. Transversal sections, 10 μm thick, were made with a rotary microtome and stained in safranin-alcian green (Hussin and Sani 1998).
All light micrographs were taken with an OLYMPUS BX50 light microscope (Olympus, Japan), equipped with photographic apparatus. Leaf epidermal and transverse sections from three different individual plants of each species were analyzed. The classification proposed by Baranova (1987, 1992) was followed in determining the type of stomata and the terminology of Wilkinson (1979) was adopted for other characters.
FTIR analysis
The methods for FTIR analysis were modified from the method described by Lu (2004). Five milligrams powdered leaves were placed directly on the germanium piece of the infrared spectrometer. Infrared analysis was performed using American NICOLET NEXUS Model 670 FTIR spectrophotometer, equipped with an OMNI sampler averaging of 256 scans. Spectral resolution was 4 cm−1, and spectra collected over the wave number ranged from 4,000 to 675 cm−1. Spectra were processed using OMNIE 5.2 Brainpower Operation software. Samples were run in triplicate using leaves from three different individual plants of each species.
After filtering out the spectra regions that were dominated by CO2 (2403.21–2272.06 cm−1 and 682.77–675 cm−1), FTIR spectra were baseline corrected so that the smallest absorbance was equal to 0. The first derivatives of these normalized spectra were calculated using the Unscrambler 9.6 (CAMO, Trondheim, 1998) with five-point smoothing. These data were then subjected to multivariate analysis. Principal component analysis (PCA) was performed using a maximum of 10 principal components. Cluster analysis was created on the dissimilarity matrix of Euclidean distances with UPGMA as the clustering algorithm using the STATICA statistical package (STATICA for Windows, StatSoft).
Results
Leaf epidermis characters
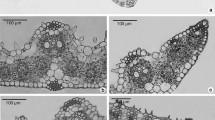
The characteristics of leaf epidermis of the section Tuberculata were listed in Table 2. It appeared that epidermal features were constant within species. The epidermal cells of Tuberculata were usually polygonal or irregular in form, with anticlinal walls straight to curved, or sinuous. The pattern of anticlinial wall varied not only in different species but also between adaxial (Ad) and abaxial (Ab) epidermis of the same species. Consistent pattern of anticlinial wall between Ad and Ab occurred in C. lipingensis (Fig. 1c, d), C. rhytidophylla (Fig. 1g, h), C. leyeensis (Fig. 1i, j) and C. ilicifolia (Fig. 2k, l).
a–x Characteristics of epidermal cells: adaxial (Ad) on the left, abaxial (Ab) on the right. a, b C. tuberculata; c, d C. lipingensis; e, f C. rhytidocarpa; g, h C. rhytidophylla; i, j C. leyeensis; k, l C. anlungensis, m, n C. rubituberculata; o, p C. acutiperulata; q, r C. acuticalyx; s, t C. atuberculata; u, v C. neriiflolia; w, x C. obovatifolia. Scale bar: 100 μm
All species in section Tuberculata were hypostomatic, and the stomata were all of the anisocytic type in that guard cells were surrounded by three subsidiary cells (Fig. 1, 2). The leaves of some species in Tuberculata were glabrous, while a small number of taxa had hairs on the abaxial lamina surface, e.g. C. tuberculata, C. lipingensis, C. rhytidocarpa, C. anlungensis, C. leyeensis, C. anlungensis, C. atuberculata, C. parvimuricata, C. hupehensis, C. zengii and C. ilicifolia (Table 2).
Leaf transverse characters
All the leaves in the sections were bifacial and were composed of wax, epidermis, palisade parenchyma, spongy parenchyma, and vascular tissue (Fig. 3a). The abaxial epidermis cells in the section were all single layered (Fig. 3a, 4), but on most adaxial surfaces there was often a multiple epidermis (Table 2) except for a single layer in C. rhytidophylla (Fig. 4d), C. leyeensis (Fig. 4e), C. obovatifolia (Fig. 4l) and C. ilicifolia (Fig. 4r).
a, b Leaf transverse sections (T.S.) in Tuberculata species. a C. parvimuricata T.S. of the leaf illustrating the general anatomy (ad adaxial epidermis, ab abaxial epidermis, pal palisade parenchyma, spo spongy parenchyma, gla gland, lb lateral bundle, scl sclereid, cry crystal); b the central vascular of C. leyeensis illustrating the general anatomy (xyl xylem, phl phloem, scl sclereid, par parenchyma). Scale bar: 150 μm
a–r Leaf transverse sections (T.S.) in Tuberculata species. a C. tuberculata; b C. lipingensis; c C. rhytidocarpa; d C. rhytidophylla; e C. leyeensis; f C. anlungensis; g C. rubituberculata; h C. acutiperulata; i C. acuticalyx; j C. atuberculata; k C. neriiflolia; l C. obovatifolia; m C. rubimuricata; n C. parvimuricata; o C. hupehensis; p C. zengii; q C. pyxidiacea; r C. ilicifolia. Scale bar: 150 μm
The mesophyll comprised a loosely arranged spongy parenchyma and one, two or seldom three layers of palisade parenchyma. The palisade cells were found immediately below the adaxial epidermis. The palisade mesophyll in C. rhytidophylla (Fig. 4d) was only a single layer thick, while there were two layers in C. parvimuricata (Fig. 4n), C. hupehensis (Fig. 4m) and C. ilicifolia (Fig. 4r). However, one, two as well as three layers of palisade cells in other Tuberculata species could occur simultaneously in a single leaf. The cells of palisade parenchyma were all vertically-elongated while the cells of spongy parenchyma were more irregular in shape with increased intercellular spacing. Only palisade cells contained many large chloroplasts. Sclereids were abundantly present in the mesophyll of Tuberculata species (Fig. 3a, b). Crystals with small spiny druses were present in most species but rare in C. ilicifolia (Fig. 4l). Moreover, the glands were found in all Tuberculata species except in C. ilicifolia (Table 2, Fig. 3a).
All species examined shared similar vascular anatomy in leaves (Fig. 3b). The midrib bundle was slightly flattened and surrounded by thick-walled tissue. Veins in the section were embedded and surrounded by parenchymatous bundle sheaths.
FTIR analysis and species discrimination
The FTIR spectra from section Tuberculata were analyzed and are given in Fig. 5a. Principle component analysis (PCA) of FTIR data was displayed in a two-dimensional plot using the first two principal components (Fig. 5b). It permitted a visualization of the degree of affinity among the species. Tentatively, the following groupings and ascriptions seemed reasonable: 14, 15, 11, 12 (11, 12 ascription unclear) C. parvimuricata subsp. hupehensis; 2, 3 C. rhytidocarpa subsp. lipingensis; 1, 10 C. tuberculata subsp. atuberculata; 13, 16, 17 (17 ascription unclear) C. rubimuricata subsp. zengii; 5, 6, 7 C. anlungensis subsp. leyeensis (7 ascription unclear); 4, 8, 9 C. acutiperulata subsp. acuticalyx (4 ascription unclear).
A hierarchical dendrogram was generated to display the relationships between plant species based on PCA of FTIR data (Fig. 5c). The dendrogram divided 18 species into two groups. C. ilicifolia was separated at the top level (Euclidean 0.245) from the other 17 Tuberculata species, indicating the sole difference of C. ilicifolia. The shortest distances between spectra of Tuberculata species were recorded within C. parvimuricata and C. hupehensis (Euclidean 0.041), which indicated their highest similarity. While C. lipingensis and C. rhytidocarpa (Euclidean 0.062), C. tuberculata and C. atuberculata (Euclidean 0.072), C. rubimuricata and C. zengii (Euclidean 0.084), C. neriiflolia, C. obovatifolia, C. parvimuricata and C. hupehensis (Euclidean 0.087), C. anlungensis and C. leyeensis (Euclidean 0.096), C. pyxidiacea, C. rubimuricata and C. zengii (Euclidean 0.099), C. acutiperulata and C. acuticalyx (Euclidean 0.111), respectively, formed a group distinct from the other plants (Fig. 5b).
Geographical distribution
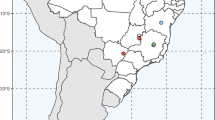
Section Tuberculata is distributed in Southwest China and the range of its distribution is limited between 22°30′–31°12′N and 103°18′–110°30′S (Fig. 6a). Figure 6a showed that Tuberculata species are distributed in the regions of Guizhou (about ten species), Yunnan (two species), Guangxi (five species), Sichuan (two species), Chongqing (two species), Hunan (two species) and Hubei (one species) provinces of China (Fig. 6a).
a–b Distribution maps and evolutionary trends of species of Camellia sect. Tuberculata. a Distribution map of Tuberculata species; b distribution range of revised Tuberculata species and its evolution trends. Arrows show evolution trends of Tuberculata. 1, C. tuberculata; 2, C. lipingensis; 3, C. rhytidocarpa; 4, C. rhytidophylla; 5, C. leyeensis; 6, C. anlungensis; 7, C. rubituberculata; 8, C. acutiperulata; 9, C. acuticalyx; 10, C. atuberculata; 11, C. neriiflolia; 12, C. obovatifolia; 13, C. rubimuricata; 14, C. parvimuricata; 15, C. hupehensis; 16, C. zengii; 17, C. pyxidiacea; 18, C. ilicifolia
The grid squares with the highest concentration of species (five species) were found on three-river regions of Guizhou province. The east Guizhou region into the northwest Hunan region had slightly lower concentrations of species (four species). Fewer species were found in western Yunnan, southern Guangxi, northern Sichuan and eastern Hunan regions (Fig. 6a).
C. tuberculata and C. atuberculata, C. hupehensis and C. parvimuricata, C. acutiperulata and C. acuticalyx, C. lipingensis and C. rhytidocarpa, C. leyeensis and C. anlungensis, C. rubimuricata and C. zengii, respectively, had similar distribution patterns which also showed consistent groupings and ascriptions with FTIR analysis in Fig. 5b, c.
Discussion
Leaf comparative anatomy evidence for subdivision of section Tuberculata
Leaves provided many anatomical characters which could be regarded as a classical source of data used in plant taxonomy (Stace 1966; Brittan 1970; Baranova 1972, 1987, 1992; Lubke and Phipps 1973; Upchurch 1984; Kong 2001; Yang and Lin 2005). Results of the study showed that there was considerable anatomical variation between the 18 species studied and some anatomical characters had diagnostic value in the section. Anisocytic stomata and sclereids were found in all 18 species studied. All were marked by glands and crystals, except C. ilicifolia. Some species had a certain number of layers (1 or 2) of palisade cells, and others varied from one to three layers. A hypodermal layer was not seen in all species, but a multiple epidermis was found in most species.
As shown in Table 2, idioblasts (sclereids and glands) were present in the abaxial mesophyll of all Tuberculalta species and were a characteristic feature of the section. Previous studies maintained that idioblasts were a well-known feature of Camellia (Foster 1944; Schofield 1968). Foster (1956) illustrated that there were three major categories of plant idioblasts, viz. excretory, tracheoid and sclerenchymatous, and their existence could serve as a diagnostic characteristic. In this aspect, Chang (1981) maintained that the only one anatomical characters that Tuberculata shared was the presence of the glands in the abaxial mesophyll. In our study, sclereids were present in all analyzed species, while glands were absent in C. ilicifolia (Table 2). Therefore, the fact that glands were absent in C. ilicifolia supports the view that C. ilicifolia is not included in section Tuberculata (Chang 1981; Chang and Ren 1991). In all analyzed species branched sclereids were frequent to abundant. This might be similar to the ‘sclerenchymatous idioblasts’ as reported by Foster (1956). According to Keng (1962), sclereids running freely in the leaf were found chiefly in a few genera of Theaceae. Metcalfe and Chalk (1972) and Luna and Ochoterena (2004) supported this idea, maintaining that the sclerenchymatous idioblasts in the parenchymatous tissues of the leaf were especially characteristic of the family. Thus, the presence of leaf sclereids might be only useful in the taxonomy of Camellia and family Theaceae, while the presence of glands has taxonomic value in section Tuberculata.
Solereder (1908) was regarded as the first botanist who gave a detailed account on the stomatal apparatus in angiosperms including Theaceae. Latter, based on Solereder’s (1908) study, Keng (1962) reported that there were two basic stomata types in Theaceae, ranunculaceous (anomocytic), and an “intermediate’’ form between anomocytic and paracytic (called ‘‘gordoniaceous’’), characterized by the presence of obliquely situated subsidiary cells. Yang et al. (2003) suggested that the intermediate type between anomocytic and paracytic more exactly fit the anisocytic stomata. Our results that anisocytic stomata were observed in all the species in this study (Fig. 1, 2) corresponded with previous views (Keng 1962; Yang et al. 2003). However, Ao et al. (2002) pointed out that all of the species examined shared the same cyclocytic stomata after observations on 36 species representing 19 sections in Camellia. It was considered here more likely that he wrongly identified the stomata type or the terminology for this characteristic was based on other classifications because stomata in their figures were much similar to anisocytic stomata as we had defined them as guard cells surrounded by three subsidiary cells.
The epidermal cell presented a few taxonomically or diagnostically useful features (Baranova 1972; Wilkinson 1979; Stace 1984; Upchurch 1984; Kong 2001; Yang and Lin 2005). However, up to now, there is still no epidermal study in Tuberculata except for the only species C. tuberculata (Ao et al. 2002). Although the usefulness of the epidermis characters in species identification appeared limited due to the high diversity of characters as seen and described in the shape of epidermis, in our study, based on a high number of species from different regions in China, it was shown that the pattern of epidermal cells between Ad and Ab in section Tuberculata was reliable.
The vascular tissue in leaf mesophyll lacked distinctive features in most species. However the epidermis and palisade parenchyma in transverse sections showed some variation for certain species. Different types of adaxial epidermal cells and different numbers of layer of palisade parenchyma were observed in different species of section Tuberculata. A multiple epidermis had proven to be of interesting diagnostic value in a few species of Rubiaceae (Kocsis et al. 2004), Sterculiaceae (Hussin and Sani 1998) and Papilionoideae (Crow et al. 1997). However, to our knowledge, few studies have illustrated the presence of the multiple epidermis in section Tuberculata, genus Camellia and even in family Theaceae. With extensive leaf anatomic study of the samplings from different regions in China, it was shown that the presence of multiple epidermis was constant in the same species. This suggested that multiple epidermis was reliable as a diagnostically useful character in Tuberculata species.
The thickness of palisade parenchyma varied significantly in the same species distributed in different ecological regions. However, the number of layers of palisade parenchyma was reliable in the same species collected from different regions in this study. Therefore, palisade parenchyma layers could be regarded as a character with systematic value at the species level within the section Tuberculata.
FTIR analysis for a subdivision of section Tuberculata
The FTIR have been used not only in microbe biology to correctly identify closely related microbial strains (Naumann et al. 1991; Goodacre et al. 1998; Wenning et al. 2002) but also in plant biology in a number of studies that include discrimination of cell-wall mutant plants (Stewart et al. 1997; Chen et al. 1998), cell-wall composition and architecture (McCann et al. 1997), mechanical properties and molecular dynamics of plant cell-wall polysaccharides (Wilson et al. 2000), and determination of the fruit content in processed foods (Wilson et al. 1993). Recently, FTIR have proven to be of great taxonomic value at the level of species and sections in the genera Hypericum and Triadenum (Lu et al. 2004), and represented phylogenetic relationships between higher plant species (Kim et al. 2004).
Multivariate analysis such as PCA and cluster analysis turned out to be a very useful tool for differentiating plants (Chen et al. 1998; Kim et al. 2004), as it revealed relationships among their FTIR spectra. In PCA, principal components 1 and 2 (PC1 and PC2) could be used to separate the 18 disputed Tuberculata species. Figure 5b showed C. ilicifolia significantly distant from other 17 species. Cluster analysis over the entire range of the first derivatives spectra also showed that C. ilicifolia and other 17 Tuberculata species were placed at the top level of the dendrogram, which determined that C. ilicifolia was separate from other Tuberculata plants. These results did not support the conclusion of Ming and Zhong (1993) in transferring C. ilicifolia Y. K. Li ex H. T. Chang from section Pseudocamellia in Chang’s (1981, 1998) classification into section Tuberculata.
Figure 5b, c showed that C. parvimuricata and C. hupehensis, C. tuberculata and C. atuberculata, C. acutiperulata and C. acuticalyx, C. lipingensis and C. rhytidocarpa, C. leyeensis and C. anlungensis, C. rubimuricata and C. zengii, respectively, formed homogeneous groups indicating their high homogeneity. These results supported Ming and Zhong (1993) who merged C. tuberculata and C. atuberculata, C. acutiperulata and C. acuticalyx, C. lipingensis and C. rhytidocarpa, C. leyeensis and C. anlungensis, and regarded C. hupehensis and C. parvimuricata as a species. Although the spectra of C. lipingensis and C. rhytidocarpa formed homogeneous clusters (Fig. 5b, c), the spectra of C. zengii were not incorporated into C. rhytidocarpa cluster (Fig. 5b, c), which was not consistent with Ming and Zhong’s (1993) merging of C. zengii into C. rhytidocarpa. The fact that C. lipingensis and C. rhytidocarpa formed a homogeneous group supported the reduction of C. zengii and C. rubimuricata to synonyms.
Although C. neriiflolia, C. obovatifolia, C. parvimuricata and C. hupehensis formed a group at the level of Euclidean distance of 0.087 (Fig. 5c), the significant differences among their leaf anatomical characters such as hairs present on abaxial epidermis or not, and the type of the epidermis and palisade parenchyma (Table 2) did not support them to be regarded as one species. Furthermore, the difference of their biogeographical distribution also did not support their merging (Fig. 6b). Similarly, C. lipingensis and C. rhytidocarpa could not be merged into C. tuberculata and C. atuberculata, and C. pyxidiacea could not be merged into C. rubimuricata and C. zengii.
Taxonomic treatment of section Tuberculata
Seventeen species were described in Chang and Ren’s (1991) taxonomic treatment of Tuberculata, while only six taxa were described after Ming and Zhong’s (1993) revision of the section based on morphological characters (Table 1). Uncertain morphological evidences resulted in the controversial circumscription. Therefore, in this study leaf comparative anatomy and multivariate analysis of leaf FTIR data were conducted to re-examine the classification of the section tuberulata.
Chang and Ren (1991), Chang (1998), Ming and Zhong (1993) and Ming (1999) agreed on the species delimitation of C. tuberculata Chien, C. anlungensis Chang, C. rhytidocarpa Chang et Liang, and C. parvimuricata Chang based on morphology. In our study, the characters of leaf comparative anatomy and multivariate analysis of leaf FTIR data also varied significantly among these species. This suggested that the delimitation of all the four species was reliable.
The major characteristics of C. ilicifolia Y. K. Li ex H. T. Chang described by Chang (1981, 1998) were smooth in ovary and fruit surface, no hairs on seeds and no glands in abaxial leaves that were regarded as the diagnostic characters of section Pseudocamellia. However, Ming and Zhong (1993), and Ming (1999) moved C. ilicifolia from section Pseudocamellia in Chang’s (1981, 1998) classification into section Tuberculata, because he stated that C. ilicifolia shared slight strumae in the ovary surface and 3–5 locules that were regarded as the diagnostic characters of section Tuberculata. Thus, C. ilicifolia Y. K. Li ex H. T. Chang was transferred by Ming and Zhong (1993), and Ming (1999) from section Pseudocamellia in Chang’s (1981, 1998) classification into section Tuberculata. It was considered here that the diversity of characters as seen and described by two authors in the shape of ovary in C. ilicifolia resulted in different species delimitation. Our results did not support Ming and Zhong’s (1993) taxonomic treatment, because multivariate analysis of FTIR data (Fig. 5b, c) and the absence of the glands on the abaxial mesophyll and crystals in mesophyll cells (Table 2) in C. ilicifolia significantly indicated its difference from Tuberculata species.
Camellia tuberculata Chien and C. atuberculata Chang were reduced to synonyms of C. tuberculata based on morphological characters by Ming and Zhong (1993). Leaf comparative anatomy and FTIR analysis in this study supported this. However, evidences from leaf comparative anatomy and FTIR analysis did not support C. rhytidophylla Chang to be merged into C. tuberculata as in Ming and Zhong’s (1993) classification. A unicellular epidermis, one layer of palisade cells and hairs absent on the abaxial epidermis in C. rhytidophylla showed the distinction with other two species. We agreed with Chang and Ren (1991) in considering C. rhytidophylla as a separate species rather than reducing it to a form of C. tuberculata as done by Ming and Zhong (1993).
Camellia hupehensis Chang and C. parvimuricata Chang were reduced to synonyms of C. parvimuricata based on morphological characters by Ming and Ming (1993). In our study, we found that characters of the leaf anatomy in the two species, such as the shape of epidermal cells, glands and hairs on abaxial surface, multiple epidermis, the number of palisade mesophyll layers and sclereid and crystals in mesophyll cells, were constant with no apparent difference (Table 2). Furthermore, all samplings analysed by multiple analysis of leaf FTIR data in two species clearly formed homogeneous groups (Fig. 5b, c). These results supported Ming and Zhong’s (1993) merging.
Although leaf anatomical characters listed in Table 2 showed no apparent difference in C. rubituberculata Chang and C. pyxidiacea Z. R. Xu et al., a significant difference was found in the FTIR analysis of their leaves (Fig. 5b, c). Furthermore, we have examined specimens of the species and found that the morphological characters of C. rubituberculata were red flowers, a hairy ovary and 1–1.3 mm thick pericarps, which was significantly different from C. pyxidiacea with white flowers, glabrous ovary and 3–7 mm thick pericarps. Thus, we did not approve Ming’s merging C. rubituberculata Chang into C. pyxidiacea Z. R. Xu et al.
The merging of C. leyeensis Chang into C. anlungensis Chang, C. lipingensis Chang into C. rhytidocarpa Chang et Liang, and C. acuticalyx Chang into C. acutiperulata Chang et Ye by Ming and Zhong (1993) based on morphology found supports in our study because of their homogeneous groups in multiple analysis of leaves FTIR data (Fig. 5b, c). However, evidence from leaf anatomical characters and multiple analysis of their leaf FTIR data did not support the merging of C. acutiperulata Chang et Ye, C. acuticalyx Chang and C. obovatifolia Chang into C. anlungensis Chang. Examination of the herbarium specimens and living materials of these species showed that C. acutiperulata Chang et Ye subsp. acuticalyx Chang was characterized by elliptic 8–13 cm long leaves, oval bracts and sepals with acute apex, and glabrous ovary and style, and C. obovatifolia Chang by obovate to lanceolate 6–9 cm long leaves, ovate bracts and sepals with round apex, and a hairy ovary with glabrous style. These descriptions were significantly different from that of C. anlungensis Chang. We observed that the major characteristics of C. anlungensis were obovate 10 cm long leaves, round sepals, and hairy ovary and style. Thus, the difference of the morphological characters also did not surpport that C. acutiperulata Chang et Ye, C. acuticalyx Chang, C. obovatifolia Chang and C. anlungensis Chang were reduced to synonyms of C. anlungensis.
Camellia rubimuricata Chang et Z. R. XU and C. zengii Chang were characterized by their consistent pattern between Ad and Ab, multiple epidermis, same number of layers of the palisade parenchyma (Table 2) and homogeneous groups in the cluster analysis (Fig. 5b, c). The above features were unique for them, supporting the view that they were one species, and that C. zengii Chang should no longer be recognized.
Geographical distribution and evolution
Section Tuberculata was regarded as an endemic group of the subtropical area of China (Chang and Ren 1991; Ming and Zhong 1993; Ming 1999). There have been some previous geographical studies carried out to determine the distribution of the Tuberculata species (Chang and Ren 1991; Ming and Zhong 1993; Ming and Zhang 1996). In their study, Chang and Ren (1991) and Ming and Zhong (1993) suggested Guizhou province of China as the center of distribution of section Tuberculata.
The present geographic survey of the Tuberculata species, based on herbarium specimens and supplemented by field work, provided useful information on the geographical distribution of the section in southwest China (Fig. 6a). The results in our study that the highest concentration of species was distributed in Guizhou province also indicated that Guizhou province was the center of distribution of section Tuberculata (Fig. 6a). Moreover, distribution maps of section Tuberculata (Fig. 6a, b) showed that the distribution trends of the section were gradually spreadings from the centers of the distribution to circumjacent regions.
Morphological (Chang 1981; Ming and Zhong 1993; Ming 1999) and chromosome-cytological characters (Chang and Ming 1999) had provided strong evidence for the close affinity of Pseudocamellia to Tuberculata. Chang (1981) suggested that section Tuberculata was derived from Pseudocamellia using phyletic approach. Similarly, Ming and Zhong (1993) stated that the section might be a substitute group from section Pseudocamellia that was distributed and had evolved towards the east, and interpreted the evolutionary trends among six Tuberculata species. Subsequently, Ming (1999) conducted a cladistic approach to analyse the phylogenetic relationships of Camellia, and confirmed that section Tuberculata was evolved from section Pseudocamellia.
According to Ming and Zhong (1993), C. pyxidiacea distributed in the three-river region of Guizhou province was regarded as the most original species because of the unconspicuous strumae in ovary surface, 5 locules per ovary and flowers more than 5 cm in size. C. anlungensis and C. tuberculata were supposed to be the advanced species because of the clear strumae in ovary surface, 3–5 locules per ovary and flowers 3–5 cm in size, and C. rhytidocarpa and C. parvimuricata were supposed to constitute the most advanced species because of the significant strumae in ovary surface, 3 locules per ovary and up to 3 cm in flower size. In our taxonomic treatment of section Tuberculata, the above five species mentioned by Ming and Zhong (1993) were also reliable. Thus, combined with their biogeographical distribution, we could draw a conclusion that Tuberculata species originated in the three-river region of Guizhou and spread to Guangxi, Yunnan, Sichuan, Chongqing, Hubei and probably Hunan before the disintegration of the Yangtze region (Fig. 6B).
References
Ao CQ, Chen GX, Chang HD (2002) Leaf epidermis morphology of camellia and its taxonomic significance. Acta Bot Yunnan 24(1):68–74
Baranova M (1972) Systematic anatomy of the leaf epidermis in the Magnoliaceae and some related families. Taxon 21:447–469
Baranova M (1987) Historical development of the present classification of morphological type of stomates. Bot Rev 53:53–79
Baranova M (1992) Principles of comparative stomatographic studies of flowering plants. Bot Rev 58: 1–99
Brittan NH (1970) A preliminary survey of the stem and leaf anatomy of Thysanotus R. Br. (Liliaceae). In: Robson NKB, Culter DF, Gregory M (eds) New research in plant anatomy. Academic Press, London, pp 57–70
Chang HT (1981) A taxonomy of the genus Camellia. J Sun Yatsen Univ 4:18–52
Chang HT (1984) New record of Camellia from South China. Act Sci Nat Univ Sunyats 2:77–80
Chang HT, Ren SX (1991) A classification on the section Tuberculata of Camellia. Act Sci Nat Univ Sunyats 30(4):86–91
Chang HT (1998) Camellia. In: Fl. Reipubl. Popularis Sin. (eds) Flora. Science Press, Beijing, pp 37–48
Chen L, Carpita NC, Reiter WD, Wilson RH, Jeffries C, McCann MC (1998) A rapid method to screen for cell-wall mutants using discriminant analysis of Fourier transformation infrared spectra. Pl J 16:385–392
Chien SS (1939) Four new ligneous plants of Szechuan. Contrib Biol Lab Sci Soc China Bot 12(2):94–99
Crow E, Stirton CH, Cutler DF (1997) Leaf anatomy of the genus Psoralea sensu stricto (Psoraleeae, Papilionoideae, Leguminosae). Bot J Linn Soc 124:155–182
Foster AS (1944) Structure and development of sclereids in the petiole of Camellia japonica L. Bull Torrey Bot Club 71(3):302–326
Foster AS (1956) Plant idioblasts: remarkable examples of cell specialization. Protoplasma 46:184–193
Goodacre R, Timmins M, Burton R, Kaderbhai N, Woodward AM, Kell DB, Rooney PJ (1998) Rapid identification of urinary tract infection bacteria using hyperspectral whole-organism fingerprinting and artificial neural networks. Microbiology 144:1157–1170
Hussin KH, Sani ZM (1998) Comparative leaf anatomical studies of some Sterculia L. species (Sterculiaceae). Bot J Linn Soc 127(2):159–174
Keng H (1962) Comparative morphological studies in Theaceae. Univ Calif Publ Bot 33:269–384
Kim SW, Ban SH, Chung H, Cho S, Chung HJ, Choi PS, Yoo OJ, Liu JR (2004) Taxonomic discrimination of flowering plants by multivariate analysis of Fourier transform infrared spectroscopy data. Pl Cell Rep 23:246–250
Kocsis M, Darók J, Borhidi A (2004) Comparative leaf anatomy and morphology of some neotropical Rondeletia (Rubiaceae) species. Pl Syst Evol 248:205–218
Kong HZ (2001) Comparative morphology of leaf epidermis in the Chloranthaceae. Bot J Linn Soc 136:279–294
Li YK, Yang MZ (1987) A new species of Camellia from Guozhou. Guihaia 7(1):13–14
Lin MJ, Lu QM (1984) New record of Camellia from Guizhou. Act Sci Nat Univ Sunyats 2:81–83
Lu HF, Cheng CG, Tang X, Hu ZH (2004) FTIR spectrum of Hypericum and Triadenum with reference to their identification. Acta Bot Sin 46(4):401–406
Lubke RA, Phipps JB (1973) Taximetrics of Loudetia (Gramineae) based on leaf anatomy. Canad J Bot 51:2127–2146
Luna I, Ochoterena H (2004) Phylogenetic relationships of the genera of Theaceae based on morphology. Cladistics 20:223–270
McCann MC, Chen L, Roberts K, Kemsley EK, Séné CFB, Carpita NC, Stacey NJ, Wilson RH (1997) Infrared microspectroscopy: sampling heterogeneity in plant cell-wall composition and architecture. Pl Physiol 100:729–738
Metcalfe CR, Chalk L (eds) (1972) 50. Theaceae and 52. Bonnetiaceae. In: Anatomy of the Dicotyledons, 2nd edn. Oxford University Press, Oxford, pp 181–191 (193–195)
Ming TL, Zhong YC (1993) A revision of genus Camellia sect. Tuberculata. Acta Bot Yunnan 15(2):123–130
Ming TL, Zhang WJ (1996) The evolution and distribution of genus Camellia. Acta Bot Yunnan 18(1):1–13
Ming TL (1999) A systematic synopsis of genus Camellia. Acta Bot Yunnan 21(2):149–159
Naumann D, Helm D, Labischinski H (1991) Microbiological characterization by FT-IR spectroscopy. Nature 351(6321):81
Pearson DL, Juliano SA (1993) Evidence for the influence of historical processes in co-occurrence and diversity of tiger beetle species. In: Rickleffs RE, Schluter D (eds) Species diversity in ecological communities; historical and biogeographical perspectives. University of Chicago Press, Chicago, pp 194–202
Solereder H (1908) Systematic anatomy of the dicotyledons (Trans. Boodle LA, Fritsch FE), 2 vols. Clarendon Press, Oxford
Schofield EK (1968) Petiole anatomy of the Guttiferae and related families. Mem New York Bot Gard 18:1–55
Sealy JR (1958) A revision of the genus Camellia. Roy Hort Soc, London, pp 147–164
Stace CA (1984) The taxonomic importance of the leaf surface. In: Heywood VH, Moore DM (eds) Current concepts in plant taxonomy, vol 25. Academic Press, London, pp 67–94
Stace CA (1966) The use of epidermal characters in phylogenetic considerations. New Phytol 65:304–318
Stewart D, Yahiaoui N, McDougall GJ, Myton K, Marque C, Boudet AM, Haigh J (1997) Fourier-transform infrared and Raman spectroscopic evidence for the incorporation of cinnamaldehydes into the lignin of transgenic tobacco (Nicotiana tabacum L.) plants with reduced expression of cinnamyl alcohol dehydrogenase. Planta 201:311–318
Upchurch GR (1984) Cuticle evolution in early cretaceous angiosperms from the potomac group of Virginia and Maryland. Ann Missouri Bot Gard 71:522–550
Wenning M, Seiler H, Scherer S (2002) Fourier-transform infrared microspectroscopy, a novel and rapid tool for identification of yeast. Appl Environ Microbiol 68:4717–4721
Wilkinson HP (1979) The plant surface (mainly leaf). In: Metcalfe CR, Chalk L (eds) Anatomy of the dicotyledons, 2nd edn. Clarendon Press, Oxford, pp 97–167
Wilson RH, Slack PT, Appleton GP, Sun L, Belton PS (1993) Determination of the fruit content of jam using Fourier transform infrared spectroscopy. Food Chem 47:303–308
Wilson RH, Smith AC, Kacurakova M, Saunders PK, Wellner N, Waldron KW (2000) The mechanical properties and molecular dynamics of the plant cell wall polysaccharides studied by Fourier-transform infrared spectroscopy. Pl Physiol 124:397–405
Xu ZR (1985) A new plant from Limestone Hill in South Guizhou. Guihaia 5(4):347–348
Xu ZR, Chen FP, Peng CY (1987) A new species of Camellia sect. Tuberculata. Guihaia 7(1):19–21
Yang ZR, Lin Q (2005) Comparative morphology of the leaf epidermis in Schisandra (Schisandraceae). Bot J Linn Soc 148:39–56
Yang SX, Liu AZ, Peng H, Wu ZY (2003) Stomata types of Pyrenaria (Theaceae) and their systematic implication. Guihaia 23:250–252
Acknowledgments
We thank Prof. J. Y. Gao (the International Camellia Species Garden of Jinhua City, China) and Prof. R. Parks (the University of North Clifford, USA), for identifying and collecting living species. We are grateful to Y. Z. Xu and D. T. Li for assistance with the FTIR and L. L. Huang for technical assistance in leaf anatomy. This work was carried out with financial support from the National Natural Science Foundation of China (grand no. 30370088) and the Natural Science Foundation of Zhejiang Province (grand no. 302101).
Author information
Authors and Affiliations
Corresponding author
Additional information
H. F. Lu and B. Jiang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Lu, H.F., Jiang, B., Shen, Z.G. et al. Comparative leaf anatomy, FTIR discrimination and biogeographical analysis of Camellia section Tuberculata (Theaceae) with a discussion of its taxonomic treatments. Plant Syst Evol 274, 223–235 (2008). https://doi.org/10.1007/s00606-008-0047-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-008-0047-6