Summary
Background
Given the paucity of evidence surrounding lateral incisional hernias (LIH), optimal techniques remain elusive. We aim to compare perioperative and mid-term outcomes of patients who underwent robotic LIH repair using three techniques.
Methods
Patients were grouped as intraperitoneal onlay (IPOM), transabdominal preperitoneal (TAPP), or retromuscular (RM). Clavien–Dindo classification and Comprehensive Complication Index (CCI®; University of Zurich, Zurich, Switzerland) were used to report postoperative complications and morbidity scores. Surgical site events (SSEs), including surgical site occurrences (SSOs) and surgical site infections (SSIs), were also compared.
Results
Of the 555 patients, 26 patients were included in the study; 5 (19.2%) underwent IPOM, 8 (30.8%) underwent TAPP, and 13 (50%) underwent RM repair. Although there were no differences regarding hernia defect size, a larger mesh size as well as a greater mesh overlap was achieved in the RM group compared to the IPOM and TAPP groups (p < 0.05). Additionally, RM repair allowed for a higher mesh-to-defect ratio than the recommended ratio of 16:1. There were no differences between groups in terms of postoperative outcomes, including SSEs, Clavien–Dindo grades, and CCI® scores.
Conclusion
No differences in mid-term outcomes between robotic IPOM, TAPP, or RM repair were noted. However, the robotic RM repair allows for significantly larger mesh size and mesh overlap, as well as a higher mesh-to-defect ratio.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lateral incisional hernias (LIH) are relatively rare compared to midline hernias [1]. A number of anatomical factors play a role in the technical difficulties faced with repair of LIH. Firstly, there is less aponeurotic tissue at the lateral abdominal wall compared to the midline portion of the abdomen. Additionally, hernias located close to the bony prominences, such as the costal margin and the iliac crest and those in close association with major neurovascular structures, may not allow for proper mesh overlap and adequate mesh fixation [1,2,3]. Therefore, the repair of laterally located incisional hernias presents a challenge for surgeons [1,2,3,4,5].
Due to the rarity of LIH, most published studies on LIH consist of case series [2, 5,6,7,8,9,10], with the most prevalent surgical techniques used being open and laparoscopic. Comparative studies are limited and the majority of studies compare defect location [11,12,13,14,15] rather than surgical technique [3, 16, 17]. Given the lack of high-quality evidence to provide reliable recommendations for these challenging hernias, optimal techniques and approaches remain unclear [1, 4, 18]. Clinical studies show that recurrence rates, wound complications, and hospital length of stay differ between open and laparoscopic approaches [18]. However, there are no published series or comparative analyses between the robotic approach to LIH and the aforementioned techniques. Our aim is to compare perioperative and midterm results of intraperitoneal onlay (IPOM), transabdominal preperitoneal (TAPP), and retromuscular (RM) in robotic LIH repair. We hypothesize that robotic RM repair will be advantageous in terms of perioperative and midterm outcomes.
Materials and methods
The data for this study were obtained from a prospectively collected institutional review board-approved database of cases between February 2013 and July 2019 and were retrospectively reviewed. From this database, patients who had surgery with the purpose of repair of a lateral incisional hernia were included. Patients who had surgery for midline hernias (according to the European Hernia Society classification [19], defined as both primary and incisional hernias between the lateral border of both rectus sheaths, the xiphoid, and the pubic bone) and those with primary (non-incisional) lateral hernias were excluded from the study. Patients who underwent concomitantly performed non-hernia procedures were also excluded for better clarification of postoperative complications. After selection, patients were grouped according to the mesh position of the index procedure (IPOM, TAPP, RM).
The database includes patients’ demographics (age, sex, body mass index [BMI], and comorbidities), hernia characteristics (etiology, hernia content, localization, hernia defect size), operative variables (procedure setting, concomitant procedures, the presence of conversion to other approaches, duration of procedures, mesh type and size, fixation method, estimated blood loss [EBL], ability to close the hernia defect, and intraoperative complications), and early postoperative results (the hospital length of stay [LOS], hospital readmission within a postoperative 30-day period, and postoperative complications). Additional calculations were performed according to intraoperative measurements including defect area in cm2 (oval formula), mesh area in cm2 (oval or rectangular formula), mesh overlap in cm in both craniocaudal and transverse directions, and mesh-to-defect ratio (M/D ratio). Postoperative complications were reviewed as documented in the surgeon’s follow-up visits, as well as the patients’ medical records and clinical charts. In patients with inadequate data retrieval, phone calls were made to gather detailed information about postoperative complications. All complications were categorized according to the Clavien–Dindo classification system [20]. Of these, surgical wound complications were further categorized according to the previously published classification of surgical site events (SSEs) [21]. SSEs were further classified as surgical site infections (SSIs; including cellulitis, superficial, deep, and organ-space infections), surgical site occurrences (SSOs; including fluid collections such as seroma and hematoma), and surgical site occurrence or infection procedural interventions (SSO/SSI-PIs; SSOs or SSIs requiring any procedural intervention such as reopening a wound, placing a drain, percutaneous aspiration, or reoperation). Postoperative morbidity score was measured using the Comprehensive Complication Index (CCI®, University of Zurich, Zurich, Switzerland) [22]. The Morales–Conde classification algorithm was used to describe the severity of a seroma complication [23]. Mid-term outcomes were assessed by phone survey according to the criteria of the ventral hernia recurrence inventory [24]. Briefly, patients were asked two questions regarding their hernia surgery: 1) “Do you have physical symptoms or pain at the site?” 2) “Do you feel or see a bulge?” If a patient responded “yes” to either of these questions, the patient was invited for an office visit to perform a physical examination and obtain further imaging studies, if necessary. For patients who could not be reached by telephone interview, the last visit date was determined to calculate the follow-up time. For other patients, the phone survey date or examination date was used for follow-up calculation.
Surgical technique
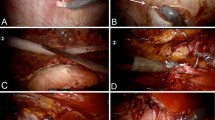
The patients were placed in the supine or semi-lateral decubitus position with flexing of the table depending on hernia localization (Fig. 1a). Following appropriate preparation, the trocars were inserted depending on the type of repair and the patient-side cart of the da Vinci surgical robotic system (Intuitive Surgical, Sunnyvale, CA, USA) was docked.
Intraperitoneal onlay mesh repair
For IPOM repair, after adhesiolysis, the defect was measured and primary closure of the hernia defect was performed by running a long-lasting absorbable barbed suture (Stratafix 0™ on CT‑1 needle, Ethicon, Somerville, NJ, USA) under reduced intraabdominal pressure (4–8 mm Hg) or de-flexing the table. The mesh was introduced and secured to the posterior fascia using barbed absorbable sutures (2–0 V-Loc™; Medtronic, Minneapolis, MN, USA) in a running fashion.
Transabdominal preperitoneal repair
For TAPP repair, the preperitoneal plane was entered and dissected circumferentially around the defect to provide space for adequate mesh deployment. After closing the hernia defect, the mesh was deployed and secured to the posterior fascia. The peritoneal flap was closed with a barbed absorbable suture (2–0 V-Loc™; Medtronic, Minneapolis, MN, USA).
Retromuscular repair
For RM repair, the procedures were performed with either transabdominal or totally extraperitoneal access. After achievement of the retrorectus plane, the dissection was carried out laterally towards the linea semilunaris and a transversus abdominis release (TAR) was performed (Fig. 1b). Neurovascular bundles of the rectus muscle were found and preserved during the TAR. The dissection plane was extended laterally (Fig. 1c, d). After the hernia defect was identified (Fig. 2a), the hernia sac was reduced into the abdominal cavity. A circumferential dissection can help complete reduction of the hernia sac without peritoneal tearing (Fig. 2b). Once the dissection was completed, primary closure of the anterior defect was accomplished by running a long-lasting absorbable barbed suture (Stratafix 0™ on CT‑1 needle, Ethicon, Somerville, NJ, USA; Fig. 2c). For TA access RM repair, the opening of the posterior rectus sheath was closed using barbed absorbable suture (2–0 V-Loc™; Medtronic, Minneapolis, MN, USA) in a running fashion. In the case of bilateral TAR, the same steps were performed for the contralateral side of the patient, to obtain tension-free closure of the posterior flaps. The mesh was then inserted, deployed, and placed in its correct position (Fig. 2d). Separate mesh was used for patients who required concomitant inguinal hernia repair (IHR). Pneumoperitoneum was released under direct vision. Any fascial incision more than 10 mm, if present, was closed along with skin incisions with absorbable sutures after administration of local anesthetic (1% bupivacaine hydrochloride) at the trocar sites.
Robotic lumbar hernia repair; a patient position, b transversus abdominis release for robotic retromuscular hernia repair, c lateral extension of the dissection plan as caudal to the hernia defect, TAM transversus abdominis muscle, FT fascia transversalis d lateral extension of the dissection plan as cranial to the hernia defect
Statistical analysis
All statistical analyses were performed using SPSS software (Statistical Package for Social Sciences for Windows Version 22, IBM® Corporation, Armonk, NY, USA). Categorical variables were represented in terms of frequency (n and/or %), while continuous variables were reported as the mean ± the standard deviation (SD) for normal distributions or the median with interquartile range (IQR) for non-normal distributions. Chi-square test or Fisher’s exact test was used for categorical variables. One-way analysis of variance (ANOVA) or the Kruskal–Wallis test was used for continuous variables, as appropriate. A p-value of <0.05 was considered as statistically significance.
Results
From a total of 555 patients who underwent a robotic ventral hernia repair, 26 patients who underwent robotic lateral incisional hernia repair were enrolled in this study. Of these, robotic IPOM repair was performed in 5 patients, robotic TAPP in 8 patients, and robotic RM repair in 13 patients. Patient demographics and mesh position details are listed in Table 1 and Fig. 3.
Three patients (3/26) in the cohort underwent robotic hernia repair in an emergency setting, of whom 1 patient underwent IPOM repair and 2 patients underwent RM repair (p = 0.453). Regarding hernia content, 14/26 patients had an incarcerated hernia: omentum in 6/14, small bowel in 7/14, and colon in 6/14 patients. There were no differences between groups regarding the presence of hernia incarceration (p = 0.940) or hernia content (omentum, small bowel, and colon: p = 0.162, p = 0.714, and p = 0.497, respectively). The comparison of hernia characteristics and operative variables between groups are summarized in Table 2. Of robotic RM repairs, while transabdominal trocar access was used in 7/13 patients, a totally extraperitoneal trocar placement was used in 6/13 patients. All robotic RM repairs were performed with a unilateral TAR technique except in one case which required a bilateral TAR repair in order to obtain tension-free closure of the posterior fascia. Four out of 26 patients required concomitant inguinal hernia repair (three bilateral, one unilateral). Of these four patients, two were in the TAPP group and the other two were in the RM group (p = 0.478).
In terms of mesh materials, polypropylene, polyester, and expanded polytetrafluoroethylene mesh was used in 5, 18, and 3 patients, respectively; there was no statistical difference between groups (p = 0.107). While no fixation material was applied in 11 patients, suture alone or absorbable tacker and suture in combination were applied in 15 patients. All robotic IPOM repairs (5/5) required circumferential mesh fixation, while 6 of the TAPP repairs (6/8) and 4 of the RM repairs (4/13) were performed by using a few interrupted sutures to be able to hold the mesh in place, as discussed later. Drains were not placed in any of the patients.
None of the patients required conversion to an open or laparoscopic approach. However, a hybrid technique was required in one patient who underwent IPOM repair to be able to close the hernia defect. The presence of intraoperative complications did not differ between groups (p = 0.453); subcutaneous emphysema occurred in 1 patient who underwent IPOM repair (1/5), and a small bowel injury occurred during adhesiolysis and was repaired primarily in 2 patients who underwent robotic RM repair (2/13).
The mean hospital length of stay was 0.65 days (range = 0–2 days). Most patients were discharged on the same day of the procedure (15/26). Neither the hospital length of stay nor the rate of same-day discharge varied between groups (p = 0.282, p = 0.427, respectively). Two patients (2/26) were readmitted within the postoperative 30-day period (1 in the TAPP group, 1 in the RM group, p = 0.713). The reasons for hospital readmission were postoperative ileus for the TAPP patient and surgical site infection for the RM patient. The Ventral Hernia Recurrence Inventory questionnaire was completed for 22/26 patients via phone conversation. There was no recurrence in the entire cohort, with the mean follow-up time being 33.3 (min–max: 2.9–76.2) months. Postoperative complications are detailed in Table 3. The Clavien–Dindo grades, the CCI® scores, and the rates of SSEs did not differ between groups. In 1/5 IPOM patients, trocar site cellulitis occurred and was treated with antibiotic medication. A superficial SSI occurred in 1 RM patient (1/13), who was readmitted to hospital and treated with percutaneous drainage. A postoperative seroma was detected in 2/26 patients using imaging methods (Morale–Conde grade 0b); another patient (1/26) had a clinically detected seroma accompanied by pain (Morales–Conde grade 3d). A hematoma occurred in 1 patient (1/26) who had taken coumadin for peripheral vascular disease. All seromas and hematoma resolved without any intervention. None of the patients experienced chronic postoperative pain.
Discussion
No single technique has been accepted as the most appropriate surgical approach for LIH. Many technical factors influence the success of this procedure, such as the type of surgical access, position of the mesh in relation to the abdominal wall, degree of mesh overlap, and use of mesh fixation [4, 25]. Most literature on LIH repair focuses on the open and laparoscopic approach. To our knowledge, this is the first study to compare outcomes of robotic LIH repair using different mesh positions.
Performing open extraperitoneal repair is technically demanding as it may require shifting from the preperitoneal to the retromuscular space, as described by Philips et al. [2]. According to their technique, the dissection began from the hernia site and the mesh was placed in the retromuscular–preperitoneal plane. They reported a superficial wound infection in 1/16 cases and a mesh infection in 1/16 cases, with a mean follow-up period of 16.8 months. Authors have suggested that obtaining a wide mesh overlap followed by permanent fixation likely eliminated hernia recurrence [2]. In our robotic TAPP group, we did not observe postoperative infection (0/8), but two patients developed a seroma (2/8). Due to the fact that the fascia propria, which is usually referred to as preperitoneal fat, has a variable thickness in different locations of the abdominal wall, it may make the preperitoneal dissection problematic; peritoneal integrity may not be maintained during dissection or, even if preserved, may result in a thin and poor-quality peritoneal flap. Moreover, it is almost impossible to perform a “pure” preperitoneal dissection near the linea semilunaris, where the peritoneum is tightly adhered to the posterior rectus sheath. Such technical challenges can cause TAPP repair to be preferred only with small hernias.
Anatomically fixed boundaries, such as the diaphragm superiorly and the pelvis inferiorly, may make it difficult to achieve proper mesh overlap and fixation [1,2,3,4,5]. Furthermore, the distribution of preperitoneal fatty tissue and aponeurotic fascia in the abdominal wall varies; when compared to the medial abdomen, there is less aponeurotic tissue and more preperitoneal fatty tissue at the lateral abdominal wall. Shekarriz et al. [6] described a laparoscopic transperitoneal preperitoneal repair of lumbar hernias in a total of 3 cases. Their technique involves closing the peritoneal flap after the fixation of a non-coated mesh to the lateral abdominal wall. This method isolates the mesh from the peritoneal contents. Some authors have applied transperitoneal or partial preperitoneal mesh placement, without complete peritoneal closure, for laparoscopic repair of laterally located hernias [5, 8].
There are no published comparisons between robotic and open TAR for LIH repair. In a retrospective review of 11 patients with a history of kidney transplantation and concomitant TAR, 2 seromas (2/11), 1 hematoma (1/11), and 1 (1/11) recurrence were reported [26]. Available studies comparing robotic TAR and open TAR procedures for ventral hernias suggest that robotic TAR offers lower postoperative morbidity and shorter hospital stay [27, 28]. In our previous study reviewing patients who underwent robotic RM ventral hernia repair, we found that the presence of incisional hernia and locating off-midline hernias were independent predictors for the need to perform an adjunctive TAR during RM repair for ventral hernias [29]. In a multivariate analysis unique to the TAR + group, we also found that a transverse mesh overlap was a factor mathematically associated with the presence of all prevalent postoperative complications (odds ratio: 1.46, 95% CI 0.9–2.2, p = 0.09). However, we did not find statistical significance between IPOM, TAPP, and RM groups in terms of postoperative outcomes, although the rate of SSEs was mathematically higher in extraperitoneal mesh placement groups (TAPP and RM). This higher rate of SSEs may be due to the necessity of extensive tissue dissection in order to place the mesh in the extraperitoneal plane. Further studies with a large sample size are needed to better understand the association between the degree of dissection and postoperative wound complications in robotic LIH repair.
Although open LIH repair carries an inherent risk of wound complications such as flap necrosis, infection, and fluid collections, some studies have shown promising results [10]. In a study with 29 patients who underwent abdominal wall reconstruction for large flank hernias with a mean follow-up period of 21.2 months, there was found to be 2/29 seromas, 2/29 wound infections, 3/29 persistent pain, and 1/29 recurrence [9]. Purnell et al. [17] reviewed a total of 31 consecutive patients repaired with a 7.5-cm wide piece of midweight macroporous polypropylene mesh over a 13-year period. The mesh was positioned between the internal and external muscles (12 patients) if internal oblique and transverse abdominal muscles were able to be closed without tension. If not, the mesh was placed intraperitoneally (19 patients). They did not report any SSIs or 30-day SSOs. However, one superficial wound infection, one wound breakdown, and one case of chronic pain were reported. In a prospective nonrandomized study conducted with a total of 16 patients, Moreno-Egea et al. [3] compared the laparoscopic approach (n = 9) with the open approach (n = 7) for secondary lumbar hernias. In the laparoscopic technique, an IPOM placement with an average of 5 cm of mesh overlap in all directions was preferred, whereas preperitoneal mesh placement with more than 2 cm of overlap was used for an open approach. They found that the laparoscopic approach is associated with a decreased morbidity rate (33% vs. 86%) and a shorter mean hospital stay (2.2 days vs. 7.1 days) compared to the open approach. While none of the patients in the laparoscopic group (0/9) experienced any chronic pain, two patients in the open group (2/7) did. The authors concluded that laparoscopic repair of lumbar hernias is more efficient and more profitable than the traditional open approach.
Previously published studies on laparoscopic ventral hernia repair have reported that the long-term durability of repair, and consequently decreased recurrence rate, may be affected by mesh overlap. There have been reported rates for mesh overlap [4, 30] and mesh-to-defect ratio [31, 32] which are valid for midline hernias. However, due to a lower incidence of laterally located hernias, a minimum mesh overlap needed to diminish the recurrence rate has yet to be discovered. As a recommendation for lateral defects which may need a large mesh overlap, the International Endo-Hernia Society guideline stated that TAR should be preferred over anterior component separation techniques [4]. In recent years, we altered our approach for ventral hernia repair from IPOM to the RM plane (Fig. 3). We observed that ample space could be obtained by dissecting the RM plane with TAR towards the central tendon of the diaphragm superiorly, the psoas muscle laterally, and the retropubic space inferiorly. Since the dissected area is usually filled with mesh, a larger mesh is used in TAR repair. Accordingly, we found that the mesh size was larger in the RM group (p = 0.046) and, thus, considerably more mesh overlap could be achieved in both craniocaudal and transverse directions (p = 0.028 and p = 0.016, respectively). Although there were no statistically significant differences between groups in terms of the M/D ratio, it was only possible to reach a ratio above the recommended ratio of 16:1 in the RM group (median = 18), suggesting that the RM approach could be the superior technique when it comes to recurrence prevention.
Intraperitoneal placement typically requires thorough mesh fixation through tackers, intracorporeal sutures, and transfascial sutures in order to prevent mesh migration. This is sometimes achieved by orthopedic drills and anchors adjacent to the iliac crests and ribs, which could be a significant source of postoperative pain and increased risk of neurovascular injury [33, 34]. In robotic IPOM repair, the mesh can easily be secured with circumferential superficial sutures. Studies on open lateral abdominal wall reconstruction emphasize that anchoring the mesh to the boundaries of the lateral abdominal wall, particularly near the bony prominences, may provide a durable reconstruction [2, 16, 35, 36]. In minimally invasive extraperitoneal hernia repairs, which are usually performed for small/moderate size hernias, minimal fixation is needed because the mesh is confined within the abdominal wall layers and intraabdominal forces act to hold the mesh in place [33]. In our extraperitoneal mesh groups, the mesh was secured with a few interrupted sutures in order to hold the mesh in place. Furthermore, we did not use any fixation material in 2/8 patients in the TAPP group and 9/13 patients in the RM group. Tailoring the mesh to occupy the entire dissected area may help to hold the mesh in place, because the borders of this area prevent displacement of the mesh. In this way, the necessity of fixation material can be minimized.
This study has several limitations. Although the sample size of the study is relatively small, it is very similar to the previously published series of LIHs. Other limitations arise from patient selection and the retrospective nature of the study, although the data of participants came from a prospectively collected database. The lack of quality of life assessment as well as the absence of cost analysis data are other limitations of this study, such that the overall burden of the presented procedures is unknown. Placing the mesh extra-peritoneally allows use of less costly uncoated mesh.
In conclusion, the present study showed that there are no differences between robotic IPOM, TAPP, and RM mesh positions in terms of perioperative and mid-term outcomes with lateral hernia repair. However, the use of a retromuscular plane may allow for a significantly larger mesh size, mesh overlap, and a higher mesh-to-defect ratio. Further larger prospective and comparative studies reporting different approaches with longer follow-up are warranted to determine the influence of mesh position on postoperative outcomes in the treatment of LIH.
References
Zhou DJ, Carlson MA. Incidence, etiology, management, and outcomes of flank hernia: review of published data. Hernia. 2018;22(2):353–61. https://doi.org/10.1007/s10029-018-1740-1.
Phillips MS, Krpata DM, Blatnik JA, Rosen MJ. Retromuscular preperitoneal repair of flank hernias. J Gastrointest Surg. 2012;16(8):1548–53. https://doi.org/10.1007/s11605-012-1890-x.
Moreno-Egea A, Torralba-Martinez JA, Morales G, Fernández T, Girela E, Aguayo-Albasini JL. Open vs laparoscopic repair of secondary lumbar hernias: a prospective nonrandomized study. Surg Endosc. 2005;19(2):184–7. https://doi.org/10.1007/s00464-004-9067-7.
Bittner R, Bain K, Bansal VK, Berrevoet F, Bingener-Casey J, Chen D, et al. Update of Guidelines for laparoscopic treatment of ventral and incisional abdominal wall hernias (International Endohernia Society (IEHS)): part B. Surg Endosc. 2019; https://doi.org/10.1007/s00464-019-06908-6.
Edwards C, Geiger T, Bartow K, Ramaswamy A, Fearing N, Thaler K, et al. Laparoscopic transperitoneal repair of flank hernias: a retrospective review of 27 patients. Surg Endosc. 2009;23(12):2692–6. https://doi.org/10.1007/s00464-009-0477-4.
Shekarriz B, Graziottin TM, Gholami S, Lu HF, Yamada H, Duh QY, et al. Transperitoneal preperitoneal laparoscopic lumbar incisional herniorrhaphy. J Urol. 2001;166(4):1267–9.
Renard Y, de Mestier L, Cagniet A, Demichel N, Marchand C, Meffert JL, et al. Open retromuscular large mesh reconstruction of lumbar incisional hernias including the atrophic muscular area. Hernia. 2017;21(3):341–9. https://doi.org/10.1007/s10029-016-1570-y.
Sun J, Chen X, Li J, Zhang Y, Dong F, Zheng M. Implementation of the trans-abdominal partial extra-peritoneal (TAPE) technique in laparoscopic lumbar hernia repair. BMC Surg. 2015;15:118. https://doi.org/10.1186/s12893-015-0104-3.
Pezeshk RA, Pulikkottil BJ, Bailey SH, Schaffer NE, Reece EM, Thornton NJ, et al. An evidence-based model for the successful treatment of flank and lateral abdominal wall hernias. Plast Reconstr Surg. 2015;136(2):377–85. https://doi.org/10.1097/PRS.0000000000001432.
Patel PP, Warren JA, Mansour R, Cobb WS IV, Carbonell AM. A large single-center experience of open lateral abdominal wall hernia repairs. Am Surg. 2016;82(7):608–12.
Veyrie N, Poghosyan T, Corigliano N, Canard G, Servajean S, Bouillot JL. Lateral incisional hernia repair by the retromuscular approach with polyester standard mesh: topographic considerations and long-term follow-up of 61 consecutive patients. World J Surg. 2013;37(3):538–44. https://doi.org/10.1007/s00268-012-1857-9.
Lal R, Sharma D, Hazrah P, Kumar P, Borgharia S, Agarwal A. Laparoscopic management of nonmidline ventral hernia. J Laparoendosc Adv Surg Tech A. 2014;24(7):445–50. https://doi.org/10.1089/lap.2013.0381.
Ferrarese A, Enrico S, Solej M, Surace A, Nardi MJ, Millo P, et al. Laparoscopic management of non-midline incisional hernia: a multicentric study. Int J Surg. 2016;33(Suppl 1):S108–13. https://doi.org/10.1016/j.ijsu.2016.06.023.
Moreno-Egea A, Carrillo-Alcaraz A. Management of non-midline incisional hernia by the laparoscopic approach: results of a long-term follow-up prospective study. Surg Endosc. 2012;26(4):1069–78. https://doi.org/10.1007/s00464-011-2001-x.
Moreno-Egea A, Carrillo A, Aguayo JL. Midline versus nonmidline laparoscopic incisional hernioplasty: a comparative study. Surg Endosc. 2008;22(3):744–9. https://doi.org/10.1007/s00464-007-9480-9.
Kapur SK, Liu J, Baumann DP, Butler CE. Surgical outcomes in lateral abdominal wall reconstruction: a comparative analysis of surgical techniques. J Am Coll Surg. 2019;229(3):267–76. https://doi.org/10.1016/j.jamcollsurg.2019.03.023.
Purnell CA, Park E, Turin SY, Dumanian GA. Postoperative flank defects, hernias, and bulges: a reliable method for repair. Plast Reconstr Surg. 2016;137(3):994–1001. https://doi.org/10.1097/01.prs.0000479987.80490.5c.
Beffa LR, Margiotta AL, Carbonell AM. Flank and lumbar hernia repair. Surg Clin North Am. 2018;98(3):593–605. https://doi.org/10.1016/j.suc.2018.01.009.
Muysoms FE, Miserez M, Berrevoet F, Campanelli G, Champault GG, Chelala E, et al. Classification of primary and incisional abdominal wall hernias. Hernia. 2009;13(4):407–14. https://doi.org/10.1007/s10029-009-0518-x.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13. https://doi.org/10.1097/01.sla.0000133083.54934.ae.
Petro CC, Novitsky YW. Classification of hernias. In: Novitsky YW, editor. Hernia surgery. Current principles. Cham: Springer; 2016. pp. 15–21.
Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien PA. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg. 2013;258(1):1–7. https://doi.org/10.1097/SLA.0b013e318296c732.
Morales-Conde S. A new classification for seroma after laparoscopic ventral hernia repair. Hernia. 2012;16(3):261–7. https://doi.org/10.1007/s10029-012-0911-8.
Baucom RB, Ousley J, Feurer ID, Beveridge GB, Pierce RA, Holzman MD, et al. Patient reported outcomes after incisional hernia repair-establishing the ventral hernia recurrence inventory. Am J Surg. 2016;212(1):81–8. https://doi.org/10.1016/j.amjsurg.2015.06.007.
Suarez S, Hernandez JD. Laparoscopic repair of a lumbar hernia: report of a case and extensive review of the literature. Surg Endosc. 2013;27(9):3421–9. https://doi.org/10.1007/s00464-013-2884-9.
Petro CC, Orenstein SB, Criss CN, Sanchez EQ, Rosen MJ, Woodside KJ, et al. Transversus abdominis muscle release for repair of complex incisional hernias in kidney transplant recipients. Am J Surg. 2015;210(2):334–9. https://doi.org/10.1016/j.amjsurg.2014.08.043.
Bittner JG IV, Alrefai S, Vy M, Mabe M, Del Prado PAR, Clingempeel NL. Comparative analysis of open and robotic transversus abdominis release for ventral hernia repair. Surg Endosc. 2018;32(2):727–34. https://doi.org/10.1007/s00464-017-5729-0.
Carbonell AM, Warren JA, Prabhu AS, Ballecer CD, Janczyk RJ, Herrera J, et al. Reducing length of stay using a robotic-assisted approach for retromuscular ventral hernia repair: a comparative analysis from the Americas Hernia Society quality collaborative. Ann Surg. 2018;267(2):210–7. https://doi.org/10.1097/SLA.0000000000002244.
Gokcal F, Morrison S, Kudsi OY. Robotic retromuscular ventral hernia repair and transversus abdominis release: short-term outcomes and risk factors associated with perioperative complications. Hernia. 2019;23(2):375–85. https://doi.org/10.1007/s10029-019-01911-1.
LeBlanc K. Proper mesh overlap is a key determinant in hernia recurrence following laparoscopic ventral and incisional hernia repair. Hernia. 2016;20(1):85–99. https://doi.org/10.1007/s10029-015-1399-9.
Tulloh B, de Beaux A. Defects and donuts: the importance of the mesh:defect area ratio. Hernia. 2016;20(6):893–5. https://doi.org/10.1007/s10029-016-1524-4.
Hauters P, Desmet J, Gherardi D, Dewaele S, Poilvache H, Malvaux P. Assessment of predictive factors for recurrence in laparoscopic ventral hernia repair using a bridging technique. Surg Endosc. 2017;31(9):3656–63. https://doi.org/10.1007/s00464-016-5401-0.
Warren JA, Love M. Incisional hernia repair: minimally invasive approaches. Surg Clin North Am. 2018;98(3):537–59. https://doi.org/10.1016/j.suc.2018.01.008.
Salameh JR, Salloum EJ. Lumbar incisional hernias: diagnostic and management dilemma. JSLS. 2004;8(4):391–4.
Mukherjee K, Miller RS. Flank hernia repair with suture anchor mesh fixation to the Iliac crest. Am Surg. 2017;83(3):284–9.
Blair LJ, Cox TC, Huntington CR, Ross SW, Kneisl JS, Augenstein VA, et al. Bone anchor fixation in abdominal wall reconstruction: a useful adjunct in suprapubic and para-iliac hernia repair. Am Surg. 2015;81(7):693–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
O.Y. Kudsi receives a teaching course and/or consultancy fees from Intuitive, Bard, Gore, outside the submitted work. N. Bou-Ayash, K. Chang, and F. Gokcal declare that they have no competing interests.
Ethical standards
All procedures performed in studies involving human participants or on human tissue were in accordance with the ethical standards of the institutional and/or national research committee and with the 1975 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. The data of this study were obtained from a database approved by the Institutional Review Board.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kudsi, O.Y., Bou-Ayash, N., Chang, K. et al. Robotic repair of lateral incisional hernias using intraperitoneal onlay, preperitoneal, and retromuscular mesh placement: a comparison of mid-term results and surgical technique. Eur Surg 53, 188–197 (2021). https://doi.org/10.1007/s10353-020-00634-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10353-020-00634-3