Abstract
The robotic platform matches or surpasses laparoscopic surgery in postoperative results. However, limited date and slow adoption are noticed in the middle east. We aimed to report outcomes of robotic and laparoscopic colorectal surgery performed by fellowship-trained robotic colorectal surgeons and compare it to larger more experienced centers. Retrospective review of prospectively collected data between 2021 and 2023 of 107 patients who had robotic-assisted or laparoscopic-assisted colorectal surgery was included in the study. The outcomes were overall morbidity, serious morbidity, mortality, conversion to open, length of hospital stay, and the quality of oncological specimen. Of 107 patients, 57 were in the robotic and 50 were in the laparoscopic surgery groups. Overall, there were no significant differences in overall morbidity (46.8 vs. 53.2%, p = 0.9), serious morbidity (10.5 vs. 8%, p = 0.7), or mortality (0 vs. 4%, p = 0.2). Regarding oncological outcomes, there were no significant difference between the two groups regarding the number of lymph node harvested (17.7 ± 6.9 vs 19.0 ± 9.7, p = 0.5), R0 resections (92.7 vs. 87.1%, p = 0.5), and the rate of complete mesorectal excision (92.7 vs. 71.4%, p = 0.19). The study found that the robotic group had an 86% reduction in conversion rate to open surgery compared to the laparoscopic group, despite including more obese and physically dependent patients (OR = 0.14, 95% CI 0.03–0.7, p = 0.01). Robotic surgery appears to be a safe and effective as laparoscopic surgery in smaller colorectal surgery programs led by fellowship-trained robotic surgeons, with outcomes comparable to those of larger programs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, minimally invasive (MIS) colorectal surgery has become the standard of care in surgical treatment for both malignant and benign disease processes. Compared to open surgery, MIS offers faster patient recovery, shorter length of hospital stay (LOS), and less perioperative morbidities without compromising oncological outcomes [1]. Rational for its superiority includes less trauma to the abdominal wall (small incisions), minimal exposure of internal organs to the external environment, minimal tissue manipulation, less tissue trauma, and easier precise sharp dissection within the confinement of the narrow pelvis [1]. However, laparoscopic surgery has its own limitations, such as relatively high conversion rate to open surgery, arguably higher positive circumferential resection margin (CRM) rates in locally advanced cancer, suboptimal visualization of deep structures due to limited two-dimensional views in association with poor stability provided by conventional laparoscopic-assisted controlled camera, and poor ergonomics of the straight tip instruments leading to enhanced tremor effect [2, 3]. The da Vinci surgical system (Intuitive Surgical, CA, USA) was the first robotic surgical system to be approved by the FDA in 2000. It was introduced as an innovative device that could overcome many of the limitations of laparoscopic surgery. The robotic platform demonstrated that it could alleviate the anatomical limitation of the bony pelvis and provide precise stable dissection in a confined pelvic space, facilitated by efficient third-arm retraction, fine instrument movement with flexible EndoWrist instruments that allowed 7 degrees of freedom and a magnified three-dimensional camera view [4, 5]. According to the current literature, employment of the robotic platform demonstrates a favorable safety profile and is associated with promising oncological outcomes [6,7,8,9,10]. Compared to laparoscopy, robotic surgery has proven to have similar postoperative morbidity and oncological outcomes, with data showing trends toward lower conversion rates when performed by experienced robotic surgeons [11].
The adoption of robotic surgery in the Middle East has been slow, and there are very limited data regarding outcomes in newly established programs. This study aims to report our experience and short-term outcomes of robotic colorectal surgery performed by fellowship-trained robotic colorectal surgeons compared to laparoscopic approach.
Methods
A retrospective review of prospectively collected data from 2021 to 2023 was performed. All operations were performed at Jabir Al-Ahmad Hospital by surgeons with experience in both open and laparoscopic surgery who underwent fellowship training in robotic colorectal surgery for a minimum of 1 year. All surgeons had at least 3 years of independent practice following fellowship. Robotic-assisted colorectal surgery was first introduced in our institution in 2021 and, in this study, we included all patients who underwent this surgical modality since then.
Study population
All patients > 18 years of age who underwent inpatient robotic- or laparoscopic-assisted colorectal procedure in our institution from 2021 to 2023 were included. We did not include patients who had emergency procedures. Colorectal procedures were identified using Current Procedural Terminology (CPT) codes. All patients who were candidates for minimal invasive surgery were offered robotic surgery. Reasons for not performing robotic surgery were either patient refusal to robotic surgery, unavailability of the robot, or patient’s primary surgeon not being robotic trained. These patients were included in the laparoscopic group.
Perioperative variables
Patient demographics, clinical characteristics, American Society of Anesthesiology (ASA) class, history of smoking, physical dependency status (independent or partial/complete dependency), 5-modified frailty index (mFI), and medical comorbidities were collected. Five variables were assessed as part of the 5-mFI: congestive heart failure, chronic obstructive pulmonary disease, hypertension requiring medication, diabetes, and non-independent functional status. An mFI score was calculated for each patient by adding the number of frailty variables present (scored 1 point per variable), resulting in a range from 0 to 5 points. Patients were stratified into two categories based on the number of variables: mFI = 0 or 1, and mFI ≥ 2. The use of surgical drains was also documented and collected. Pathological specimen quality data including TNM classification, R0 resection, number of lymph nodes harvested, circumferential resection margin (CRM) status, and completeness of total mesorectal excision (TME) was recorded and collected. Thirty-day morbidity and mortality were collected as well.
Outcomes
Primary outcomes include overall 30-day morbidity, serious morbidity, mortality, and conversion to open. Overall morbidity includes minor (Clavien–Dindo score 1 or 2) and serious morbidity (Clavien–Dindo score 3 or 4). Conversion was defined as the unplanned change from any minimally invasive technique to an open approach. Oncological outcomes including quality of resection and number of lymph nodes harvested were reported. In addition, length of hospital stay (LOS) was reported.
Perioperative care pathway
A standardized care pathway was followed by all patients: the day prior to the surgery, patients received mechanical bowel preparation accompanied by oral antibiotics, hair removal, and a chlorhexidine body wash. Moreover, preoperative anticoagulation and intravenous antibiotic administration were provided to all patients. Following the surgical procedure, patients were started on a regimen of clear liquid diet at the post-recovery unit, and given nonsteroidal anti-inflammatory drugs, paracetamol, and patient-controlled analgesia for pain control. Ambulation was encouraged once the patient was transferred to the floor, and Foley catheter removal occurred on postoperative day 1.
Surgical technique
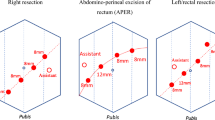
For robotic-assisted surgery, we used the Da Vinci SI robot (Intuitive Surgical Inc.) to perform all cases. A right hemicolectomy was counted as robotic if the ileocolic vessels ligation, medial dissection, lateral dissection, and the hepatic flexure dissections were performed by the robot. For left colon resection, the splenic flexure mobilization and dissection left colic and/or inferior mesenteric arteries (IMA) must have been performed by the robot. For anterior and low anterior resections, the IMA and the total mesorectal excision (TME) dissection must have been performed by the robot. With regards to the robotic port configuration, for anterior resection, low anterior resection, abdominoperineal resection, and rectopexy a right lower quadrant, umbilical, left upper quadrant, left lateral (level of anterior axillary line) robotic ports and right upper quadrant assistant port were used (Fig. 1). For left hemicolectomy cases, a right lower quadrant, umbilical, epigastric and left lateral (level of midclavicular line) robotic ports and right upper quadrant assistant port were used (Fig. 2). For right hemicolectomy, robotic ports were placed in the epigastric, umbilical, left lower quadrant, and right lower quadrant regions, in addition to a left upper quadrant assistant port (Fig. 3). In all robotic right hemicolectomies, the anastomosis was done intracorporeally. For laparoscopic right hemicolectomies, the anastomosis was done both intracorporeally and extracorporeally depending on the surgeon preference and case circumstances. Left colectomies anastomosis were done extracorporeally if side-to-side anastomosis was performed. For end-to-end anastomosis, the anvil was applied to the proximal limb extracorporeally, and then, the anastomosis was performed intracorporeally. For rectosigmoid and rectal resections, the anvil was applied to the proximal limb extracorporeally, and then, the anastomosis was performed intracorporeally. Figure 4 illustrates extracorporeal anastomosis and specimen extraction sites.
Statistical analysis
Continuous variables were expressed as means and standard deviation (SD) or median and interquartile range (IQR) as appropriate. Numbers and percentages were used for categorical variables for descriptive statistics of the study population. Pearson Chi-squared tests or Fisher’s exact tests were used to compare categorical variables, whereas t tests or Wilcoxon–Mann–Whitney tests were used to compare continuous versus categorical variables, as appropriate. Backward stepwise multivariable logistic regression (p value exit criteria = 0.05) analysis was performed to evaluate the association between robotic vs laparoscopic surgery and the following outcomes: conversion to open and the number of lymph node harvested, adjusting for the following variables: age, gender, body mass index (BMI), dependency, and ASA. Statistical significance was considered as a p value < 0.05. The data were analyzed using STATA statistical software package (STATA version 17, STATA Corporation, College Station, TX, USA).
Results
Univariate analysis
Demographics
One hundred and seven patients were included in the study, of whom 50 patients were in the laparoscopic group and 57 (53.3%) patients were in the robotic group. The robotic group had heavier patients (mean BMI 28.6 kg/m2) than the laparoscopic group (mean BMI 27.7 kg/m2), though this was not statistically significant, p value = 0.5. Drains were significantly more frequently used in the robotic group compared to the laparoscopic group, 35 vs. 19 patients, respectively, p value = 0.04. In the robotic group, most drains were used for proctectomies (64%), diverticular disease (14%), and rectopexies (14%). In the laparoscopic group, drains most drains were used following proctectomies (60%) and diverticular disease (20%). In addition, there were statistically significant more dependent patients in the robotic group compared to the laparoscopic group; 19 (33.3%) vs. 5 (10%), p value = 0.004. However, when we compared frailty scores (mFI scores), we found no significant difference between the two groups (Table 1).
Conversion to open
Out of 107 cases, just over 10% (n = 12) were converted to open. We found that laparoscopic cases were significantly more likely to be converted to open than robotic cases; 20% (n = 10) vs. 3.5% (n = 2), p value = 0.01). For the robotic cases, the cause of conversion was cardiac arrhythmias due to pneumoperitoneum in one case and technical difficulties in the other. For the laparoscopic group, one conversion was due to bulky tumor in the sigmoid not allowing for proper dissection around the tumor. The rest were due to technical difficulties such as narrow pelvis in male patients, and extensive adhesions.
Oncology
In the series, there were 72 oncology cases of which 41 and 31 were robotic and laparoscopic, respectively. In both groups, most lesions were T3. In the robotic group, the distribution was T4 (15%), T3 (61%), and T2/1 (24%). In the laparoscopic group, the distribution was 16, 58, and 26%, for T4, T3, and T2/1 lesions, respectively. There were no significant differences between the two groups in relation to the number of lymph nodes harvested, rate of R0 resection, and the rate of complete mesorectal excision (Table 2).
Morbidity and mortality
Overall complication rate in the series was 26%. There was no significant difference between the two the groups and both were approximately 26%. There was two anastomotic leaks in the robotic group. One after rectosigmoid resection, which was managed by reoperation and end colostomy. In the second case, the patient had proximal colonic conduit ischemia following low anterior resection and hand-sewn coloanal anastomosis, which was managed with reoperation and end colostomy. In the laparoscopic group, there was one anastomotic leak after low anterior resection for rectal cancer, which was managed by drains, since the patient had diverting loop ileostomy. When evaluating serious morbidity, the overall rate was 9.4%, including 8% in the laparoscopic group and 10.5% in the robotic group, and the difference was not statistically significant. Overall mortality rate was low, 1.95% (Table 2).
Length of hospital stay
The median length of hospital stay was 6 days. There was no significant difference between the two groups.
Multivariate analysis
Conversion to open, morbidity and mortality
On multivariate logistic regression analysis, we found that robotic surgery was associated with 86% reduction in conversion rate to open compared to the laparoscopic group, OR 0.14, 95% CI 0.03–0.7, p = 0.01). We found no difference between the two groups in terms of overall complications (OR 1.17, 95% CI 0.38–1.57), serious complications (OR 0.80, 95% CI 0.15–4.10), or mortality (Table 3).
Discussion
To our knowledge, this is the first and largest comparative study of robotic versus laparoscopic colorectal surgery in the Middle East by colorectal surgeons trained in both minimally invasive approaches. Our overall conversion rate (10%) seems to be similar to other studies (ranging between 5.9 and 34%). In our practice, we found the robotic approach to be superior, with 86% reduction in the odds of conversion compared to laparoscopy. Gravililidis et al. systematic review and meta-analysis of 25 studies of rectal cancer reported 74% reduction in the odd of conversion in the robotic group [12]. Phan et al. systemic review and meta-analysis of 6 randomized control trials and five propensity-score matching studies reported that robotic surgery was significantly associated with 62% decrease in the odds of conversion to open; 6.7 vs. 14.5%, OR 0.38, 95% CI 0.30–0.46 [13]. Ng et al.’s systematic review and meta-analysis of 76 studies found that robotic surgery was associated with 60% reduction in the odd of conversion (OR 0.40, 95% CI 0.30–0.50) [14]. When only RCTs were included, no significant difference was observed. However, the reduction in the rate of conversion was replicated in several other studies [15,16,17]. The ROLAAR study did not identify a significant difference between robotics (8.1%) and laparoscopic (12.2%) techniques; however, a subgroup analysis identified that surgeons with over 100 prior robotic surgeries had significantly lower conversion rate, with 70% reduced rate in the odds of conversion (OR 0.304, 95% CI 0.094–0.988) [11]. This significant finding was not replicated in surgeons with prior fewer than 100 robotic cases. The positive effect of training on robotic surgery colorectal outcomes was reported by multiple studies [18, 19], which suggests that the learning curve in robotic surgery is a significant cofounder for colorectal surgery outcomes. We believe that in the field of colorectal surgery, robotic surgery is associated with reduction in the rate of conversion, especially if it was conducted by experienced and/or robotic fellowship-trained surgeons. This supports the hypothesis that proper utilization of the robotic superior ergonomics and visual field can yield significant benefits over laparoscopy. It is not clear why the significant reduction in risk of conversion did not translate to significant reduction in morbidity or length of hospital stay, though this was the situation in most other studies. Our morbidity and mortality rates appear to be on bar with other larger groups [11, 15, 19, 20]. In our cohort, there was no significant difference between robotic and laparoscopic surgery with regards to both overall complications (both 26%) and serious complications (10.5 vs. 8%). Gorsek et al. reported similar trends to ours regarding nonsignificant difference in serious complications (2.2 vs. 8.1%) [21]. Palomba et al. reported no difference in their geriatric’s series in regards to overall complications (29 vs. 25%) [17]. Fleming et al.’s propensity-score matching study reported no difference regarding both overall complications (29.7 vs. 31.3%) and serious complications [22].
When we reviewed our specimen’s oncological quality, which can be used as predictor for long-term outcomes, such as local recurrence, disease-free survival, and overall survival, we found no significant difference between the two groups. Regarding both R0 resection and complete mesorectal excision, there were trends toward lower rates in the robotic groups. Our R0 resection was 92.7% in the robotic group compared to 87.1% in the laparoscopic group, and our complete mesorectal excision was 92.3% compared to 71.4% in the robotic and laparoscopic group respectively. Other studies, including the ROLAAR study, reported similar outcomes to ours. However, Kethman et al.’s multicentric propensity-score matching study reported that both open and robotic techniques were associated with lower oncological success than laparoscopy [11, 23]. Specifically, they reported distal margin as the Achilles heel for this inferiority. In their discussion, they attributed this discrepancy between laparoscopy and robotics due to the learning curve. We concur, as the superior ergonomics and exposure provided by the robot over laparoscopy can only be utilized with proper training. The robotic approach compared to other techniques in colorectal surgery is still in its infancy worldwide and is facing similar challenges to when laparoscopic techniques were adopted initially.
Strengths and limitations
This is a retrospective review of prospectively collected data and the first cohort comparative study of robotic colorectal surgery in Kuwait and our region. It is unique, because it shines the light on what type of outcomes to expect if a new robotic program, led by fellowship-trained robotic colorectal surgeons, was to be established in small country/city with relatively small population. Our results are limited by the small sample size and lack of long-term outcomes. However, the quality of our surgical specimen would likely translate to acceptable long-term outcomes.
Conclusion
The robotic approach compared to other techniques in colorectal surgery is still in its infancy worldwide and is facing similar challenges to when laparoscopic techniques were adopted initially. When colorectal robotic surgery is conducted by experienced and/or fellowship-trained robotic colorectal surgeons, the risk of conversion to open is significantly reduced. Therefore, we gain the benefits of avoiding open surgery which is known to be associated with higher morbidity, longer hospital stay, and higher overall health costs. The morbidity, mortality, and pathological specimen’s quality in our robotic colorectal case series are comparable to larger and more established robotic programs. In addition, our robotic conversion rate was lower than reported by some previous larger studies. Based on available literature, this is likely attributed to our program being conducted by fellowship-trained robotic colorectal surgeons. We believe that new robotic programs similar to ours should adopt a similar strategy to ensure safe and equivalent outcomes. In this context, analyzing larger sets of data looking into the above outcomes in addition to long-term outcomes such as local recurrence, disease-free survival, and overall survival is warranted in future studies.
Data availability
No datasets were generated or analysed during the current study.
References
Silva-Velazco J, Dietz DW, Stocchi L, Costedio M, Gorgun E, Kalady MF, Kessler H, Lavery IC, Remzi FH (2017) Considering value in rectal cancer surgery: an analysis of costs and outcomes based on the open, laparoscopic, and robotic approach for proctectomy. Ann Surg 265(5):960–968. https://doi.org/10.1097/SLA.0000000000001815
Mak TWC, Lee JFY, Futaba K, Hon SSF, Ngo DKY, Ng SSM (2014) Robotic surgery for rectal cancer: a systematic review of current practice. World J Gastrointest Oncol 6(6):184–193. https://doi.org/10.4251/wjgo.v6.i6.184
Jayne DG, Thorpe HC, Copeland J, Quirke P, Brown JM, Guillou PJ (2010) Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg 97(11):1638–1645. https://doi.org/10.1002/bjs.7160
Weber PA, Merola S, Wasielewski A, Ballantyne GH (2002) Telerobotic-assisted laparoscopic right and sigmoid colectomies for benign disease. Dis Colon Rectum 45(12):1689–1694. https://doi.org/10.1007/s10350-004-7261-2. (discussion 1695-1696)
Kim JC, Kwak JY, Yoon YS, Park IJ, Kim CW (2014) A comparison of the technical and oncologic validity between robot-assisted and conventional open abdominoperineal resection. Int J Colorectal Dis 29(8):961–969. https://doi.org/10.1007/s00384-014-1916-9
Pigazzi A, Ellenhorn JDI, Ballantyne GH, Paz IB (2006) Robotic-assisted laparoscopic low anterior resection with total mesorectal excision for rectal cancer. Surg Endosc 20(10):1521–1525. https://doi.org/10.1007/s00464-005-0855-5
deSouza AL, Prasad LM, Marecik SJ, Blumetti J, Park JJ, Zimmern A, Abcarian H (2010) Total mesorectal excision for rectal cancer: the potential advantage of robotic assistance. Dis Colon Rectum 53(12):1611–1617. https://doi.org/10.1007/DCR.0b013e3181f22f1f
Lim DR, Bae SU, Hur H, Min BS, Baik SH, Lee KY, Kim NK (2017) Long-term oncological outcomes of robotic versus laparoscopic total mesorectal excision of mid-low rectal cancer following neoadjuvant chemoradiation therapy. Surg Endosc 31(4):1728–1737. https://doi.org/10.1007/s00464-016-5165-6
Saklani AP, Lim DR, Hur H, Min BS, Baik SH, Lee KY, Kim NK (2013) Robotic versus laparoscopic surgery for mid-low rectal cancer after neoadjuvant chemoradiation therapy: comparison of oncologic outcomes. Int J Colorectal Dis 28(12):1689–1698. https://doi.org/10.1007/s00384-013-1756-z
Memon S, Heriot AG, Murphy DG, Bressel M, Lynch AC (2012) Robotic versus laparoscopic proctectomy for rectal cancer: a meta-analysis. Ann Surg Oncol 19(7):2095–2101. https://doi.org/10.1245/s10434-012-2270-1
Jayne D, Pigazzi A, Marshall H, Croft J, Corrigan N, Copeland J, Quirke P, West N, Rautio T, Thomassen N, Tilney H, Gudgeon M, Bianchi PP, Edlin R, Hulme C, Brown J (2017) Effect of robotic-assisted vs conventional laparoscopic surgery on risk of conversion to open laparotomy among patients undergoing resection for rectal cancer: the ROLARR Randomized Clinical Trial. JAMA 318(16):1569–1580. https://doi.org/10.1001/jama.2017.7219
Gavriilidis P, Wheeler J, Spinelli A, de’Angelis N, Simopoulos C, Di Saverio S (2020) Robotic vs laparoscopic total mesorectal excision for rectal cancers: has a paradigm change occurred? A systematic review by updated meta-analysis. Colorectal Dis 22(11):1506–1517. https://doi.org/10.1111/codi.15084
Phan K, Kahlaee HR, Kim SH, Toh JWT (2019) Laparoscopic vs. robotic rectal cancer surgery and the effect on conversion rates: a meta-analysis of randomized controlled trials and propensity-score-matched studies. Tech Coloproctol 23(3):221–230. https://doi.org/10.1007/s10151-018-1920-0
Ng KT, Tsia AKV, Chong VYL (2019) Robotic vs. conventional laparoscopic surgery for colorectal cancer: a systematic review and meta-analysis with trial sequential analysis. World J Surg 43(4):1146–1161. https://doi.org/10.1007/s00268-018-04896-7
Al-Temimi MH, Chandrasekaran B, Agapian J, Peters WR, Wells KO (2019) Robotic vs. laparoscopic elective colectomy for left side diverticulitis: a propensity score-matched analysis of the NSQIP database. Int J Colorectal Dis 34(8):1385–1392. https://doi.org/10.1007/s00384-019-03334-x
Solaini L, Bocchino A, Avanzolini A, Annunziata D, Cavaliere D, Ercolani G (2022) Robotic vs. laparoscopic left colectomy: a systematic review and meta-analysis. Int J Colorectal Dis 37(7):1497–1507. https://doi.org/10.1007/s00384-022-04194-8
Palomba G, Dinuzzi VP, Capuano M, Anoldo P, Milone M, De Palma GD, Aprea G (2022) Robotic vs. laparoscopic colorectal surgery in elderly patients in terms of recovery time: a monocentric experience. J Robot Surg 16(4):981–987. https://doi.org/10.1007/s11701-021-01332-2
Panteleimonitis S, Miskovic D, Bissett-Amess R, Figueiredo N, Turina M, Spinoglio G, Heald RJ, Parvaiz A, Collaborative EARCS (2021) Short-term clinical outcomes of a European training programme for robotic colorectal surgery. Surg Endosc 35(12):6796–6806. https://doi.org/10.1007/s00464-020-08184-1
Aradaib M, Neary P, Hafeez A, Kalbassi R, Parvaiz A, O’Riordain D (2019) Safe adoption of robotic colorectal surgery using structured training: early Irish experience. J Robot Surg 13(5):657–662. https://doi.org/10.1007/s11701-018-00911-0
Chang W, Wei Y, Ren L, Jian M, Chen Y, Chen J, Liu T, Huang W, Peng S, Xu J (2020) Short-term and long-term outcomes of robotic rectal surgery-from the real word data of 1145 consecutive cases in China. Surg Endosc 34(9):4079–4088. https://doi.org/10.1007/s00464-019-07170-6
Grosek J, Ales Kosir J, Sever P, Erculj V, Tomazic A (2021) Robotic versus laparoscopic surgery for colorectal cancer: a case-control study. Radiol Oncol 55(4):433–438. https://doi.org/10.2478/raon-2021-0026
Fleming CA, Ullah MF, Chang KH, McNamara E, Condon E, Waldron D, Coffey JC, Peirce CB (2021) Propensity score-matched analysis comparing laparoscopic to robotic surgery for colorectal cancer shows comparable clinical and oncological outcomes. J Robot Surg 15(3):389–396. https://doi.org/10.1007/s11701-020-01116-0
Kethman WC, Harris AHS, Morris AM, Shelton A, Kirilcuk N, Kin C (2020) Oncologic and perioperative outcomes of laparoscopic, open, and robotic approaches for rectal cancer resection: a multicenter, propensity score-weighted cohort study. Dis Colon Rectum 63(1):46–52. https://doi.org/10.1097/DCR.0000000000001534
Funding
The authors declare that no funds, grants, or other supports were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design: AA, DS, OA, AA, MA, KA, ZC, DS, BA, SA, and SA. Material preparation, data collection, and analysis were primarily undertaken by KA, AA, MA, ZC, and DS. The statistical analysis was expertly managed by OA. The first draft of the manuscript was authored by AA and DS. Both AA and DS played pivotal roles in editing, revising, and finalizing the manuscript. All authors provided crucial feedback, participated in revising subsequent drafts, and approved the final manuscript for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study was approved by Kuwait Ministry of Health Ethical Review Board (Ethical Approval No. 2228/2023).
Consent to participate
Given the observational nature of this study, which involved no disclosure of personal data, participant consent was not required. Therefore, no specific consent statements are included in the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alkhamis, A., Soliman, D., Alkandari, O. et al. Outcomes in robotic-assisted compared to laparoscopic-assisted colorectal surgery in a newly established colorectal tertiary center: a retrospective comparative cohort study. J Robotic Surg 18, 152 (2024). https://doi.org/10.1007/s11701-024-01908-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11701-024-01908-8