Abstract
Robotic colorectal surgery is gaining popularity. The objective of this study was to compare clinical and cancer outcomes in propensity-score matched cohorts (PSM-1:1) undergoing colorectal cancer (CRC) surgery performed using laparoscopic or robotic surgery in a single institution. A PSM cohort comparison was performed in a tertiary referral cancer and National accredited rectal cancer surgery centre. Patients with CRC undergoing laparoscopic or robotic resection with curative intent from 2016 to 2019 (inclusive) were assessed for inclusion. Matched cohorts were selected using a 1:1 ratio. Statistical analysis was performed using SPSS, version 22. 128 patients were analysed [laparoscopic (n = 64); robotic (n = 64)]. Median age was 64 years (29–84 years). 55% (n = 70) of patients were male, 45% female (n = 58). SSI rates were slightly lower in the robotic group [10.9% (n = 7) v 12.5% (n = 8) p = 0.40]. Anastomotic leak rates were equal in both groups [5.4% (n = 3)]. All but one patient received an R0 resection in each group, median LNY was 14 in the robotic group and 12 in the laparoscopic group (p = 0.004) and no difference in disease recurrence (p = 0.465) or survival (p = 0.886) was observed. Structured introduction of a robotic colorectal programme over a 3-year period has resulted in equivalent outcomes with an established laparoscopic programme for CRC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer accounts for 1.8 million cancer diagnoses worldwide per year [1]. Outcomes in colorectal cancer are improving with five-year survival rates as high as 90% in early-stage cancer [2]. Robotic colorectal surgery (most commonly using the da Vinci surgical system, Intuitive, Sunnyvale, Ca.) is gaining popularity [3]. In rectal surgery, robotic techniques are proposed to offer better access to the pelvis (most notably in a narrow male pelvis) and are thus a favourable option for rectal cancer surgery in particular. With a 3D-binocular view, overall visualisation and depth perception are improved and the system can significantly reduce the effect of tremor. Furthermore, the 7° motion offered by endo-wristed instruments combined with the above is proposed to facilitate improved dexterity, the precision of technique and quality of surgery [3]. In recent years, an evolving role for robotic surgery in colectomy and general surgical procedures is emerging, offering benefits including higher lymph node yields and increased ability to complete intracorporeal anastomosis [4, 5].
In clinical terms, robotic surgery has not shown superiority to laparoscopic surgery in a randomised controlled trial for rectal cancer surgery [6]. However, while laparoscopic colorectal surgery is the most widely performed technique for colorectal cancer resection (and is widely considered gold standard), it has never shown superiority to open surgery in terms of cancer outcomes either [7,8,9,10]. There are clear benefits to minimally invasive techniques including smaller incisions leading to reduced pain, faster recovery of mobility and earlier discharge from hospital [11]. There is also established evidence that robotic surgery is associated with improved functional outcomes following rectal cancer surgery compared to laparoscopic surgery, most notably urinary and sexual function likely due to the improved precision it offers which allows the autonomic nerves to be better visualised and preserved [12,13,14,15].
It is crucial when introducing new surgical technology that a safe, structured, quality-assured approach is adopted. When introducing a new technology it is vital to ensure that benchmarking to standard practice is regularly performed. Thus the aim of this study was to compare both clinical and oncological outcomes in robotic and laparoscopic colorectal cancer surgery in our institution, three years following the introduction of a robotic-assisted colorectal surgery programme and over a decade of practice of laparoscopic colorectal surgery. A 1:1 propensity-score matched system was chosen to reduce the risk of bias.
Methods
This is a single centre propensity score-matched cohort comparison study. Data were recorded prospectively as part of standard quality assurance and governance structures in the surgical management of colorectal cancer in keeping with the National Cancer Control Programme (NCCP) guidelines in Ireland.
Patient selection
Patients undergoing elective laparoscopic or robotic resection for colorectal cancer with curative intent from 2016 to 2019 (inclusive) were assessed for study inclusion. Exclusion criteria were patients undergoing open surgery, emergency surgery, local or transanal excision, palliative resection or those with distant metastases at the time of diagnosis. All patients underwent clinical examination, colonoscopy and CT thorax, abdomen and pelvis (CT TAP) for staging at diagnosis, i.e. pre-operatively. For rectal cancers specifically, pelvic MRI was also performed for staging and those with locally advanced rectal cancer (T3, T4, node-positive) received standard long-course neoadjuvant chemoradiotherapy (NACRT) followed by curative resection 6–8 weeks after completion of NACRT. All cases were discussed at a multidisciplinary colorectal cancer meeting following diagnosis and consensus group decision made on the best treatment strategy. For analysis propensity-score matched cohorts (age, gender, stage and anatomical location of disease, year of surgery, procedure performed, neoadjuvant and adjuvant therapies) were randomly selected.
Laparoscopic and robotic-assisted surgery programmes
This study was performed in University Hospital Limerick, a 514-bed university teaching hospital and tertiary referral cancer centre for the mid-western region of Ireland. Up until the introduction of the robotic-assisted programme, laparoscopic colorectal surgery was performed for colorectal cancer as standard and open surgery where appropriate by four fellowship-trained colorectal surgeons with significant experience in minimally invasive colorectal surgery. Approximately 200 colorectal cancers are treated per year and all cancer surgery was performed following oncological principles of high vascular ligation and enbloc mesocolic or mesorectal resection. Patients were managed with a pragmatic approach to enhanced recovery principles.
The robotic-assisted surgery programme commenced in June 2016 using the DaVinci Xi dual-console surgical system. This was the first hospital in Ireland to introduce robotic-assisted colorectal surgery and robotic surgery was performed by three colorectal consultants. A structured governance programme was established including monthly quality assurance meetings and maintenance of a prospective database including a wide range of demographic, operative, clinical and pathological outcome data. Prior to commencing live robotic surgery, colorectal surgeons undertook structured training specified by Intuitive Surgical and guided by guidelines from the European Academy of Robotic Colorectal Surgery (EARCS) [16].
Clinical and oncological outcomes
The following clinical outcomes were recorded and analysed: surgical site infection (SSI), anastomotic leak, total hospital length of stay (LOS) calculated from the day of admission to the day of medical discharge. Complications were classified as per the Clavien-Dindo system [17]. Surgical site infection was defined as an infection at the incision or organ space that occured following surgery and was clinically detected (± confirmed on microbiological assessment) [18]. Anastomotic leak was defined as a defect of the intestinal wall at the anastomotic site leading to a communication between the intra- and extraluminal compartments that was clinically, radiologically or endoscopically detected [19]. As data were retrospectively analysed, ileus was reported pragmatically as diagnosed by the surgical team clinically based on the delay of the first postoperative flatus lasting for > 72 h post-operatively or any other status requiring intervention for treatment for ileus [20]. Oncological outcomes that were compared included: resection margin status, lymph node yield, recurrence and survival data. All pathological specimen were reported in keeping with the TNM classification of malignant tumours [21]. Resection margin was defined as follows: R0, complete resection, tumour > 2 mm from resection margin; R1, tumour within 1 mm of resection margin; R2, tumour at or involving the resection margin. Recurrence was reported as local (pelvic, endoluminal, anastomotic) or distant (regional lymph nodes, distant organ disease).
Statistical analysis
A propensity score-matched analysis using a 1:1 selection ratio was utilised to control for potential bias in baseline patient, clinical, pathological and treatment characteristics between patient groups undergoing laparoscopic and robotic resection for colorectal cancer. This approach removed conventional biases associated with a standard multivariate model and analysis [22]. Standard propensity scores were computed by logistic regression modelling using the odds of undergoing robotic colorectal cancer resection as the dependent variable and the following independent variables were selected: age, gender, stage and anatomical location of disease, year of surgery, procedure performed and whether neoadjuvant and adjuvant therapies were administered. All robotic and laparoscopic colorectal cancer cases performed since the commencement of the robotic surgery programme were eligible for inclusion in the PSM selection process. Statistical analysis was performed using SPSS, version 22 and graphs generated using GraphPad Prism, version 8. Comparison of variables was performed using the χ2 or Fisher’s exact test for categorical variables and the student’s t test or Mann–Whitney U test for continuous variables (where appropriate) with significance observed at p < 0.05. Kaplan–Meier curves were created to compare survival data.
Results
Patient characteristics and procedures performed
A total of 128 patients were analysed: laparoscopic (n = 64) and robotic (n = 64) groups were directly compared (Table 1). Clinical and procedural characteristics are summarised in Table 1. Median age overall was 64 years (29–84 years). 51.6% (n = 33) of patients were male, 48.4% female (n = 31) in the laparoscopic group compared to 57.8% (n = 37) males and 42.8% (n = 27) females in the robotic group (p = 0.504). Each matched group included the following procedures: 20% (= 13) right hemicolectomies; 36% (n = 23) anterior resections, 27% (n = 17) low/ultra-low anterior resections, 8% (n = 5) APRs and 9% (n = 6) other procedures. One-quarter of patients were node-positive on final histology, two-thirds of rectal patients received NACRT and 18–20% of patients proceeded to adjuvant chemotherapy post-operatively.
Clinical outcomes
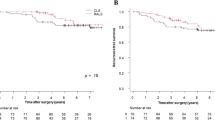
Comparative clinical outcomes are summarised in Table 2. Length of stay was similar between the two groups when all patients were analysed [median 7 days (IQR 7) in the laparoscopic group and 6 days (IQR 7) in the robotic group (p = 0.073)]. When cases who developed complications were excluded, a significant difference was observed between LOS in colonic cases [median 5 days in robotic cases and 7 days in laparoscopic (p = 0.016)] as outlined in Fig. 1. A similar trend was not observed in rectal cases.
The total post-operative complication rate was 29.7% (n = 19) in the robotic group compared to 31.3% (n = 20) in the laparoscopic group (p = 0.853). A 5.4% (n = 3) anastomotic leak was observed in each group. In the robotic group, one leak occurred following a right hemicolectomy which required a laparotomy and end-ileostomy formation. Two occurred in rectal cancer patients both of whom received NACRT and subsequently underwent low and ultra-low anterior resection. The low anterior resection required laparotomy and end-colostomy formation, the ultra-low case was successfully managed with an examination under anaesthesia (EUA), transanal washout and drain insertion. This patient had a loop ileostomy fashioned at the time of ultra-low anterior resection. Similarly in the laparoscopic group, one leak occurred following right hemicolectomy (laparotomy and end-ileostomy formation) and two low anterior resections who had received NACRT (one requiring laparotomy and end-colostomy formation and one managed successfully with interventional radiology drainage).
Surgical site infection (SSI) rates were also similar [laparoscopic group 12.5% (n = 8) v 10.9% (n = 7) p = 0.40]. All SSIs were managed with antibiotics and ward level wound management. While the overall median LOS was 7 days, the LOS was significantly increased in the setting of SSI [robotic: median 11 days (IQR10); laparoscopic: median 11 days (IQR7)]. Table 2 shows comparable 30-day readmission and re-operative rates and there were no 30-day moralities.
Oncological outcomes
As outlined in Table 1 there was no significant difference in tumour stage and nodal status between the two groups or between the number of patients who received NACRT or adjuvant chemotherapy. Oncological outcomes are summarised in Table 3. Follow-up was four months shorter in the robotic group [laparoscopic 20.5 months, robotic 16.5 months, p = 0.961]. A clear R0 resection was obtained in all but one patient in each group (98.4% R0 rate). Median nodal harvest was greater in the robotic group (14 nodes) compared to the laparoscopic group [(12 nodes), p = 0.004], however, the minimum recommended harvest of twelve nodes was obtained in both groups. A significant difference in disease recurrence was not observed [n = 5 in the laparoscopic group, n = 3 robotic group, p = 0.465] or in the interval to recurrence when such occurred [11 months in the laparoscopic group, 8 months in robotic group, p = 0.428]. Hazard ratio for disease-specific mortality was 1.15 in both groups, p = 0.886 (Fig. 2).
Discussion
Robotic-assisted colorectal surgery offers an alternative surgical platform for both benign and malignant disease. In this study, we identified that the initial introduction of a robotic-assisted colorectal surgery programme can achieve similar clinical and cancer outcomes compared to an established laparoscopic surgery programme for colorectal cancer. Robotic surgery was associated with shorter LOS in colonic cases and higher lymph node yields with trends towards lower SSI and post-operative ileus rates also observed. Overall, all outcomes in both groups were acceptable and in keeping with, if not more favourable than, International data [6,7,8,9,10]. It is clear that both laparoscopic and robotic surgery are safe methods of performing colorectal cancer surgery and robotic surgery may be more beneficial in the pelvis.
To discuss the overall role and potential for robotic surgery in the treatment of rectal cancer (where it is deemed to have the greatest benefit), it is important to consider the evidence comparing laparoscopy to open surgery in rectal cancer. Laparoscopy in this setting has been a source of debate as studies have shown mixed results over open surgery, yet it is widely adopted as the gold standard. In 2007, the CLASICC trial (randomized trial of 794 patients comparing laparoscopic versus open surgery for rectal cancer) showed no significant difference in the outcome of overall survival, disease-free survival, or local recurrence at three years [10]. Similarly, the Comparison of Open versus laparoscopic surgery for mid or low REctal cancer After Neoadjuvant chemoradiotherapy non-inferiority (COREAN) trial (randomized 340 patients to open or laparoscopic surgery) found no significant differences between CRM positivity rates or quality of TMEs [9]. Two subsequent large multicentre non-inferiority RCTs published in 2015, the Australasian Laparoscopic Cancer of the Rectum trial (ALaCaRT) and the American College of Surgeons Oncology Group (ACOSOG) Z605 trial investigated if laparoscopy was non-inferior to robotic surgery for rectal cancer [7, 8]. In the ALaCaRT trial, the laparoscopic approach did not demonstrate inferiority when comparing the achievement of clear margins (R0) and completeness of TME [7]. Controversially, the ACOSOG study could not demonstrate non-inferiority (statistically) of laparoscopic resection compared to open surgery and therefore concluded that the evidence did not support the use of laparoscopy for rectal cancer [8]. One may conclude that randomised controlled trial evidence does not show any clinical outcome findings for the superiority of laparoscopy compared to open surgery for rectal cancer yet we perform laparoscopic surgery everyday as it does have other perceived benefits such as smaller incisions, better pain scores and a marginally reduced LOS but conversion rates remain high and often are difficult to define [23].
Robotic surgery with endo-wristed instruments is deemed to offer improved dexterity compared to standard laparoscopy which is most beneficial in narrow spaces e.g. male pelvis [3]. There is an abundance of current literature comparing robotic, laparoscopic, and open resections for rectal cancer including systematic reviews, meta-analyses, large retrospective series, and single centre reviews [24]. To summarise, robotic surgery appears to achieve clinical and cancer outcomes comparable to open and laparoscopic surgery with better pain scores, shorter recovery and return to work and shorter LOS [25]. Most notably it is reported that the return of urological and sexual function may be better in those who undergo rectal cancer surgery robotically compared to a laparoscopic approach [26,27,28]. Regarding sexual function, studies have shown better recovery of sexual function (using the International Index of Erectile Function (IIEF) score) at both three and six months post-operatively following robotic resection compared with laparoscopic [27, 28]. Earlier return of urinary functioning has also been shown using the International Prostate Symptom Score (IPSS) [27]. In colon-specific surgery, with a growing interest in intra-corporeal anastomosis this appears to be better facilitated with robotic surgery owing to improved dexterity [4].
There are a number of limitations to this data. We did not analyse whether patients had previous surgery or grade of adhesions that were present and may have impacted on the surgical technique. Obesity indicators and BMI were not recorded. As this was a retrospective analysis of prospectively maintained database we did not have access to quality of life data. The median follow-up of 14–20 months means that further medium to long-term cancer recurrences or mortalities that may occur are not included.
Conclusion
In conclusion, the structured introduction of a robotic-assisted colorectal surgery programme over a three-year period has resulted in equivalent outcomes with an established laparoscopic programme for colorectal surgery. Clinical and short-term oncological outcomes in both groups are favourable, we can therefore conclude that both techniques are appropriate in expert hands.
References
I. agency for research in Cancer (2019) International agency for research in cancer. [Online]. https://gco.iarc.fr/today/online-analysis-map?v=2018&mode=population&mode_population=continents&population=900&populations=900&key=asr&sex=0&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&nb_items=5&grou. Accessed 02 Sep 2019
ASCO “Cancer Net.” [Online]. https://www.cancer.net/cancer-types/colorectal-cancer/statistics. Accessed 2 Sep 2019
Sivathondan PC, Jayne DG (2018) The role of robotics in colorectal surgery. Ann R Coll Surg Engl 100:42–53
Ozben V et al (2020) Robotic complete mesocolic excision for transverse colon cancer can be performed with a morbidity profile similar to that of conventional laparoscopic colectomy. Tech Coloproctol
Sheetz K, Claflin J, Dimick J (2020) Trends in the adoption of robotic surgery for common surgical procedures. JAMA Netw Open 3(1):e1918911
Jayne D et al (2017) Effect of robotic-assisted vs conventional laparoscopic surgery on risk of conversion to open laparotomy among patients undergoing resection for rectal cancer the rolarr randomized clinical trial. JAMA J Am Med Assoc 318(16):1569–1580
Stevenson ARL et al (2015) Effect of laparoscopic-assisted resection vs open resection on pathological outcomes in rectal cancer: the ALaCaRT randomized clinical trial. JAMA J Am Med Assoc 314(13):1356–1363
Fleshman J, Branda M, Sargent DJ et al (2015) Effect of laparoscopic-assisted resection vs open resection of stage II or III rectal cancer on pathologic outcomes: the ACOSOG Z6051 randomized clinical trial. JAMA J Am Med Assoc 314(32):1346–1355
Kang SB, Ji WP, Jeong SY et al (2010) Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol 11:637–645
Guillou PJ, Quirke P, Thorpe H et al (2005) Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial). Lancet 365(9472):1728–1726
Talamini MA, Hanly EJ (2004) Robotic abdominal surgery. Am J Surg 188(4):19–26
Kim NK, Kim YW, Cho MS (2015) Total mesorectal excision for rectal cancer with emphasis on pelvic autonomic nerve preservation: expert technical tips for robotic surgery. Surg Oncol 24(3):172–180
Panteleimonitis S, Ahmed J, Harper M, Parvaiz A (2016) Critical analysis of the literature investigating urogenital function preservation following robotic rectal cancer surgery. World J Gastrointest Surg 8(11):744
Kim HJ, Choi GS, Park JS, Park SY, Yang CS, Lee HJ (2018) The impact of robotic surgery on quality of life, urinary and sexual function following total mesorectal excision for rectal cancer: a propensity score-matched analysis with laparoscopic surgery. Color Dis 20(5):O103–O113
Luca F et al (2018) Sexual and urinary outcomes in robotic rectal surgery: review of the literature and technical considerations. Updates Surg 70(3):415–421
Miskovic D et al (2019) European consensus on the standardization of robotic total mesorectal excision for rectal cancer. Color Dis 21(3):270–276
Strasberg S, Clavien PA, Sanabria JR (1992) Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery 111(5):518–526
Berriós-Torres SI et al (2017) Centers for disease control and prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg 152(8):784–791
Rahbari NN, Weitz J, Hohenberger W, Heald RJ, Moran B, Ulrich A, Holm T, Wong WD, Tiret E, Moriya Y, Laurberg S, den Dulk M, van de Velde C (2010) Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery 147(3):339–351
Chapman SJ, Thorpe G, Vallance AE, Harji DP, Lee MJ, Fearnhead NS (2019) Systematic review of definitions and outcome measures for return of bowel function after gastrointestinal surgery. BJS Open 3(1):1–10
Wittekind C, Sobin LH, Gospodarowicz MK (2009) TNM classification of malignant tumours, 7th edn. Wiley-Blackwell
D’Agostino RB Jr (1998) Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 17:2265–2281
Agha R, Muir G (2003) Does laparoscopic surgery spell the end of the open surgeon? J R Soc Med 96(11):544–546
Connelly TM, Malik Z, Sehgal R, Byrnes G, Coffey JC, Peirce C (2019) The 100 most influential manuscripts in robotic surgery: a bibliometric analysis. J Robot Surg 0123456789
Khan JS, Banerjee AK, Kim S-H, Rockall TA, Jayne DG (2018) Robotic rectal surgery has advantages over laparoscopic surgery in selected patients and centres. Color Dis 20(10):845–853
Broholm M, Pommergaard HC, Gögenür I (2015) Possible benefits of robot-assisted rectal cancer surgery regarding urological and sexual dysfunction: a systematic review and meta-analysis. Color Dis 17(5):375–381
Park SY, Choi GS, Park JS, Kim HJ, Ryuk JP (2014) Urinary and erectile function in men after total mesorectal excision by laparoscopic or robot-assisted methods for the treatment of rectal cancer: a case-matched comparison. World J Surg 38:1834–1842
D’Annibale A, Pernazza G, Monsellato I, Pende V, Lucandri G, Mazzocchi P et al (2013) Total mesorectal excision: a comparison of oncological and functional outcomes between robotic and laparoscopic surgery for rectal cancer. Surg Endosc 27(6):1887e1895
Acknowledgements
I would like to thank all members of the laparoscopic and robotic-assisted surgery programmes at University Hospital Limerick for their contribution to the clinical practice upon which this research was based.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Christina A Fleming, Muhammad F Ullah, Kah Hoong Chang, Emma McNamara, David Waldron, Eoghan Condon, J Calvin Coffey, Colin Peirce declares that s/he has no conflict of interest.
Ethical approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.
Informed consent
Informed consent was obtained from all patients for being included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fleming, C.A., Ullah, M.F., Chang, K.H. et al. Propensity score-matched analysis comparing laparoscopic to robotic surgery for colorectal cancer shows comparable clinical and oncological outcomes. J Robotic Surg 15, 389–396 (2021). https://doi.org/10.1007/s11701-020-01116-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11701-020-01116-0