Abstract
Non-midline abdominal wall hernias present unique anatomic challenges, making repair more complex. The constraints of the peritoneal cavity, pelvis, and costal margin limit the utility of intraperitoneal mesh repair, and extra-peritoneal repairs have traditionally been performed using open techniques, often resulting in higher wound morbidity. Advances in minimally invasive surgery make visualization and dissection of such complex cases feasible, with all the attendant benefits of a minimally invasive over an open approach. In this study, we examined the use of the robotic platform to repair non-midline hernias. Retrospective review of all non-midline abdominal wall hernias was performed robotically at Prisma Health, excluding parastomal hernias. Study conducted and outcomes reported according to STROBE statement. Repair was performed in the retro-rectus (n = 3) or retro-rectus + transversus abdominis release (TAR) (n = 39), pre-peritoneal (n = 22), and intraperitoneal (n = 1). Mean hernia width was 9.4 cm, permanent synthetic mesh used for all repairs. Mean LOS was 1.5 days. Surgical-site occurrence (SSO) occurred in 49.2%, 78% of which were simple seroma. Three patients (4.6%) developed surgical-site infection (SSI). Two recurrences were identified with a mean follow-up of 11 mos. The robotic platform facilitates complex dissection to allow minimally invasive, extra-peritoneal repair of complex non-midline hernias. This approach overcomes the anatomic constraints of intraperitoneal mesh repair and the wound morbidity of open repair.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lateral abdominal wall hernias may develop as primary lumbar hernias, but more commonly derive from prior subcostal or flank incisions, ostomy sites, traumatic abdominal wall injury, or trocar sites [1,2,3]. Non-midline hernias are often complex and may be larger and more symptomatic than midline defects. Repair is technically demanding due to anatomic boundaries of the bony pelvis, retroperitoneum, and costal margin, often resulting in higher risk of intraoperative and perioperative complications [4, 5]. Achieving defect closure and adequate mesh reinforcement requires utilizing sometimes unfamiliar tissue planes in the lateral abdominal wall and retroperitoneum. Physiologically, the ratio of muscle to aponeurotic tissue in the lateral abdominal wall is higher, resulting in a lower effective tensile strength, which affects both defect closure integrity and the strength and durability of mesh fixation. Furthermore, lateral hernias are subjected to asymmetric forces that may result in higher risk of recurrence [6].
The European Hernia Society (EHS) classification system defines lateral hernias as below the costal margin, above the iliac crest, and lateral to the semilunar line. Midline hernias occur between the xiphoid and pubic symphysis and bounded by the semilunar line laterally [3]. While this is system is a useful standard for reporting, it does not necessarily correlate with surgical technique required for repair. Most notably, when using a retro-rectus (RR) approach to ventral hernia repair (VHR), the linea semilunaris limits the lateral dissection and therefore mesh overlap of the hernia. For hernias occurring through the linea alba, this is typically adequate. However, for hernias occurring through the rectus sheath but not directly through the linea alba, such as prior stoma sites, repair technique likely includes additional lateral dissection with a transversus abdominis release (TAR), which increases the complexity and may account for different outcomes compared to RR repair alone. For this reason, we define hernias for this study as non-midline rather than simply lateral. We previously reported outcomes for open pre-peritoneal and retro-rectus/muscular repair of lateral abdominal wall hernias, resulting in 13% risk of surgical-site infection (SSI) and 11.5% recurrence rate [7]. More recently, we have adopted a robotic approach for many of these hernia defects. The robotic platform provides excellent visualization, enhances the ability to dissect abdominal wall layers, closes the hernia defect, and widely reinforces the repair with mesh [8]. This study evaluates our surgical approach and clinical outcomes of robotic repair of non-midline abdominal wall hernias.
Methods
We retrospectively identified all patients undergoing repair of non-midline ventral/incisional hernias between August 2013 and March 2020. All surgeries were performed at Prisma Health Upstate by senior authors JAW and AMC. Patient demographics, hernia characteristics, technical details, and clinical outcomes were maintained prospectively in the Abdominal Core Health Quality Collaborative (AHSQC), a prospectively maintained national hernia registry. Any patient with a non-midline hernia was included, including those with a concurrent midline hernia defect (n = 26). Patients with parastomal hernias were excluded. Outcome measures were selected to determine the safety and efficacy of the robotic surgical approach. Primary outcomes were Surgical-Site Occurrence (SSO) and infection (SSI). Secondary outcomes were operative time, hernia recurrence, conversion to open repair, and other complications associated with repair. Study design and outcomes reporting were performed according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [9]. This study was approved by the Prisma Health Institutional Review Board.
Surgical technique
Technique varied according to the location of the hernia defect and presence or absence of a concurrent midline hernia. The single patient repaired with intraperitoneal mesh followed standard surgical approach that is well described elsewhere. Selection of the optimal approach is key, and preoperative computed tomography (CT) imaging is essential (Fig. 1).
Computed tomography imaging of non-midline hernias. A Concurrent midline hernia and hernia extending through the linea semilunaris; dashed arrow—medial edge of obliques; solid arrow—lateral edge of rectus abdominis. B Lumbar hernia; dashed arrow—posterior iliac crest; solid arrow—lateral edge of obliques. C Non-midline hernia occurring through the rectus sheath; dashed arrow—linea semilunaris; solid arrow—lateral portion of rectus abdominis muscle. D Recurrent flank hernia; dashed arrow—prior mesh in the intraperitoneal position with posterolateral hernia recurrence; solid arrow—intact aponeurotic portion of the posterolateral external oblique
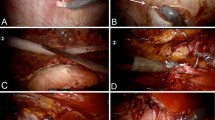
The eTEP technique involves laparoscopic entry directly into the retro-rectus space without immediate entry into the peritoneal cavity (Fig. 2). The RR space is developed to accommodate additional trocar placement. Depending on the location of the hernia, this may be a unilateral or bilateral dissection, and trocar placement varies according to the technique required for repair. For hernias without concurrent midline defect that are true lateral hernias, a unilateral dissection is often adequate, with placement of trocars along the medial rectus sheath with dissection progressing medial-to-lateral, a transversus abdominis release (TAR), and lateral dissection continuing in the pre-peritoneal/retroperitoneal space (Fig. 3A). For hernias off-midline but within the confines of the rectus sheath, such as prior ostomy sites, or patients with a concurrent midline hernia, a bilateral RR dissection is needed to ensure adequate mesh overlap in all directions (Fig. 3B, C).
Schematic of extra-peritoneal repair options. A eTEP approach with unilateral retro-rectus dissection and TAR for hernias at or beyond the linea semilunaris. B eTEP approach with bilateral retro-rectus dissection across the midline and contralateral TAR for hernias within the rectus sheath. C eTEP approach with bilateral retro-rectus dissection and unilateral TAR for concurrent midline and non-midline hernia. D Transabdominal approach with retro-rectus or pre-peritoneal dissection for non-midline hernias
Transabdominal repair begins with intraperitoneal placement of trocars with incision of the posterior rectus sheath (for RR repair) or peritoneum (for pre-peritoneal repair) to enter the extra-peritoneal plane for repair and mesh placement (Fig. 3D).
Results
We identified 65 patients who underwent robotic repair of non-midline hernias. Mean age was 62 years (range 21–91) and included 27 female and 38 female patients. Mean body mass index (BMI) was 33.8 kg/m2 (21.9–59.3). Comorbidities include hypertension (53.8%), diabetes (29.2%), chronic obstructive pulmonary disease (6.2%), and active smokers (16.9%). Patient characteristics are detailed in Table 1.
Hernias were located subcostal (L1; n = 7), flank (L2; n = 20), iliac (L3; n = 20), lumbar (L4; n = 13), or multiple sites (n = 5). Concurrent midline hernias were present in 26 patients. Hernia types included primary lumbar hernias (n = 2), traumatic flank hernias (n = 6), and incisional hernias (n = 57). Incisional hernias occurred after urologic procedures (n = 9), spine procedures (n = 3), prior ostomy site (n = 16), cholecystectomy (n = 5), appendectomy (n = 4), trocar sites (n = 8), or other incisional (n = 12). Robotic repairs were performed using a transabdominal RR/retro-muscular (RM) approach 27 patients (41.5%; RR alone = 2; RR + TAR = 25), transabdominal pre-peritoneal (rTAPP) in 22 (33.8%), extended view totally extra-peritoneal (eTEP) in 15 (23.1%; RR alone in 1; RR + TAR in 14), or intraperitoneal onlay of mesh (IPOM) (n = 1; 1.5%). The mean hernia defect size was 111 cm2 (9.1 cm mean length; 9.4 cm mean width). Permanent synthetic mesh was used for repair in all cases. Conversion to open approach was required in 9 cases (13.8%) due to development of hypercarbia (n = 3), poor visualization (n = 5), and concern bowel injury during adhesiolysis (n = 1). Five of these cases occurred during eTEP repair and 4 during transabdominal retro-muscular repair. Surgical details are shown in Table 2.
Mean operative time was 220.4 min (range 80–484 min). Fascial closure was achieved in 63 cases (96.9%). No bowel injuries occurred. Mean length of stay (LOS) was 1.5 days (median 1 day). SSI occurred in three patients (4.6%), two superficial and one deep space infection. All SSIs were treated with antibiotics and one required wound opening. No mesh infections occurred, and no mesh removal was needed. SSOs developed in 32 patients (49.2%), 25 of which were seromas. Wound debridement was required in 1 case, percutaneous drainage in three, and antibiotics in four patients (two due to concurrent SSI, two empiric). One patient developed an enterocutaneous fistula that ultimately closed spontaneously after percutaneous drainage and did not require mesh removal. Two hernias recurred (3.1%), and one patient developed a trocar-site hernia. Mean follow-up was 11 months (range 0.5–60.9 months). Surgical outcomes are shown in Table 3.
Discussion
While primary non-midline hernias, Spigelian or lumbar hernias, are rare, non-midline secondary hernias can result from a variety of surgical procedures or trauma. Etiology of the hernia will certainly play a role in complexity of repair, and the heterogeneity of surgical techniques and hernia morphology should be taken into account when analyzing clinical trials data [9]. Non-midline abdominal wall hernias present with unique anatomic challenges during repair. Hernias occurring lateral to the anterior axillary line (EHS classification L4) may not be well visualized from an intraabdominal approach due to the lateral peritoneal attachments of the colon, and lateral mesh overlap is limited for the same reason. Dissection into the retroperitoneal space is required for mesh placement, or an alternative approach such as an onlay is required. When dissecting into this space, it is critical to consider the course of the ureter, gonadal and iliac vessels, and retroperitoneal neural anatomy, all of which may be distorted due to the hernia. Intimate knowledge of these structures is needed to perform a safe and efficacious repair. In particular, the iliohypogastric, ilioinguinal, and lateral femoral cutaneous nerves are at risk of injury for hernias in this location, particularly if found more caudal near the iliac crest. Posterior dissection should allow for at least 5 cm of mesh overlap and typically will extend over the psoas and/or quadratus lumborum. Inferior dissection will typically extend to the iliac fossa, where the inguinal nerves are particularly at risk, as are the spermatic structures. Superiorly, dissection of the peritoneum from the diaphragm may be needed to allow adequate mesh overlap under the costal margin. Interestingly, many lateral abdominal wall hernias will present as partial thickness defects with the external oblique layer still intact (Fig. 1D, Fig. 4). This feature actually adds benefit to a robotic approach, as the defect can be difficulty to locate with an open approach when the external oblique is intact. This should also be distinguished from denervation injury that can mimic the appearance of a lateral hernia, but without true musculo-fascial defect. This particular pathology is beyond the scope of this study, as all of our patients did have a true hernia defect.
Hernias in the lateral aspects of the rectus sheath, such as parastomal or prior ostomy-site hernias, are still defined by the EHS as midline hernias [3]. However, if repaired with a RR Rives–Stoppa approach, lateral mesh overlap is limited by the linea semilunaris, and additional lateral myofascial release is required. This is accomplished using the transversus abdominis release (TAR) and RM mesh placement. The posterior rectus sheath is incised medial to the intercostal neurovascular bundles along the semilunar line, dividing the posterior lamella of the internal oblique and the transversus abdominis muscle / aponeurosis to enter the pre-peritoneal or pre-transversalis plane. Dissection is then extended laterally in the extra-peritoneal plane below the transversus abdominis muscle to allow for adequate mesh overlap and myofascial mobilization for hernia defect closure. For this reason, hernias technically classified as midline (M1–5) by the EHS are included in this report, as the addition of a TAR adds significant complexity to the repair and is necessary for most hernias that occur lateral to the linea alba.
Selection of the optimal surgical approach depends on the location and size of the hernia relative to these anatomic limitations. Strong consideration should be given to preoperative computed tomography imaging to delineate the extent of the hernia. Current published series of lateral abdominal wall hernia repair are limited, and there is no current consensus on optimal repair owing to these limited data and the heterogeneity of these hernias. We previously reported outcomes of 61 non-midline hernias repaired in an open extra-peritoneal fashion [7]. Patients developed SSO in 49.2% of cases with SSI in 13.1% and hernia recurrence of 11.5% with a mean of 15.4 months. Others have reported similar outcomes for open flank hernia repair. Moreno–Egea et al. reported similar SSO and recurrences in 20 patients with lumbar hernias, with 40% and 15%, respectively [10]. Their group also reported a small series of 7 patients with lumbar hernia repaired using the open technique and yielded a 42.9% recurrence [11]. Veyrie et al. reported the repair of 61 patients with non-midline incisional hernias using a retro-muscular approach. Early perioperative morbidity was 18% with a recurrence rate of 4.9% with median follow-up of 47 mos [12].
Minimally invasive surgery, and hernia repair in particular, has repeatedly demonstrated lower risk of SSI compared with open surgery. There are limited data on laparoscopic repair of lateral hernias, however, due to the anatomic considerations mentioned above, which make minimally invasive repair difficult. Moreno-Egea et al. published a series of 55 patients who underwent laparoscopic (35 patients) and open (20 patients) repairs of lumbar hernias, demonstrating benefit of the laparoscopic approach over open repair in terms of both wound complications (seroma 20 vs. 40%) and hernia recurrence (2.9 vs. 15%)[13]. A second study looking more broadly at non-midline hernias demonstrated a recurrence rate of 8.2% overall, with 25% of subcostal hernias developing a recurrence [14]. The authors do note the increased complexity of pre-peritoneal dissection and limitations of mesh fixation in these hernias, which likely contribute to hernia recurrence.
These anatomic and technical concerns can largely be mitigated with the utilization of extra-peritoneal mesh placement. This is technically difficulty to accomplish with traditional laparoscopy but is greatly facilitated by the robotic platform. The enhanced visualization and ability to perform a complex dissection with articulated instruments in an ergonomically friendly manner has enabled expansion of minimally invasive techniques to include complex abdominal wall reconstruction. The ability to achieve fascial closure, aided by lateral abdominal myofascial release and extensive extra-peritoneal dissection, is key to the success of robotic repair. Others have reported similar outcomes in limited series [15]. Di Giuseppe et al. reported outcomes of robotic repair of seven incisional flank hernias [16]. With a mean follow-up of 6 months, no seromas nor recurrences were reported, and no cases required conversion to open surgery. Another more recent smaller study reported no recurrence of non-midline hernias repaired robotically, with data presented up to 24 months post repair [17].
The decision to proceed with a TAPP approach versus a RR approach (rRR or eTEP, with or without TAR) is largely at the surgeons’ discretion, though several factors deserve discussion. The peritoneum is fairly easy to dissection in the lateral abdominal wall and the upper and lower midline due to the relatively higher pre-peritoneal fat content. However, along the midline periumbilical area and underlying the rectus sheath, the peritoneum can be quite thin and its integrity difficult to maintain. Thus, for hernias with a midline component or within the lateral rectus sheath but not through the linea alba, we prefer a RR approach rather than pre-peritoneal. For true lateral hernias (EHS L1–4), the rTAPP or eTEP with unilateral RR dissection is typically employed, as long as there is adequate ability to develop the pre-peritoneal space at least 5 cm medial to the hernia defect. Schematically, this is represented in Fig. 3A, D. The choice of eTEP vs. rTAPP is made based on both surgeon preference and rectus abdominis width; a narrow rectus abdominis muscle (< 5 cm) limits the working space and favors a transabdominal approach.
There are several limitations of our study. Selection bias is inherent to any retrospective analysis. Clinical perception of technical difficulty of a given hernia repair will influence the decision to pursue open versus robotic repair, and this description is not necessarily representative of non-midline hernia repair outcomes on the whole. Additionally, this study is conducted in a high-volume hernia-specific practice at a single center, which may limit generalizability. We also performed several variations in technique. Though similar in concept, there may be important differences in outcome and technique that cannot be elucidated due to the small sample size and may increase bias in the results. As there are no controls for the current study, future case-matched studies may be beneficial to compare outcomes of robotic repair with open and laparoscopic repairs. Finally, our follow-up duration is short, and long-term analysis is needed to confirm the risk of hernia recurrence. Because of the increased capability of different institutions in performing robotic surgeries in addition to the gap of knowledge in robotic repair of non-midline hernias, timely report of the safety and efficacy of the robotic approach was a main factor for reporting our current findings. Follow-up is ongoing. Larger studies with long-term follow-up are needed to confirm our initial experiences with robotic lateral hernia repairs.
Conclusion
Non-midline abdominal wall hernia repair is difficult due to anatomic and technical constraints to commonly employed techniques. The evolution of advanced myofascial release techniques and the robotic platform enables reconstruction of these complex hernia defects in a minimally invasive fashion, minimizing perioperative morbidity while adhering to well-established principles of hernia repair. Overall, it is evident from our study that the robotic platform is safe and efficacious and will become an integral tool in repairing non-midline abdominal wall hernias. Further study is needed to optimize patient selection and determine long-term outcomes.
Data Availability
Data collected and analyzed for this study are available from the corresponding author upon reasonable request.
References
Hope WW, Tuma F (2022) Incisional hernia. StatPearls Publishing, Treasure Island (FL)
Dennis RW, Marshall A, Deshmukh H, Bender JS, Kulvatunyou N, Lees JS, Albrecht RM (2009) Abdominal wall injuries occurring after blunt trauma: incidence and grading system. Am J Surg. https://doi.org/10.1016/j.amjsurg.2008.11.015
Muysoms FE, Miserez M, Berrevoet F, Campanelli G, Champault GG, Chelala E, Dietz UA, Eker HH, El Nakadi I, Hauters P, Hidalgo Pascual M, Hoeferlin A, Klinge U, Montgomery A, Simmermacher RKJ, Simons MP, Smietański M, Sommeling C, Tollens T, Vierendeels T, Kingsnorth A (2009) Classification of primary and incisional abdominal wall hernias. Hernia. https://doi.org/10.1007/s10029-009-0518-x
Moreno-Egea A, Carrillo A, Aguayo JL (2008) Midline versus nonmidline laparoscopic incisional hernioplasty: a comparative study. Surg Endosc. https://doi.org/10.1007/s00464-007-9480-9
Slater NJ, Montgomery A, Berrevoet F, Carbonell AM, Chang A, Franklin M, Kercher KW, Lammers BJ, Parra-Davilla E, Roll S, Towfigh S, van Geffen E, Conze J, van Goor H (2014) Criteria for definition of a complex abdominal wall hernia. Hernia. https://doi.org/10.1007/s10029-013-1168-6
Kapur SK, Butler CE (2018) Lateral abdominal wall reconstruction. Semin Plast Surg. https://doi.org/10.1055/s-0038-1666801
Patel PP, Warren JA, Mansour R, Cobb WS, Carbonell AM (2016) A large single-center experience of open lateral abdominal wall hernia repairs. Am Surg 82:608–612
Donkor C, Gonzalez A, Gallas MR, Helbig M, Weinstein C, Rodriguez J (2017) Current perspectives in robotic hernia repair. Robot Surg. https://doi.org/10.2147/RSRR.S101809
von Elm E, Altman DG, Egger M et al (2007) The strengthening the reporting of observational studies in epidemiology (STROBE) statement; guidelines for reporting observational studies. Lancet 370:1453–1457. https://doi.org/10.1016/S0140-6736(07)61602-X
Moreno-Egea A, Alcaraz AC, Cuervo MC (2013) Surgical options in lumbar hernia: laparoscopic versus open repair. A long-term prospective study. Surg Innov. https://doi.org/10.1177/1553350612458726
Moreno-Egea A, Torralba-Martinez JA, Morales G, Fernández T, Girela E, Aguayo-Albasini JL (2005) Open vs laparoscopic repair of secondary lumbar hernias: a prospective nonrandomized study. Surg Endosc. https://doi.org/10.1007/s00464-004-9067-7
Veyrie N, Poghosyan T, Corigliano N, Canard G, Servajean S, Bouillot J (2013) Lateral incisional hernia repair by the retromuscular approach with polyester standard mesh: topographic considerations and long-term follow-up of 61 consecutive patients. World J Surg. https://doi.org/10.1007/s00268-012-1857-9
Stabilini C, Cavallaro G, Dolce P, Capoccia Giovannini S, Corcione F, Frascio M, Sodo M, Merola G, Bracale U (2019) Pooled data analysis of primary ventral (PVH) and incisional hernia (IH) repair is no more acceptable: results of a systematic review and metanalysis of current literature. Hernia. https://doi.org/10.1007/s10029-019-02033-4
Moreno-Egea A, Carrillo-Alcaraz A (2012) Management of non-midline incisional hernia by the laparoscopic approach: results of a long-term follow-up prospective study. Surg Endosc. https://doi.org/10.1007/s00464-011-2001-x
Kirkpatrick T, Zimmerman B, LeBlanc K (2018) Initial experience with robotic hernia repairs: a review of 150 cases. Surg Technol Int 33:139
Di Giuseppe M, Mongelli F, Marcantonio M, La Regina D, Pini R (2020) Robotic assisted treatment of flank hernias: case series. BMC Surg. https://doi.org/10.1186/s12893-020-00843-3
Cabrera ATG, Lima DL, Pereira X, Cavazzola LT, Malcher F (2021) Robotic transabdominal preperitoneal approach (TAPP) for lateral incisional hernias. Arq Bras Cir Dig. https://doi.org/10.1590/0102-672020210002e1599
Funding
No funding was received for any portion of this study.
Author information
Authors and Affiliations
Contributions
Study conception and design was done by JAW, WSC and AMC; acquisition of data by AAG, DI, and LJ; analysis and interpretation of data by AAG and JAW; drafting of manuscript by JAW and AAG; and critical revision by AAG, JAW, WSC, and AMC. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Drs. Warren and Carbonell have received honoraria for speaking and teaching from Intuitive Surgical. Drs. Carbonell and Cobb have received honoraria and consulting fees from W.L. Gore.
Ethical approval
The databased used for this study was approved by the Prisma Health Institutional Review Board.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, A.A., Isaac, D., Jaraczewski, L. et al. Robotic repair of non-midline hernias. J Robotic Surg 17, 1021–1027 (2023). https://doi.org/10.1007/s11701-022-01509-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11701-022-01509-3