Abstract
Drought and salinity are two major limiting factors of plant growth and agricultural productivity. An efficient way to improve salt tolerance of plants is by expression of the Na+/H+ antiporter gene AtNHX1 from Arabidopsis thaliana, introduced via genetic transformation. Similarly, expression of the coding genes of betaine aldehyde dehydrogenase (BADH) cloned from Graminaceous or Chenopodiaceous weeds appears to increase salt tolerance of plants. Unlike AtNHX1 gene, the transformation of BADH genes additionally enhances osmotic stress tolerance to the transformants. To better understand the differences of their capacities in promoting plant salt tolerance, the HvBADH1 gene from Hulless barley and the AtNHX1 gene, were introduced into a glycophyte species Cichorium intybus L. We investigated the traits of osmotic adjustment and antioxidation ability in the transformed plants under salt stress condition. The results indicated that both AtNHX1 and HvBADH1-transformed plants showed similar Na+ and K+ accumulations, but HvBADH1-transformed plants exhibited better osmotic adjustments to salt stress. And the AtNHX1 overexpression lines exhibited superior membrane protection and relative calli growth, delivering better NaCl tolerance to the plants under conditions of severe salt stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Salinity is one of the major abiotic stresses negatively affecting plant growth and reducing agricultural productivity all over the world. Environmental salt stress can cause severe problems such as ion toxicity, water deficit and oxidative stress, resulting in cellular damage, growth reduction, and even plant death (Li et al. 2013). Higher plants have developed a set of biochemical and molecular mechanisms to deal with salt stress by selectively expressing specific stress-responding genes and accumulating compatible organic solutes such as soluble sugars, free proline and glycine betaine (GB) (Türkan and Demiral 2009).
AtNHX1, a vacuolar Na+/H+ antiporter in Arabidopsis thaliana, has been found to influence plant development and contribute to salt tolerance by mediating the transport of Na+ and K+ into the vacuoles (Sottosanto et al. 2007). Overexpression of AtNHX1 in many plants observably improves their salt tolerance. Compared to wild types (WT), transgenic tomato seedlings harboring AtNHX1 were able to grow, flower and even produce fruit under the salt stress triggered by 200 mM NaCl (Zhang and Blumwald 2001). In transgenic plants, the overexpression of AtNHX1 also significantly enhanced the salt tolerance of transformants (Asif et al. 2011; Chen et al. 2007). The study of transgenic Buckwheat showed that transformants overexpressing AtNHX1 were not only able to grow and flower normally, but also able to accumulate more rutin than WT in the presence of 200 mM NaCl (Chen et al. 2008). While stressed by 200 mM NaCl, transgenic tobacco lines expressing the AtNHX1 gene were able to grow and significantly accumulate sodium ions in their leaves (Zhou et al. 2011). The traits of enhanced salt tolerance could propagate for more than six generations in soybean lines that overexpressed AtNHX1 (Xing et al. 2010).
Betaine aldehyde dehydrogenase (BADH) is one of the key enzymes involved in the biosynthesis of GB, a compatible organic solute synthesized in response to salt, drought and temperature stresses in a large number of plant species (Wang et al. 2013). Responding to salt and drought stresses, the endogenous BADH level is upregulated several-fold in plant species which could synthesize betaine naturally (Fan et al. 2012). Transformed by the BADH genes, many betaine-deficient plants have been confirmed to accumulate different levels of GB in cells, and shown promoted tolerance to either salt or drought stress (Singh et al. 2010).
Because of its very high altitude, the extreme environment of the Tibetan Plateau in China is believed to be one of the harshest conditions for plant growth on this planet. Tibet is also considered to be the original habitat for cultivated barley and many wild Barleys (Wang et al. 2009). Tibetan Hulless barley (Hordeum vulgare var.nudum.hook.f.), the most important crop in the residents’ diets (Thomason et al. 2009), differs from the regular hulled barley with a bared caryopsis not covered by hulls in morphology. Unlike the cultivated barley, Tibetan Hulless barley has evolved a general resistance system to deal with all kinds of environmental stresses, and shown the strongest resistance of all plants belonging to genus Hordeum. However, the mechanism of this stress defense system is still unknown.
We have first reported the cloning of a pivotal-resistant gene HvBADH1 (GenBank No. EF492983), which encoded an isoenzyme of BADH in Tibetan Hulless barley (Zhao et al. 2008). To test the ability of HvBADH1 in plant stress resistance, we overexpressed HvBADH1 gene in non-halophyte chicory (Cichorium intybus L.) by the method of Agrobacterium tumefaciens-mediated transformation, and several transformed lines with enhanced salt stress resistance were obtained (data unpublished). To make this salt stress improvement of transformants sensible, we compared the salt tolerance of HvBADH1 or AtNHX1 overexpression chicory (Zhao et al. 2009) by assessing the different responses of these plant lines to gradient NaCl stresses. To achieve this purpose, we measured Na+ and K+, malondialdehyde (MDA), soluble sugar, free proline, chlorophyll contents and the relative electrical conductivity (REC) in T2 generation seedlings, as well as the relative growth rate of calli in different transgenic lines and WT under 0–200 mM NaCl.
Materials and methods
Plant materials and growth conditions
WT and T2 generation transgenic plants of chicory containing AtNHX1 or HvBADH1 were grown in a greenhouse of Shaanxi Provincial Key Laboratory of Biotechnology, Shaanxi Province, China. The seeds of these plant lines were harvested, soaked in tap water for 16 h, and surface sterilized with 70 % ethanol for 30 s and 0.1 % HgCl2 for 10 min. After rinsing for three times with sterile water, the seeds were placed on MS agar medium, and incubated at 25 °C under a 16/8 h L/D photoperiod (about 100 μmol m−2 s−1 provided by cool white fluorescent lamps) for about 14 days until the cotyledons fully expanded.
The construction of plant expression vectors harboring AtNHX1 or HvBADH1
Seeds of Arabidopsis thaliana and Hulless barley were surface sterilized using 0.1 % HgCl2, and then germinated on water soaked filter paper for 7 days. The seedlings were treated with 200 mM NaCl for 24 h at room temperature, and the leaves of Arabidopsis thaliana or Hulless barley were frozen in liquid nitrogen and preserved at −70 °C. Total RNA was isolated from Arabidopsis thaliana and Hulless barley seedlings using TRIzol Reagent (Invitrogen) according to the manufacturer’s protocol. The first strand cDNA was synthesized using a RevertAid H Minus Synthesis kit K1631 (Fermentas). The cDNA was then used as template DNA for PCR amplification of the AtNHX1 (AF056190, 1,617 bp) and HvBADH1 (EF492983, 1,512 bp) ORFs. The sequences of the primers for AtNHX1 amplification were 5′-ggAATTCCATATgTTggATTCTCTAgTgTCgAAAC-3′ and 5′-CgggATCCTCAAgCCTTACTAAgATCAggAggg-3′. The primers for HvBADH1 were 5′-ggAATTCCATATggTCgCgCCggCCAAgATCC-3′ and 5′-CgggATCCCTAgTTAgCCggAgCCTTgTACCAC-3′. PCR was performed using Pyrobest DNA Polymerase (Takara, Dalian China). The PCR products corresponding to AtNHX1 and HvBADH1 were extracted from agarose gel, and then sequenced. All sequences were consistent with previously reported gene sequences of AtNHX1 and HvBADH1 (Zhao et al. 2008, 2009).
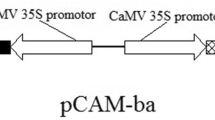
The amplified ORFs of AtNHX1 and HvBADH1 were then constructed into the multiple cloning sites of a commercial binary vector pRI101-AN using NdeI and BamHI restriction sites (Takara, Dalian China). In the final plasmid constructs, the target ORFs are under the control of the CaMV35S promoter (Fig. 1). These new plasmids, pRI101-AN-CaMV35S-AtNHX1 and pRI101-AN-CaMV35S-HvBADH1, were then separately introduced into Agrobacterium tumefaciens LBA4404 by electroporation using a Multiporator (Eppendorf), according to the manufacturer’s instructions. The subsequent Agrobacterium tumefaciens-mediated plant transformation and transformant identification were performed as described previously (Zhao et al. 2008).
Determination of Na+ and K+ contents in transgenic chicory
The transgenic HvBADH1 (H1, H2) and AtNHX1 (A1, A2) lines and WT chicory seedlings were cultured on MS medium for 4 weeks after germination and then transferred to MS liquid medium containing different concentrations of NaCl (0, 50, 100, 150 or 200 mM) for 24 h. The seedlings were rinsed with distilled water to remove NaCl from the surface, and the leaves of these seedlings were harvested and dried at 45 °C overnight. Na+ and K+ contents were measured by an atomic absorption spectrophotometer (AA-6300C from Shimadzu).
Measurement of MDA, free proline, soluble sugar and chlorophyll contents in transgenic chicory leaves
Transgenic lines and WT chicory seedlings were cultured on filter paper wetted with MS liquid medium for 28 days. Then all the plants were irrigated with MS liquid medium containing different concentrations of NaCl (0, 50, 100, 150 or 200 mM) for 24 h. 0.1 g fresh leaves from seedlings of transgenic lines and WT were used as materials for the following tests of each physiological indicator. The MDA levels were measured by a thiobarbituric acid (TBA) reaction method (Garcia et al. 2005), free proline levels were determined as described by Bates et al. (1973), and soluble sugar was measured by anthrone colorimetry as described by Yemm and Willis (1954). The same leaf samples were homogenized in a mortar and pestle containing 80 % acetone and the amount of chlorophyll was measured as the value of OD652 as described by Myers et al. (2013).
Determination of relative electrical conductivity
To assess membrane permeability, REC was determined according to Korkmaz et al. (2010). After being treated with NaCl as described in 2.3, leaf discs (1 cm in diameter) from transgenic lines and WT lines were taken from the middle portion of the fully developed youngest leaf. Then the discs were placed in individual test tubes containing 30 ml of distilled water. After incubating the samples at room temperature on a shaker (150 rpm) for 3 h, the electrical conductivity of the bathing solution (EC1) was determined by an electrical conductivity detector (model DDS-303A, Kangyi, Shanghai, China). The same samples were then placed in boiling water for 30 min and a second reading (EC2) was determined after cooling the solution to room temperature. The electrolyte leakage was calculated as REC = EC1/EC2 × 100 %.
Salt tolerance of transgenic calli
The hypocotyls of different transgenic lines and WT seedlings about 28 days old were cut into 0.5 cm segments and inoculated on MS medium supplemented with 6-BA 1.5 mg L−1, NAA 0.2 mg L−1, and ascorbic acid 100 mg L−1 for calli induction under 100 μmol m−2 s−1 illumination, 16/8 h L/D photoperiod and 25 ± 2 °C. The fresh calli were segregated and subcultured on the same medium for 6 weeks. After weighing, the calli were treated with gradient NaCl for 28 days. Subsequently, the calli’s weight was remeasured, and the relative growth rates of calli were calculated as: Relative growth rate = final weight/primary weight. 25 calli were treated in each replicate and 3 replicates were performed for each experiment.
Determination of GB
To prepare sample solutions for GB measurement, 1.0 g fresh leaves were ground by mortar and pestle and extracted in 20 mL deionized water for 24 h at 37 °C with gentle shaking. These extracts were then centrifuged at 4,500×g for 15 min, and the supernatants were filtrated with waterman filter paper and 0.45 μm filter membrane. The pH of the sample solutions was adjusted to 1.0 by adding concentrated hydrochloric acid and incubated at 4 °C for 30 min. After centrifugation at 4,500×g for 15 min, 3 mL supernatants from sample solutions were mixed with 5 mL concentrated Reinecke’s salt (NH4[Cr(NH3)2(CNS)4]·H2O; China National Pharmaceutical Group Corporation), and reacted at 4 °C for 3 h. Subsequently, the solutions were centrifuged again and the supernatant was discarded. The GB crystals were dissolved in 5 mL 70 % acetone and the optical density of these solutions was measured at 365 nm.
The preparation of a standard curve was performed using GB samples from Sigma with the unit as mg/g FW (Martinez 1983).
Statistical analysis
To analyze the variance of the physiological indicators between transgenic plant strains, we used an ANOVA method of Duncan’s multiple comparison procedure. In addition, paired sample t tests were used to evaluate the statistical significance of the difference between each transgenic line and the WT at each NaCl level. The significant levels were defined as * when P ≤ 0.05, and ** while P ≤ 0.01. Six plants were used for each treatment. Each experiment was repeated three times. All the data used in the statistical analysis were an average of three replicates and analyzed by the SPSS statistical software package v19.0.
Results
Determination of Na+ and K+ contents
The accumulation of Na+ and K+ in WT and T2 generation transgenic seedlings treated with NaCl was measured. The results suggested that together with the increasing environmental NaCl, the Na+ contents increased in leaves of all the transgenic and WT plants. Though transgenic plants could accumulate much more Na+ than the WT at every stress levels, no observable differences were found between CaMV35S:HvBADH1 and CaMV35S:AtNHX1 lines (Fig. 2a).
Corresponding to increased NaCl stresses from 0 to 200 mM, the K+ contents in the transgenic and WT plants rose rapidly. Similar to the situation of Na+ contents, significant differences of K+ content were found between transgenic and WT plants at each level of salt stress, but there were no noticeable differences between transgenic lines (Fig. 2b). In addition, the K+/Na+ ratios in transgenic plants were also higher than those of WT at each stress level, but the differences between transformed HvBADH1 and AtNHX1 lines were not significant (Table 1).
Measurement of MDA contents
MDA is the product of lipid peroxidation and relates closely to the oxidative degradation of polyunsaturated fatty acids of cell membrane. Changes in cellular MDA levels during abiotic stress are coordinated with the process of membrane damage and reflect the antioxidative capacity of plant cells (Tian et al. 2011). In our study, the contents of MDA increased dramatically in all the tested plants corresponding to the increase of environmental NaCl levels. Under each stress level, the MDA contents of transgenic lines were lower than the WT, but no noticeable differences were observed between CaMV35S:HvBADH1 and CaMV35S:AtNHX1 lines when stressed with 50–150 mM NaCl. However, the CaMV35S:AtNHX1 lines showed a significant higher accumulation of MDA than HvBADH1 lines until the NaCl stress reached 200 mM (Fig. 3a).
Determination of free proline contents
As a physiological response to hostile environment, plant cells usually produce abundant free proline to raise their cellular osmotic potential. Triggered by NaCl stress, the contents of free proline in leaves of transgenic and WT plants increased rapidly, and the proline levels of both transgenic lines were higher than the WT at each stress level. In addition, CaMV35S:HvBADH1 lines displayed a significantly higher ability to accumulate more free proline than the CaMV35S:AtNHX1 lines at all the tested stress levels (Fig. 3b).
Determination of soluble sugar contents
The young leaves of transgenic and WT plants grown under different NaCl stresses were used to measure soluble sugar contents. When no additional NaCl was supplied, all the plants contained nearly the same soluble sugar contents. Similar to the MDA and proline measurements, soluble sugar contents also increased with NaCl concentration in both transgenic lines. The soluble sugar content of CaMV35S:HvBADH1 leaves showed 1.67-fold higher values than that of CaMV35S:AtNHX1 when exposed to 150 mM NaCl and 1.47-fold to 200 mM NaCl (Fig. 3c).
Measurement of chlorophyll contents
To estimate the damage on the photosynthetic system caused by the salinity stress, the amounts of chlorophyll content were measured in transgenic lines and WT treated with different concentrations of NaCl. With increased salt, the chlorophyll contents decreased in all tested plants. Not surprisingly, more dramatic decline in chlorophyll content was found in WT leaves compared to both transgenic lines under each stress level. Contents of chlorophyll in CaMV35S:HvBADH1 lines were slightly higher than that of AtNHX1 transformants when environmental NaCl level was lower than 150 mM, but significant differences were observed between these two lines at NaCl concentrations of 200 mM (Fig. 3d).
Determination of relative electrical conductivity
For the purpose of evaluating the protective function of HvBADH1 and AtNHX1 on the cell membrane under osmotic stress, REC was measured in two transgenic lines and WT. Without salt stress, the values of REC in all tested plants were similar. Under conditions of 50–200 mM NaCl, however, the REC data from each plant strain showed dramatic differences. The values of REC in all plant strains increased with rising NaCl concentration, and the level of REC in WT was always significantly higher than that of transgenic lines. Again no significant differences in REC were detected between the CaMV35S:AtNHX1 and CaMV35S:HvBADH1 lines when stressed by the 50–150 mM NaCl. However, the REC of CaMV35S:HvBADH1 plants was much higher than the CaMV35S:AtNHX1 plants under 200 mM of NaCl stress (Fig. 4a).
Salt tolerance of transgenic calli
The relative growth rates of transgenic and WT calli were measured to evaluate the salt tolerance of different transgenic lines at the cellular level. In the control group (without NaCl), the relative growth rates of transgenic calli were similar to WT. With increased NaCl concentration, relative growth rates declined rapidly in all the tested calli lines, though that of the transgenic lines was significantly higher than WT at each stressed level. By comparing the data from different transgenic calli grown under 100–200 mM NaCl, we confirmed that overexpression of AtNHX1 could promote the survival rates of calli to a greater extent than the HvBADH1 gene. At 200 mM of salt stress, the WT calli turned brown and only kept 2.97 % growth rates whereas most of the transgenic calli survived and even maintained growth rates of 10.7–22.3 % (Fig. 4b).
Determination of GB
The accumulation of GB in WT and T2 generation transgenic seedlings was measured. Compared to the values of WT and CaMV35S:AtNHX1 lines under regular growth conditions, a 6.77-fold higher GB content was observed in HvBADH1 lines, but no noticeable accumulation of GB was found in WT or CaMV35S:AtNHX1 lines (Fig. 5). Growing under NaCl-stressed condition, HvBADH1 lines, as well as the WT and AtNHX1 lines, showed the same GB content level with their control plantlets which grew under non-stressed condition.
Discussion
Many efforts have been made to promote the tolerance of cultivars by transgenic methods, and quite a lot of genes involved in stress responses have been cloned for this purpose (Yu et al. 2012). Na+/H+ anti-transporter genes function in a specific salt stress-resistant pathway by transporting excessive cytosolic Na+ into vacuoles (Apse et al. 1999), while BADH genes are believed to play a key role in a general osmotic stress-resistant pathway. Genetic transformations of both anti-transporter genes and BADH genes have already been used in many attempts to promote the salt stress resistance of plants (Wang et al. 2013). High altitude plants have evolved over millions of years to develop a complex defense system to adapt to environmental stresses, such as lower O2, high UV radiation and extreme temperature, and understanding the mechanisms of the specific stress-resistance system in these plants may provide deep insight into how to promote the salt stress resistance of other major crops. In this work, we evaluated the salt stress tolerance in transgenic plants of HvBADH1 from highland Hulless barley (Hordeum vulgare var.nudum.hook.f.) and AtNHX1 from Arabidopsis thaliana.
A resting state with intracellular higher [K+] and lower [Na+] is important to maintain the activities of many cytosolic enzymes, as well as a regular membrane potential. When salt stress occurs, the efficiency of Na+/H+ anti-transporters is affected by excessive Na+ in cytosol and expression of genes encoding Na+ transporters, such as AtNHX1, is upregulated to increase the amount of these anti-transporters on the membrane of vacuoles (Zhu 2003). Little difference was found between CaMV35S:AtNHX1 and CaMV35S:HvBADH1 lines in this study for Na+, K+ contents or K+/Na+ ratio, although the data from both transgenic lines were much higher than WT under stress conditions. By overexpressing these two genes, the transgenic cells could maintain cytosolic enzyme activity and plasma membrane integrity against the ion toxic effect and prevent the inhibition of K+ uptake of cells caused by salt stress. Compared to the effect of Na+ compartmentalization caused by AtNHX1 overexpression, the osmotic potential promoting process caused by overaccumulation of GB made a similar contribution to increase the salt tolerance by modulating both the levels of K+ and Na+ and keeping the K+/Na+ balance mainly in the leaves.
As a by-product of the membrane damage process triggered by reactive oxygen species (ROS), MDA is released to the cytosol soon after stress (Wang et al. 2011). In the present study, the MDA levels suggested that the overexpression of HvBADH1 gene and subsequent overaccumulation of GB in the cytosol raised the osmotic potential and decreased the generation of ROS in cytosol. This effect allowed the HvBADH1 lines to show a superior ability than AtNHX1 strains to minimize membrane damage under high salt stress. However, because of its ability to transport redundant Na+ into the vacuoles, the overexpressed AtNHX1 exhibited a similar antioxidative capacity to HvBADH1 at salt concentrations less than 150 mM NaCl. Thus, both transgenic lines showed protective activities against oxidative damage to the cell membrane, though HvBADH1 had higher protection under severe stress conditions.
To protect themselves from various abiotic stresses, plants responsively synthesize and accumulate some organic osmolytes in the cells, such as soluble sugar, free proline and GB (Rodríguez-Calcerrada et al. 2011), which helps plants maintain cell turgor during salt and osmotic stresses (Zheng et al. 2009). Overaccumulated soluble sugar and free proline in protoplast can protect macromolecules from being hurt by various abiotic stresses such as dehydration and salinity; proline also takes part in the endogenous radical scavenging system. In our research, increased soluble sugar and free proline triggered by salt stress modulated the maintenance of osmotic equilibrium in protoplasts of the transgenic lines. The values of these two osmolytes showed a jump in the HvBADH1 lines, but not in AtNHX1 lines at 150–200 mM NaCl stresses, suggesting that overexpressing HvBADH1 may attribute to the salt tolerance of transformants by maintaining the osmotic potential in cells to severe NaCl stresses.
The contents of chlorophyll reflect the physiological status of plants, which are also used to estimate the tolerance of plants to salinity (Gitelson et al. 2003). Lower chlorophyll contents in leaves directly limit both the photosynthetic activity and efficiency of plants encountered with salt stress. The redundant Na+ and Cl− ions permeating into cytoplasm reduce the chlorophyll levels by interfering the activity of chlorophyll-synthesizing enzymes (Garriga et al. 2014). In the present study, the contents of chlorophyll in HvBADH1 lines were slightly higher than that of AtNHX1 lines, which suggested that transformation of HvBADH1 was better to protect the plant photosynthesis system from salt damage.
REC is another important physiological status indicator that directly reflects the extent of cell membrane damage under osmotic stress. At levels of NaCl stress lower than 150 mM, no noticeable differences were detected between the transgenic plant strains. The significant lower level of REC in AtNHX1 lines at 200 mM NaCl indicated that the overexpression of AtNHX1 provided the cell membrane with a better protection than HvBADH1 lines.
Plant tissue tolerance is thought to be a major component to determination of overall plant response to salinity (Getnet Dino Adem et al. 2014). The measurement of relative growth rate of calli showed that the AtNHX1 lines could endure severe salinity stress better than the HvBADH1 lines. But under slight salt stress conditions, the two lines responded more similarly. These phenotypes may be explained by a hypothesis that the toxic effect of overaccumulated ions, such as Na+ and Cl− might be major obstacles to physiological metabolism and cell growth under severe salt stress conditions, while osmotic stress inhibits plant growth under slight salt stress. The precise segmentation point between severe and slight stress conditions might vary for different plant species or different physiological indicators, but in this study, this segmentation point was 100 mM NaCl.
GB is a common osmolyte that accumulates in many plant species, and the levels of GB in the plant tissues are correlated with salt tolerance. Genetic evidence that GB improves salinity tolerance has been obtained in barley and maize (Zhang et al. 2009). In the present study, CaMV35S:HvBADH1 lines exhibited an over accumulation of GB in leaves under stressed conditions, which may correspond to enhanced salt stress tolerance. Our results are consistent with the theory that osmoprotection caused by osmolyte accumulation may play a pivotal role in natural stress environments where stress does not affect plant survival (Neha Gupta et al. 2014).
A recent report found that NHX1 and NHX2 genes, the homologues of AtNHX1 in barley, may not necessarily confer the same protein(s) function as AtNHX1 in Arabidopsis, though they have similar nucleotide sequence (Getnet Dino Adem et al. 2014). Therefore, we chose AtNHX1 from Arabidopsis as a reference to evaluate the contribution of HvBADH1 from Hulless barley to the ionic and osmotic stress parameters determining salinity tolerance.
In conclusion, we evaluated how overexpressing AtNHX1 and HvBADH1 affected the salt tolerance of transgenic plants by measuring eight different physiological indicators in CaMV35S:AtNHX1 and CaMV35S:HvBADH1 chicory lines. The determination of calli growth rate and REC revealed that the overexpression of AtNHX1 gene promoted the tolerance of transgenic calli, and increased the ability of transgenic plants to maintain homeostasis against membrane injury under severe salinity condition. In contrast, the HvBADH1 overexpressing plants exhibited better capacity to maintain the osmotic potential of cytoplasm by synthesizing and accumulating more soluble sugar and free proline. The MDA and chlorophyll levels showed that the overexpression of HvBADH1 also enhanced the ability of transgenic plants to protect the integrity of membrane system and photosynthesis system when threatened by NaCl stress. In summary, our results suggest that AtNHX1 is more suitable for severe salinity, while HvBADH1 seems superior for dealing with general abiotic stresses. However, further field tests will be required to determine the long-term productivities of HvBADH1 and AtNHX1 lines. These results support the idea that a successful progress in the breeding of crops with enhanced NaCl stress tolerance can only be achieved by targeting several complementary traits together (Getnet Dino Adem et al. 2014). Because these two genes confer different responses to stress, it is a logical next step to assess the salt-resistant phenotype of plants that expresses both HvBADH1 and AtNHX1 genes.
Author contribution statement
Yuwei Zhao designed the research. Fang Zhang, Xiaolong Li and Pan Lai performed experiments. Fang Zhang, Xiaolong Li and Pengfei Li analyzed data. Fang Zhang wrote most of the article.
References
Adem GD, Roy SJ, Zhou MX, Bowman JP, Shabala S (2014) Evaluating contribution of ionic, osmotic and oxidative stress components towards salinity tolerance in barley. BMC Plant Biol 14:113
Apse MP, Aharon GS, Snedden WA, Blumwald E (1999) Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 285:1256–1258
Asif MA, Zafar Y, Iqbal J, Iqbal MM, Rashid U, Ali GM, Arif A, Nazir F (2011) Enhanced expression of AtNHX1, in transgenic Groundnut (Arachis hypogaea L.) improves salt and drought tolerance. Mol Biotechnol 49:250–256
Bates LS, Waldren RP, Teare JD (1973) Rapid determination of proline for water stress studies. Plant Soil 39:205–207
Chen H, An R, Tang JH, Cui XH, Hao FS, Chen J, Wang XC (2007) Over-expression of a vacuolar Na+/H+ antiporter gene improves salt tolerance in an upland rice. Mol Breed 19:215–225
Chen LH, Zhang B, Xu ZQ (2008) Salt tolerance conferred by overexpression of Arabidopsis vacuolar Na+/H+ antiporter gene AtNHX1 in common buckwheat (Fagopyrum esculentum). Transgenic Res 17:121–132
Fan WJ, Zhang M, Zhang HX, Zhang P (2012) Improved tolerance to various abiotic stresses in transgenic sweet potato (Ipomoea batatas) expressing spinach betaine aldehyde dehydrogenase. PLoS One 7:e37344
Garcia YJ, Rodriguez-Malaver AJ, Peñaloza N (2005) Lipid peroxidation measurement by thiobarbituric acid assay in rat cerebellar slices. J Neurosci Meth 144:127–135
Garriga M, Retamales JB, Romero-Bravo S, Caligari PDS, Lobos GA (2014) Chlorophyll, anthocyanin and gas exchange changes assessed by spectroradiometry in Fragaria chiloensis under salt stress. J Integr Plant Biol 56:505–515
Gitelson AA, Gritz Y, Merzlyak MN (2003) Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J Plant Physiol 160:271–282
Gupta N, Thind SK, Bains NS (2014) Glycine betaine application modifies biochemical attributes of osmotic adjustment in drought stressed wheat. Plant Growth Regul 72:221–228
Korkmaz A, Korkmaz Y, Demirkıran AR (2010) Enhancing chilling stress tolerance of pepper seedlings by exogenous application of 5-aminolevulinic acid. Environ Exp Bot 67:495–501
Li B, He LZ, Guo SR, Li J, Yang YJ, Yan B, Sun J, Li J (2013) Proteomics reveal cucumber Spd-responses under normal condition and salt stress. Plant Physiol Biochem 67:7–14
Martinez SEV (1983) Simultaneous determination of choline and betaine in some fish materials. Analyst 108:1114–1119
Myers JA, Curtis BS, Curtis WR (2013) Improving accuracy of cell and chromophore concentration measurements using optical density. BMC Biophys 22:4
Rodríguez-Calcerrada J, Shahin O, Rey MC, Rambal S (2011) Opposite changes in leaf dark respiration and soluble sugars with drought in two Mediterranean oaks. Funct Plant Biol 38:1004–1015
Singh A, Singh PK, Singh R, Pandit A, Mahato AK, Gupta DK, Tyagi K, Singh AK, Singh NK, Sharma TR (2010) SNP haplotypes of the BADH1 gene and their association with aroma in rice (Oryza sativa L.). Mol Breed 26:325–338
Sottosanto JB, Saranga Y, Blumwald E (2007) Impact of AtNHX1, a vacuolar Na+/H+ antiporter, upon gene expression during short- and long-term salt stress in Arabidopsis thaliana. BMC Plant Biol 7:18
Thomason WE, Brooks WS, Griffey CA, Vaughn ME (2009) Hulless barley seeding rate effects on grain yield and yield components. Crop Sci 49:342–346
Tian N, Wang J, Xu ZQ (2011) Overexpression of Na+/H+ antiporter gene AtNHX1 from Arabidopsis thaliana improves the salt tolerance of kiwifruit (Actinidia deliciosa). S Afr J Bot 77:160–169
Türkan I, Demiral T (2009) Recent developments in understanding salinity tolerance. Environ Exp Bot 67:2–9
Wang AH, Yu ZY, Ding Y (2009) Genetic diversity analysis of wild close relatives of barley from Tibet and the Middle East by ISSR and SSR markers. C R Biol 332:393–403
Wang XP, Geng SJ, Ri YJ, Cao DH, Liu J, Shi DC, Yang CW (2011) Physiological responses and adaptive strategies of tomato plants to salt and alkali stresses. Sci Hortic-Amsterdam 130:248–255
Wang JY, Lai LD, Tong SM, Li QL (2013) Constitutive and salt-inducible expression of SlBADH gene in transgenic tomato (Solanum lycopersicum L. cv. Micro-Tom) enhances salt tolerance. Biochem Biophys Res Co 432:262–267
Xing LT, Yue Z, Hua L, Ting WU, Bin LW, Xia ZH (2010) Stable expression of Arabidopsis vacuolar Na+/H+ antiporter gene AtNHX1, and salt tolerance in transgenic soybean for over six generations. Chin Sci Bull 55:1127–1134
Yemm EW, Willis AJ (1954) The estimation of carbohydrates in plant extracts by anthrone. Biochem J 57:508–514
Yu S, Wang W, Wang B (2012) Recent progress of salinity tolerance research in plants. Russ J Genet 48:497–505
Zhang HX, Blumwald E (2001) Transgenic salt-tolerant tomato plants accumulate salt in foliage but not in fruit. Nat Biotechnol 19:765–768
Zhang HJ, Dong HZ, Li WJ, Sun Y, Chen SY, Kong XQ (2009) Increased glycine betaine synthesis and salinity tolerance in AhCMO transgenic cotton lines. Mol Breed 23:289–298
Zhao YW, Hao JG, Bu HY, Wang YJ, Jia JF (2008) Cloning of HvBADH1 gene from Hulless Barley and its transformation to tobacco. Acta Agron Sin 34:1153–1159
Zhao YW, Wang YJ, Bu HY, Hao JG, Jia JF (2009) Transformation of Cichorium intybus with the AtNHX1 gene and salinity tolerance of the transformants. Acta Prataculturae Sin 18:103–109
Zheng YH, Xu XB, Wang MY, Zheng XH, Li ZJ, Jiang GM (2009) Responses of salt-tolerant and intolerant wheat genotypes to sodium chloride: Photosynthesis, antioxidants activities and yield. Photosynthetica 47:87–94
Zhou S, Zhang Z, Tang Q, Lan H, Li Y, Luo P (2011) Enhanced V-ATPase activity contributes to the improved salt tolerance of transgenic tobacco plants overexpressing vacuolar Na(+)/H (+) antiporter AtNHX1. Biotechnol Lett 33:375–380
Zhu JK (2003) Regulation of ion homeostasis under salt stress. Curr Opin Plant Biol 6:441–445
Acknowledgments
This work was supported by National Natural Science Foundation of China (Grant number: 31200091, J1210063), the Research Project of Provincial Key Laboratory of Shaanxi (Grant number: 12JS104), the Research Project of Key Laboratory of Resource Biology and Biotechnology in Western China (Grant number: ZS14010), Provincial Natural Science Foundation of Shaanxi Province (Grant number: 2014JM3061), Technology Foundation for Selected Overseas Chinese Scholar, Ministry of Personnel of China (Grant number: DBL14003), the Provincial Undergraduate Innovation Research Project of Shaanxi Province (Grant number: 0804).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Moriguchi.
Rights and permissions
About this article
Cite this article
Zhang, F., Li, X., Lai, P. et al. Comparison of salt tolerance between Cichorium intybus L. transformed with AtNHX1 or HvBADH1 . Acta Physiol Plant 37, 8 (2015). https://doi.org/10.1007/s11738-014-1755-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-014-1755-x