Abstract
New eco-friendly approaches were proposed for the synthesis of cis-cyclopentene-annulated heterocyclic compounds containing a tetrahydroquinoline moiety. For the first time we implemented a one-pot three-component cyclocondensation of aromatic amines (aniline, 5-aminoquinoline, o-phenylenediamine), aldehydes, and cyclopentadiene (CPD) in water and in the ionic liquid. The effect of synthesized substituted 3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinolines on the reproduction of house fly imago and on the initial stage of offspring ontogeny was evaluated in comparison with the effect of natural insect hormone using biological screening (Musca domestica). The most probable factors of stabilization of prepared compounds 7–10 in the active site of the Heliothis virescens receptor were identified using AutoDock 4.2, AutoDock Vina, and GOLD Suite molecular docking software. According to the results of three scoring functions, 4-(3-chlorophenyl)-8-fluoro-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinoline forms the most stable complex with the chosen receptor. The results of bioassays and molecular docking indicate that these compounds may be considered as potential ecdysone agonists.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The growing demand for the development of environmentally safe chemical processes more and more often attracts the attention of synthetic chemists focusing on the synthesis of pure chemical compounds for health care, increasing life standards, and environmental safety. The humanistic nature of green chemistry is reflected by its twelve rules, proposed in 1998 by P. Anastas and J. Warner, for the development of new chemical processes ranging from the used feedstock to efficiency and waste safety (Anastas and Eghbali 2010).
Natural and synthetic compounds with a tetrahydroquinoline moiety possess a broad spectrum of biological activities (Ghashghaei et al. 2018; Muthukrishnan et al. 2019), such as anti-inflammatory (Gosmini et al. 2014), antitumor (Chen et al. 2016; Dayal et al. 2020; Hanashalshahaby et al. 2019), antituberculosis (Chavan et al. 2019; Kumar et al. 2011), antifungal (Chander et al. 2016; Chavan et al. 2019; Ozaki et al. 2019), antibacterial (Diaz et al. 2018; Kimura et al. 2019; Martínez et al. 2019; Onyedibe et al. 2021), and antimalarial (Bendale et al. 2007; Van Voorhis et al. 2007) activities. Not long ago, it was found that tetrahydroquinoline derivatives are non-steroidal agonists of Aedes albopictus mosquito ecdysone receptor, responsible for their development and reproduction (Kitamura et al. 2014; Ueno et al. 2021; Yokoi et al. 2019), and that they possess a larvicidal activity, which offers prospects for practical use for the development of new pest control agents (Fig. 1).
The most popular and optimal approach to the synthesis of substituted tetrahydroquinolines is the Povarov (aza-Diels–Alder) reaction, which consists in formal [4 + 2]-cycloaddition of aromatic imines (Schiff bases) to electron-rich olefins catalyzed by Lewis or Brønsted acids (Povarov 1967). The three-component one-pot version of the Povarov reaction proved to be a synthetically convenient and atom-economic method for the preparation of this type of structures (Glushkov and Tolstikov 2008; Tolstikov et al. 2014b). However, the catalysts and solvents used in this reaction (Glushkov and Tolstikov 2008) often suffer from drawbacks such as high cost, water sensitivity, poor availability, and toxicity. This complicates scaling up of the synthesis for both engineering and economic reasons. Therefore, it is necessary to develop modern approaches that would comply with green chemistry principles (Anastas and Eghbali 2010; Van Aken et al. 2006) for the synthesis of tetrahydroquinoline derivatives. The search for cheap acid catalysts free from heavy metals and for green solvents for the Povarov reaction and the synthesis of polysubstituted tetrahydroquinoline derivatives is a relevant task (Petronijević 2017). Ionic liquids (ILs) have occupied a decent place in organic synthesis in recent decades and are postulated as green reagents, owing to their stability, low toxicity (Rogers and Seddon 2003), and reusability (Sheldon 2005). Examples of the use of ILs to promote aza-Diels–Alder reactions with microwave or electrochemical assisted are described (Bortolami et al. 2021; Mert-Balci et al. 2013).
In order to develop eco-friendly approaches to the synthesis of polycyclic compounds containing a tetrahydroquinoline moiety, we implemented a one-pot three-component cyclocondensation of aromatic amines (aniline, 5-aminoquinoline, o-phenylenediamine), aldehydes, and cyclopentadiene (CPD) in water and in ILs. Biological screening of the obtained tetrahydrocyclopenta[c]quinolines was carried out using the Musca domestica insect. The results are analyzed in comparison with the data of molecular docking.
Results and discussion
The possibility of synthesizing Schiff bases from aromatic aldehydes and S-methyl, S-benzyl, and S-n-octyl-dithiocarbazates and thiosemicarbazides both in water and with lemon juice catalyst was reported previously (Ali et al. 2020). The authors noted that the yield of the target product in the developed eco-friendly procedure was higher than that in the conventional procedure implying heating in ethanol. In another study (Petronijević 2017) describing the syntheses of 3,4-dihydro-2(1H)-quinoxalinones and 3,4-dihydro-1,4-benzoxazin-2-ones, diluted (1:10) freshly squeezed lemon juice was used as the biocatalytic medium for the condensation of aromatic amines with enolates.
Analysis of the published protocols stimulated us to implement one-pot cyclocondensation (Povarov reaction) under conditions complying with the green chemistry principles. Previously, tetrahydro-3H-cyclopenta[c]quinolines were synthesized in acetonitrile or using CF3CO2H as the catalyst (Tolstikov et al. 2014b). There are few reported examples of eco-friendly atom-economic one-pot synthesis of polycycles with a tetrahydroquinoline moiety (Li et al. 2015). We accomplished three-component cyclocondensation of 4-fluoroaniline 1 with an equimolar amount of ethyl glyoxylate 3 and a threefold molar excess of cyclopentadiene (CPD) in aqueous lemon juice (1:1), which afforded the target cyclopentene-annulated tetrahydroquinoline 7 in 40% yield within 15 min (Scheme 1). When the reaction mixture was heated to 70℃for 20 min, the yield of the target product considerably increased.
The reaction is diastereoselective, as indicated by the homo-and heterocorrelation 1H and 13C NMR spectra of the cyclocondensation product 7. Single-crystal X-ray diffraction data for 7 unambiguously prove the structure of rel-(3aR*, 4S*, 9bS*)-4-ethoxycarbonyl-8-fluoro-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinoline (Fig. 2).
An attempt to carry out three-component cyclocondensation of 4-fluoroaniline 1 with aromatic aldehydes 4–6 under the developed conditions resulted in the formation of only Schiff bases (Celik and Kuzu 2019; Kerner et al. 2016).
The synthesis of 4-aryl-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinolines 8–10 was affected when the three-component cyclocondensation was carried out in IL (1-ethyl-3-methylimidazolium tetrachloroaluminate). The reaction of amine 1, 2 with aromatic aldehyde 4–6 and CPD in 1 mL of IL for 15 min resulted in a quantitative and diastereoselective formation of cis-cyclopentene-annulated tetrahydroquinolines 8–10 (Scheme 1). The formation of these products was confirmed by coincidence of the physicochemical assignment results with the data reported previously (Tolstikov et al. 2014b). The spin–spin coupling constant values 3 J (3a, 9b) = 8, 3J (4, 3a) = 3.2 Hz (Tolstikov et al. 2014b) of compounds 8, 9 and 3J ((3a, 9b), (4, 3a) = 8 Hz of 10, proved their cis-orientation and 3aR*, 4S*, and 9bS* relative configurations of the stereogenic centers.
In order to extend the potential of the cyclocondensation reaction taking place in aqueous lemon juice and ILs, 5-aminoquinoline 11 and o-phenylenediamine 12 were used as the amine components. This gave cycloadducts 13a, 13c and 15a, 15b (Scheme 2). The formation of cyclopentene-annulated 4-ethoxycarbonyl-3a,4,5,11b-tetrahydro-3H-cyclopenta[c]-1,7-phenanthroline 13a in aqueous lemon juice occurred with a low yield (7%), and heating and increase in the reaction time did not affect the reaction pathway, which led to imine 13b as the major product. A change in the reaction pathway toward the formation of the target product was attained in IL, in which the three-component condensation of p-trifluoromethylbenzaldehyde, aminoquinoline 11, and CPD resulted formation of 4-[4-(trifluoromethyl)phenyl]-3a,4,5,11b-tetrahydro-3H-cyclopenta[c][1,7]phenanthroline 13c (81%). It is noteworthy that the product of the eco-friendly cyclocondensation formed diastereoselectively (dr ˃ 95%) and in a higher yield than under the conditions (CF3CH2OH, CF3COOH, Ar, ~ 25 °C) developed previously for the one-pot synthesis of cyclopenta[c]1,7-phenanthrolines (65%) (Tolstikov et al. 2014a). The spin–spin coupling constants (J (3a,4) = 2.4 and J (3a,11b) = 8,4 Hz) of the vicinal protons at the newly formed C(3a), C(4), and C(11b) asymmetric centers of compound 13c correspond to their cis relative positions.
The condensation of o-phenylenediamine with ethyl glyoxylate and CPD in aqueous lemon juice afforded Schiff base 14. The target annulation product was formed in an ionic liquid. The structure molecules 15a, b are characterized by double set of proton and carbon signals of equal intensity in the 1H and 13C NMR spectra, which is indicative of syn/anti (1:1, 1H NMR)-isomer composition (Savchenko et al. 2022). Under these conditions, the yield of the summarized 4,7-bis((4-(trifluoromethyl)phenyl)-3,3a,4,5,6,7,7a,8,10a,12b-decahydrodicyclopenta[c,i]-1,10-phenanthroline 15a, b was 64%.
Thus, we developed an efficient diastereoselective one-pot approach to the three-component cyclocondensation of arylamines, aldehydes, and cyclopentadiene consistent with green chemistry principles.
Bioassay
The synthesized compounds 7–10 were tested with respect to 24-h-old image of Sh gen strain (F 182) Musca domestica. Once out of the puparium, the adult insects were placed into 300 cm3 cages (three pairs in each cage; two repetitions). The reference compound, 20-hydroxyecdysone (20E), and the test compounds were added to the drinking bowls in concentrations of 1 × 10−8 M during 4 days. Then the solutions in drinking bowls were replaced by pure water, and the insect reproduction was monitored for 10 days by placing standard containers with egg-laying substrate into the cages. The development of laid eggs was observed up to the preparation of larvae for pupation. The control cages and drinking bowls contained pure water.
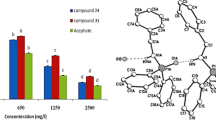
The assessment of effects of tetrahydroquinolines 7–10 on the adult house fly and the initial stage of offspring ontogeny demonstrated that the test compounds tend to accelerate the reproductive maturation; as a result, the egg-laying period started 2–4 days earlier than that in the control. The most pronounced acceleration was observed in the presence of compounds 10 (Fig. 3). It is noteworthy that this effect was retained when mixtures with 20E were used, and ecdysterone, which has a stimulating activity toward the house fly imago, completely counterbalanced the adverse effect of the compounds on the fecundity: Compound 8 only completely suppressed reproduction. As a result, the specific fecundity of the females treated with mixtures of 7–10 with 20E did not differ from that for 20E alone (Savchenko et al. 2015). However, this acceleration of the reproduction apparently took a lot of resources of the organism and, consequently, the lifespan of the imago markedly decreased. The decrease of the lifespan by one-third of the normal value was enhanced by combination with 20E.
The beneficial effect of these compounds was also noted for embryogenesis: The hatching of eggs laid by females was almost 100%, while in the control groups, no more than 70–80% of eggs were hatched (Fig. 4). The differences between the compounds were observed in the delayed effects: Only compounds 8 (in mixture with 20E) and 10 accelerated the development of larvae emerging from eggs and induced the arrival of the larval–pupal transformation phase 3–4 days earlier compared to the control.
All of the effects that we detected indicate that compounds 7–10 are incorporated into the regulation of protein biosynthesis in the same way as 20E, that is, via the interaction with the insulin signaling pathway (Cai et al. 2016; Mendes and Mirth 2016). The facts of shortening of the imago lifespan and decrease in the female fecundity, which confirm too fast resource utilization, may be indicative of incorporation of the obtained compounds into the regulation of carbohydrate and lipid metabolism, resulting in disturbance of metabolism synchronization with the energy demand of reproduction (Hou et al. 2015).
Molecular docking
The ecdysone receptor (EcR) has a remarkable ability to structurally adapt to various types of ligands. EcR binds to ecdysteroids, including 20-hydroxyecdysone (20E) and ponasterone A, and non-steroidal synthetic agonists such as dibenzoyl hydrazine (DBH) insecticides, e.g., N'-tert-butyl-N'-(3,5-dimethylphenyl)carbonyl-5-methyl-2,3-dihydro-1,4-benzodioxine-6-carbohydrazide (HWG) (Kitamura et al. 2014) (Fig. 5). The concept of receptor adaptability and plasticity of ligands was considered in a previous study (Billas et al. 2003). The subsequent structural observations provided a rational interpretation of the flexible ability of EcR to bind to and to be activated by chemically and structurally diverse ligands (Browning et al. 2021). Here we report a molecular docking study of EcR adaptability toward binding to potential synthetic agonists of the tetrahydroquinoline type in comparison with known data for native ecdysteroid and DBH-type synthetic agonist (HWG).
The synthesized structures 7–10 were docked into the active site of the target protein that was obtained from the isolated crystal structure of the moth Heliothis virescens EcR-USP heterodimer complexed with ponasterone A (PonA) (1r1k) with a resolution of 2.9 Å. For comparison, we also estimated the binding affinity in silico of PonA and the synthetic ligand HWG to the same active site of protein macromolecule EcR. The computing experiment was carried out using the AutoDock 4.2 (www.ebi.ac.uk), AutoDock Vina, and GOLD Suite (www.ccdc.cam.ac.uk) software programs.
Known, that three-component Povarov reaction, catalyzed by Lewis or Brønsted acids between aromatic amine, aldehyde, and CPD affords the preferential formation of endo/exo,cis- tetrahydroquinoline and its minor trans-isomer (Glushkov and Tolstikov 2008). Although in our eco-compatible experiments we observe the formation of diastereomer I (endo/exo-cis) mainly (NMR 1H, dr ˃ 98%), we decided to conduct molecular docking for the probable trans-isomer II too. The isomers endo-cis I and exo-cis I (Fig. 6) have the same NMR spectra, and their specific rotation is equal by zero.
The values of the three scoring functions and the root-mean-square deviations between the solutions obtained for endo/exo-cis I and endo/exo-trans II isomers are presented in Table 1 and Table 2 SI, respectively. A comparison of the results of molecular docking (Table 2 SI) of exo-cis I and exo-trans II isomers of the most active compounds 9 and 10 shows high values of the root-mean-square deviation (RMSD ≥ 2), which indicates a low probability of validity of the data obtained and makes it possible to exclude the consideration of exo-cis/trans isomers in the subsequent study.
In most cases, the coordinates of synthesized structures calculated using two different scoring functions (GOLD Suite and AutoDock Vina) coincided within the error, RMSD = 0.5 Å (Table 1). In the case of estimation of the ligand affinity to the target protein using three scoring functions (GOLD Suite, AutoDock 4.2, and AutoDock Vina), an allowable deviation of the obtained coordinates from one another was observed for stereoisomers 9(I), 9(II), and 10(II) (Table 1). The best agreement between the docking solutions regarding both the calculated ligand coordinates and binding energies to the protein was found for ligands 9(I), 9(II), and 10(II). The most pronounced differences between the estimated coordinates and scoring functions were observed for ligands 7(I, II) 8(I, II), and 10(I) (Table 1, RMSD ≥ 2). For example, the convergence of solutions obtained for ligands 8(I) and 9(II) was demonstrated using discussed docking programs (Fig. 7). This differences in the docking results are due to different methods for estimating the steric and energy matching of the ligands to the ligand-binding cavity of EcR inherent in these software programs.
The results of simulation of EcR complexes with compounds 7–10, obtained using three scoring functions of various types (AutoDock 4.2 (ADT), AutoDock Vina (AV), and GOLD), are summarized in Table 3 SI. Same Table 3 SI gives detailed description for all factors ensuring effective binding of the test ligands to the receptor active site.
As an example, Fig. 8 shows the docking solution for tetrahydroquinoline 9(I), which resides in the same spatial area of the protein as the cyclic part of the native ligand ponasterone A. Stabilization of the position of studied ligand in the EcR active site is enhanced by hydrogen bonds and by hydrophobic interactions with the nearby amino acid residues of the H1, H3, H5, and H6 helices and β-sheet (Table 3 SI). In particular, this ligand is stabilized in the active site of the receptor owing to hydrogen bonds with THR346, MET380; hydrophobic interactions with VAL395, ARG387, VAL384, LEU420; and to \({\uppi }\)-\({\uppi }\)-interactions with PHE397.
The endo-isomers (I, II) of compounds 7–10 in the in silico obtained EcR complexes are arranged as two closely spaced clusters. For example, Fig. 9 shows the location of the 9(I) and 9(II) isomers belonging to different clusters relative to ponasterone A and HWG in the active site of target protein. Compounds 7(I)–10(I) form the first cluster of docking solutions. These ligands occupy an area of the cyclic part of ponasterone A and interact with the amino acid residues of H1, H3, H5, and H6 helices and β-sheet. In the considered case, a high level of steric complementarity with EcR according to the results of three scoring functions was found for ligand 9(I) (see Table 1). The docked positions of ligands 7(I)–10(I) relative to PonA and HWG are illustrated in SI (Fig. 1).
Compounds 7(II)–10(II) form the second cluster of docking simulations, because the positions of these ligands are shifted toward the side chain of PonA in the docked model. One of the aromatic rings of these molecules occupies the same cavity of the binding pocket as the HWG ligand’s aromatic moiety (Fig. 10). According to the results of the three computational methods, stereoisomers 9(II) and 10(II) show the highest binding affinity to the target protein among the tested products of the second cluster. The docked position of the synthetic ligand (HWG) takes place of the side chain of PonaA in the binding site of EcR (Fig. 9), and it demonstrates a lower level of affinity in silico to the EcR compared to ponasterone A (Table 1).
Thus, docking results against Heliothis virescens EcR-USP heterodimer complexes with the synthesized compounds 7–10 were indicated that all exceed the synthetic HWG ligand in terms of affinity in silico, but they are inferior to natural ecdysteroids. However, the difference in the values of the scoring functions that were obtained for natural ligand and for the considered tetrahydroquinoline molecules indicates the potential strength of the interaction between protein with synthesized ligand.
Experimental
General methods
One-dimensional (1H and 13C) and two-dimensional (COSY, NOESY, HSQC, and HMBC) NMR spectra of compounds were recorded on Bruker Avance 400 HD Ascend spectrometer (400.13 MHz for 1H and 100.62 MHz for 13C) and Bruker Avance II 500 HD Ascend spectrometer (500.17 MHz for 1H and 125.77 MHz for 13C) using standard Bruker pulse sequences. For NMR data, the chemical shifts are reported in δ (ppm) referenced to residual solvent protons and 13C signals in deuterated chloroform or methanol. Coupling constants (J) are expressed in Hertz (Hz). High-resolution mass spectra (HRMS) were measured on an instrument («MaXis impact», Bruker) using a time-of-flight mass analyzer (TOF) with electrospray ionization (ESI). In experiments on selective collisional activation, the activation energy was set at maximum abundance of fragment peaks. A syringe injection was used for solutions in MeCN (flow rate 5 µL/min). Nitrogen was applied as a dry gas; the interface temperature was set at 180 °C. Column chromatography and TLC were performed using silica gel (< 0.06 mm) and pre-coated silica gel (Silufol plates), respectively; spots were processed by treatment with a solution of 4-hydroxy-3-methoxybenzaldehyde in ethanol, acidified with sulfuric acid. Melting points were determined on Boetius hot-stage microscope. Crystal of the compound 7 mounted on glass fiber was studied with an Xcalibur Gemini Eos automated four-circle diffractometer (graphite monochromator, MoKα radiation, λ = 0.71073 Å, ω-scan mode, 2θmax = 62°) at ambient temperature (293–298 K). Collected data were processed using the program CrysAlisPro (CrysAlisPRO 2012). Structures determination was carried out with the OLEX2 program (Dolomanov et al. 2009). The structures were solved by direct methods and refined using the full-matrix least-squares method in the anisotropic approximation for non-hydrogen atoms. All hydrogen atoms are generated using the proper HFIX command and isotropically refined using the riding model. The calculations were performed using the SHELX program package (Sheldrick 2008). The key crystallographic data and X-ray experiment details for compound 7 are presented in Supplementary data. The molecular plots were drawn using Mercury (Macrae et al. 2020). Crystallographic data from compound have been deposited with the Cambridge Crystallographic Data Center as Supplementary Material number CCDC–2210899 (7). Copies of the data can be obtained free of charge on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK. E-mail:deposit@ccdc.cam.ac.uk.
Molecular docking
The crystal structure of the heterodimer EcR/USP of the moth Heliothis virescens was considered as a target protein, which was obtained from Protein Data Bank in the form of an heterodimer complex with PonA (1r1k) with a resolution of 2.9 Å (https://www.rcsb.org/). The computational experiment was carried out using the programs AutoDock 4.2 (Goodsell et al. 1990; Khairutdinov et al. 2014; Morris et al. 2009), AutoDock Vina (Gaillard 2018; Trott and Olson 2010), and GOLD Suite (Gaillard 2018; Trott and Olson 2010). Ligands for calculations were selected taking into account the most probable stereoisomerism. The structures of the natural ecdysteroid, 20-hydroxyecdysone, were positioned in the same active center as well as the native ligand of the complex EcR–Ponasteron A (taking into account the stereoisomerism of these biologically active substances).
The geometric parameters of ligands 7–10 were optimized by molecular mechanics by application of the MMFF94 force field using the MarvinSketch program, version 19.19 (www.chemaxon.com), and by the semiempirical PM6 method using the GAUSSIAN09 (Frisch M. J. 2009). The subsequent preparation of the structures of ligands 7–10 and 1r1k macromolecules was carried out using the AutoDockTools (Goodsell et al. 1990; Khairutdinov et al. 2014; Morris et al. 2009) and HERMES (www.ccdc.cam.ac.uk) software. Prior to calculations, all water molecules were removed from the protein. The files with the results were converted to Mol2 and PDBQT formats with addition of the lacking hydrogen atoms and partial atomic charges calculated by the Gasteiger method (Gaillard 2018; Goodsell et al. 1990; Khairutdinov et al. 2014; Morris et al. 2009; Trott and Olson 2010).
In AutoDockTools, a three-dimensional box (26 Å size) was generated, and the test ligands were placed into the box. The position of the reference HWG agonist was taken as the center of the box (www.rscb.org). The range for the ligand-binding cavity of the target protein in the GOLD Suite program was 12 Å; in this case, the position of ponasterone A was taken as the center.
The optimal positions of the ligands in the receptor active site were identified using the Lamarckian genetic algorithm (Fuhrmann et al. 2010; Kerzmann et al. 2008; Morris et al. 2009, 2008; Rurainski et al. 2009) and the Broyden–Fletcher–Goldfarb–Shanno (BFGS) local search algorithm. The docking procedure took into account the crystal structure of the target protein and implied full conformational flexibility of the ligands.
The estimation of the ligand affinity to the target protein in the GOLD program was based on calculation of the piecewise linear potential (CHEMPLP) scoring function. The AutoDock 4.2 and AutoDock Vina scoring functions acted as tools for parameterization of the energy contributions of receptor–ligand interactions according to AutoDock and AutoDock Vina, respectively.
When searching for potentially bioactive conformers using the evaluation function of the AutoDock 4.2 program, the angle of internal rotation around all single bonds in ligands was 30°, and the movement of ligand molecules as a whole in space was also carried out with an angle of 30° relative to the initial conformations. In the AutoDock Vina and GOLD Suite programs, molecular docking was performed with default parameters. Ligand conformations characterized by the minimum value of evaluation functions were taken as the optimal solution for the search of bioactive conformation in the AutoDock 4.2 and AutoDock Vina software. In the GOLD Suite program, the values of the obtained affinity parameters were directly dependent on the steric complementarity and energy correspondence between the studied compounds and the EcR protein. The assessment of the quality of ligand positioning in the active center of EcR was characterized by the RMSD value, which is the standard deviation of the position of the ligand after docking from its native position in the simulated complexes. Docking solutions were clustered based on RMSD = 2.0 Å. The RMSD value estimated by comparing the coordination of ponasterone A calculated by the molecular docking method with its native position in the active center of the receptor was in the range of 0.010 – 0.180 Å, which indicates the applicability of the evaluation functions of the AutoDock 4.2, AutoDock Vina, and GOLD programs to the modeling of agonists and antagonists of the receptor.
Procedure for lemon juice-catalyzed Povarov reaction
Freshly distilled cyclopenta-1,3-diene (3 mmol, 0.25 mL) and ethyl glyoxylate 3 (1 mmol, 0.1 mL) were added sequentially to a solution of amine 1 (1 mmol, 0.095 mL) or 5-aminoquinoline 11 (1 mmol, 144 mg) in hot lemon juice (5 mL). The mixture was stirred while heating to 70–80 °C for 0.25 h until the amine disappeared (TLC monitoring). The reaction mixture was extracted with CH2Cl2 (3 × 5 mL), the solvent was evaporated, the residue was purified by column chromatography (SiO2, petroleum ether/ethyl acetate, 10:1) to isolate products 7 and 13a.
rel-(3aR*,4S*,9bS*)-4-ethoxycarbonyl-8-fluoro-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinoline 7
White solid, Yield: 76%, m.p. 72–74 °C (hexane). 1H NMR (500 MHz, CDCl3) δ 6.69–6.77 (m, 2H, H-7, H-9), 6.58–6.61 (m, 1H, H-6), 5.71 (m, 2H, H-1, H-2), 4.24–4.38 (m, 2H, OCH2), 4.06 (m, 2H, H-4, H-9b), 3.34–3.36 (m, 1H, H-3a), 2.33–2.39 and 2.46–2.52 (m, 2H, H-3), 1.28–1.36 (m, 3H, CH3). 13C NMR (125 MHz, CDCl3) δ 171.74 (OCO), 156.58 (d, J = 235 Hz, C-8), 140.09 (C-5a), 133.57 (C-1), 130.37 (C-2), 127.38 (d, J = 6 Hz, C-9a), 116.49 (d, J = 7 Hz, C-6), 114.71 (d, J = 22 Hz, C-7), 113.13 (d, J = 22 Hz, C-9), 61.25 (OCH2), 56.81 (C-4), 46.71 (C-9b), 40.41 (C-3a), 32.67 (C-3), 14.26 (CH3). HRMS (ESI-TOF) m/z [M+] Calculated for C15H16FNO2: 261.1165; Found: 261.8601.
rel-(3aR*,4S*,11bS*)-4-ethoxycarbonyl-3a,4,5,11b-tetrahydro-3H-cyclopenta[c][1,7]phenanthroline 13a
Yellow solid, Yield: 7%, m.p. 114–116 °C (hexane). 1H NMR (400 MHz, CDCl3) δ 8.77–8.78 (m, 1H, H-8), 8.15–8.18 (m, 1H, H-6), 7.49–7.51 (m, 1H, H-10), 7.34–7.36 (m, 1H, H-11), 7.26–7.29 (m, 1H, H-7), 5.84 (s, 1H, H-1), 5.64 (s, 1H, H-2), 4.94 (s, 1H, NH), 4.24–4.29 and 4.33–4.37 (m, 2H, CH2), 4.21 (m, 2H, H-4,11b), 3.39–3.42 (m, 1H, H-3a), 2.31–2.35 and 2.45–2.50 (m, 2H, H-3), 1.31–1.35 (m, 3H, CH3). 13C NMR (100 MHz, CDCl3) δ 171.94 (CO), 149.42 (C8), 147.54 (C5a), 137.93 (C9a), 133.88 (C1), 130.62 (C11), 130.09 (C2), 128.65 (C6), 120.74 (C11a), 120.03 (C10), 119.66 (C7), 118.68 (C5b), 61.44 (CH2), 55.98 (C4), 46.63 (C11b), 41.07 (C3a), 32.21 (C3), 14.30 (CH3). HRMS (ESI-TOF) m/z [M+] Calculated for C18H18N2O2: 294.1368; Found: 294.1365.
Ethyl-(E)-2-(quinoline-5-ylimino) acetate 13b
Yellow solid, Yield: 76%, amorphous. 1HNMR (400 MHz, CDCl3) δ 9.31–9.33 (m, 1H, H-2). 8.51 (d, 1H, J = 8.4 Hz, H-4), 8.13 (m, 1H, H-10), 8.04–8.08 (m, 1H, H-8), 7.92–7.95 (m, 1H, H-7), 7.73–7.78 (m, 1H, H-3), 7.78 (d, 1H, J = 7.6 Hz, H-6), 4.27–4.48 (m, 2H, H-12), 1.39 (t, J = 7 Hz, 3H, H-13). HRMS (ESI-TOF) m/z [M + H]+ Calculated for C13H12N2O2; 228.0898; Found: 229.0985.
Diethyl-2,2'-(1,2-phenylenedi(nitrilo)diacetate 14
White solid, Yield: 70%, m.p. 172–174 °C (EtOH). 1HNMR (400 MHz, MeOD) δ 8.21 (s, 2H, CH = N), 7.84 (d, 2H, J = 8.0 Hz, CH-Ar), 7.57–7.61 (m, 2H, CH-Ar), 4.20–4.25 (m, 4H, OCH2), 1.31 (t, 6H, J = 6.8 Hz, CH3). 13C NMR (100 MHz, MeOD3) δ 169.06 (OC = O), 150.51 (C = N), 132.68 (C-Ar), 128.72 (C-Ar), 123.79 (CAr), 60.99 (OCH2), 12.96 (CH3). HRMS (ESI-TOF) m/z [M]+ Calculated for C14H16N2O4: 276.1110: Found: 276.1115.
General procedure of synthesis 4-aryl-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinolones 8–10
Freshly distilled cyclopenta-1,3-diene (3 mmol, 0.25 mL) and appropriate aldehydes 4–6 (1 mmol) were added sequentially to a solution of amine 1 or 2 (1 mmol) in ionic liquid (2 mL). The mixture was stirred while room temperature for 0.5 h until the amine disappeared (TLC monitoring). The reaction mixture was treated with water and extracted with CH2Cl2 (3 × 5 mL), the solvent was evaporated, the residue was purified by column chromatography (SiO2, petroleum ether/ethyl acetate, 10:1) to isolate products 8–10.
rel-(3aR*, 4S*, 9bS)-4-((4-trifluoromethyl)phenyl)-8-fluoro-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinoline 8
Yellow solid, m.p. 74–76 °C (hexane). 1HNMR (400 MHz, CDCl3) δ 7.67 (d, J = 8.0 Hz, 2H, H-3', H-5'), 7.59 (d, J = 8.4 Hz, 2H, H-2', H-6'), 6.82 (d, J = 2.8 Hz, 1H, H-9), 6.75 (d, J = 8.4 Hz, 1H, H-7), 6.62 (d, J = 8.8 Hz, 1H, H-6), 5.85 (br.s, w1/2 = 8 Hz, 1H, H1), 5.71 (br.s, w1/2 = 8 Hz, 1H, H-2), 4.68 (d, J = 3.2 Hz, 1H, H-4), 4.13 (d, J = 8.8 Hz, 1H, H-9b), 3.03 (d, J = 8.8 Hz, 1H, H-3a), 1.82 and 2.62 (d, J = 8.8, J = 9.2 Hz, 2H, H-3). 13C NMR described 11b(Tolstikov et al. 2014b).
rel-(3aR*,4S*,9bS*)-4-((3-chlorophenyl)-8-fluoro-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinoline 9
Yellow solid, m.p. 88–90 °C (hexane). 1HNMR (400 MHz, CDCl3) δ 7.52 (s, 1H, H-2'), 7.42 (s, 1H, H-4'), 7.36–7.38 (m, 2H, H-5',6'), 6.86 (d, J = 2.4 Hz, 1H, H-9), 6.79 (d, J = 8.4 Hz, 1H, H-7), 6.60–6.62 (m, 1H, H-6), 5.88 (m, 1H, H-1), 5.74 (m, 1H, H-2), 4.59 (d, J = 3.2 Hz, 1H, H-4), 4.13 (d, J = 8.8 Hz, 1H, H-9b), 3.03 (d, J = 8.8 Hz, 1H, H-3a), 2.66 and 1.88 (d, J = 8.8, J = 9.2 Hz, 2H, H-3). 13C NMR described (Tolstikov et al. 2014b).
rel-(3aR*4S*9bS*)-4-(2-fluorophenyl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinoline 10
Yellow solid, Yield: 79%, m.p.106–108 °C (hexane). 1HNMR (400 MHz, CDCl3) δ 7.66 (t, J = 7.2 Hz, 1H, H-6'), 7.28–7.32 (m, 1H, H-4'), 7.21–7.25 (m, 1H, H-5'), 7.08–7.13 (m, 2H, H-7, H-3'), 7.03–7.06 (m, 1H, H-6), 6.82 (t, J = 7.2 Hz, 1H, H-8), 6.68 (d, J = 7.6 Hz, 1H, H-9), 5.89 (br. s, w1/2 = 8.0 Hz, 1H, H-1), 5.69 (br. s, w1/2 = 7.6 Hz, 1H, H-2), 5.02 (br. s, w1/2 = 10 Hz, 1H, H-4), 4.18 (d, J = 8 Hz, 1H, H-9b), 3.63 (br. s, w1/2 = 16 Hz, 1H, NH), 3.20 (d, J = 8 Hz, 1H, H-3a), 2.63–2.69 and 1.83–1.90 (m, 2H, H-3). 13CNMR (100 MHz, CDCl3) δ 160.14 (d, J = 245 Hz, C-F), 145.53 (C-5a), 134.15 (C-1), 130.19 (C-2), 129.97 (d, J = 12 Hz, C-1'), 129.04 (C-7), 128.46 (d, J = 8 Hz, C-4'), 127.24 (d, J = 4 Hz, C-6'), 126.34 (C-6), 126.25 (C-9a), 124.22 (d, J = 3 Hz, C-5'), 119.47 (C-8), 116.09 (C-9), 115.25 (d, J = 21 Hz, C-3'), 51.04 (C-4), 46.19 (C-9b), 43.35 (C-3a), 31.76 (C-3). HRMS (ESI-TOF) m/z [M+] Calculated for C18H16FN: 265.1266; Found: 265.8655.
rel-(3aR*,4S*,11bS*)-4-[4-(trifluoromethyl)phenyl]-3a,4,5,11b-tetrahydro-3H-cyclopenta[c][1,7]phenanthroline 13c
Freshly distilled cyclopenta-1,3-diene (3 mmol, 0.25 mL) and aldehyde 4 (1 mmol, 0.14 mL) were added sequentially to a solution of amine 11 (1 mmol, 144 mg) in ionic liquid (2 mL). The mixture was stirred while room temperature for 0.5 h until the amine disappeared (TLC monitoring). The reaction mixture was treated with water and extracted with CH2Cl2 (3 × 5 mL), the solvent was evaporated, the residue was purified by column chromatography (SiO2, petroleum ether/ethyl acetate, 10:1) to isolate product 13c. Yellow solid, Yield 81%, m.p. 120–122 °C (hexane). 1HNMR (400 MHz, CDCl3) δ 8.84 (d, J = 3.2 Hz, 1H, H-8), 8.16 (d, J = 8.0 Hz, 1H, H-6), 7.69 (d, 4H, J = 8.8 Hz, H-2',3',5',6'), 7.59 (d, J = 8.8 Hz, 1H, H-10), 7.46 (d, J = 8.8 Hz, 1H, H-11), 7.35 (d, J = 8.0 Hz, 1H, H-7), 5.99 (m, 1H, H-1), 5.70 (m, 1H, H-2), 4.84 (d, J = 2.4 Hz, 1H, H-4), 4.31 (d, 1H, J = 8.4 Hz, H-11b), 3.14 (d, J = 8.4 Hz, 1H, H-3a), 2.69 and 1.88 (d, 2H, J = 8.4 Hz, H-3). HRMS (ESI-TOF) m/z [M+] Calculated for C22H17F3N2: 366.1343; Found: 366.7878, 13C NMR described (Tolstikov et al. 2014a).16
4,7-Bis-(4-(trifluoromethyl)phenyl)-3,3a,4,5,6,7,7a,8,10a,12b-decahydrodicyclopenta[c,i]-1,10-phenantroline 15
Freshly distilled cyclopenta-1,3-diene (6 mmol, 0.5 mL) and aldehyde 4 (2 mmol, 0.28 mL) were added sequentially to a solution of amine 12 (1 mmol, 108 mg) in ionic liquid (2 mL). The mixture was stirred while room temperature for 0.5 h until the amine disappeared (TLC monitoring). The reaction mixture was treated with water and extracted with CH2Cl2 (3 × 5 mL), the solvent was evaporated, the residue was purified by column chromatography (SiO2, petroleum ether/ethyl acetate, 10:1) to isolate product 15. Yield: 64%, yellow solid, m.p. 108–110 °C. 1HNMR (500 MHz, CDCl3) δ 7.61–7.66 м (8H, Ar), 6.73 and 6.67 (s, 2H, H-11,12), 5.92 and 5.89 (m, 2H, H-1,10), 5.66 (m, 2H, H-2,9), 4.73 and 4.59 (s, 2H, H-4,7), 4.16 and 4.14 (d, J = 9 Hz, 2H, H-10a,12b), 3.11 and 2.98 (m, 2H, H-3a,7a), 2.59, 2.49, 1.97 and 1.80 (m, 4H, H-3,8). HRMS (ESI-TOF) m/z [M + H]+ Calculated for C32H26F6N2: 553.2073; Found: 553.3938, 13C NMR described (Savchenko et al. 2022).
Conclusion
Thus, this paper addresses eco-friendly approaches to the synthesis of new tetrahydroquinoline compounds 7–10. The biological assays of the products carried out using the model Musca domestica insect and analysis of molecular docking solutions make it possible to consider the prepared compounds as potential EcR-USP agonists.
References
Ali N, Hamid M, Putra N, Adol H, Mirza A, Usman A, Siddiquee T, Hoq MdR (2020) Efficient eco-friendly syntheses of dithiocarbazates and thiosemicarbazones. Green Chem Lett Rev 13:129–140. https://doi.org/10.1080/17518253.2020.1737252
Anastas P, Eghbali N (2010) Green chemistry: principles and practice. Chem Soc Rev 39:301–312. https://doi.org/10.1039/b918763b
Bendale P, Olepu S, Suryadevara PK, Bulbule V, Rivas K, Nallan L, Smart B, Yokoyama K, Ankala S, Pendyala PR, Floyd D, Lombardo LJ, Williams DK, Buckner FS, Chakrabarti D, Verlinde CLMJ, Van Voorhis WC, Gelb MH (2007) Second generation tetrahydroquinoline-based protein farnesyltransferase inhibitors as antimalarials. J Med Chem 50:4585–4605. https://doi.org/10.1021/jm0703340
Billas IML, Iwema T, Garnier J-M, Mitschler A, Rochel N, Moras D (2003) Structural adaptability in the ligand-binding pocket of the ecdysone hormone receptor. Nature 426:91–96. https://doi.org/10.1038/nature02112
Bortolami M, Mattiello L, Scarano V, Vetica F, Feroci M (2021) In situ anodically oxidized [bmim-BF4]: a safe and recyclable BF3 source. J Org Chem 86:16151–16157. https://doi.org/10.1021/acs.joc.1c00932
Browning C, McEwen AG, Mori K, Yokoi T, Moras D, Nakagawa Y, Billas IML (2021) Nonsteroidal ecdysone receptor agonists use a water channel for binding to the ecdysone receptor complex EcR/USP. J Pest Sci 46:88–100. https://doi.org/10.1584/jpestics.D20-095
Cai M-J, Zhao W-L, Jing Y-P, Song Q, Zhang X-Q, Wang J-X, Zhao X-F (2016) 20-Hydroxyecdysone activates forkhead box o to promote proteolysis during helicoverpa armigera molting. Development 143:1005–1015. https://doi.org/10.1242/dev.128694
Celik H, Kuzu M (2019) Microwave assisted synthesis of N-(methyl and methoxy) benzylidene-4-fluoroaniline derivatives and their carbonic anhydrase I and II inhibition properties. Org Commun 12:210–216. https://doi.org/10.25135/acg.oc.66.19.07.1327
Chander S, Ashok P, Zheng Y-T, Wang P, Raja KS, Taneja A, Murugesan S (2016) Design, synthesis and in vitro evaluation of novel tetrahydroquinoline carbamates as HIV-1 RT inhibitor and their antifungal activity. Bioorg Chem 64:66–73. https://doi.org/10.1016/j.bioorg.2015.12.005
Chavan P, Pansare D, Jadhav S, Rai M (2019) Synthesis and biological activities of new tetrahydroquinoline and pyrimidine derivatives. Eur Chem Bull 8:257. https://doi.org/10.17628/ecb.2019.8.257-264
Chen C, Zingales S, Wang T, Yuan M, Wang D, Cai L, Jiang Q (2016) Synthesis and in vitro evaluation of 4-substituted furano[3,2-c] tetrahydroquinolines as potential anticancer agents. J Enzym Inhib Med Chem 31:853–858. https://doi.org/10.3109/14756366.2015.1064120
CrysAlisPRO (2012) CrysAlis PRO (2012), (revision 1.171.37.35) A.T.L., Yarnton, Oxfordshire.
Dayal N, Wang M, Sintim HO (2020) HSD1787, a tetrahydro-3H-pyrazolo[4,3-f]quinoline compound synthesized via Povarov reaction, potently inhibits proliferation of cancer cell lines at nanomolar concentrations. ACS Omega 5:23799–23807. https://doi.org/10.1021/acsomega.0c03001
Diaz G, Miranda I, Sartori S, Dias G, Kohlhoff M, Purgato G, Nogueira M (2018) Unprecedented one-pot sequence for the synthesis of tetrahydroquinoline alkaloids and preliminary evaluation of their antibacterial activity. J Brazil Chem Soc 29:2646–2656. https://doi.org/10.21577/0103-5053.20180145
Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H (2009) OLEX2: a complete structure solution, refinement and analysis program. J Appl Crystallogr 42:339–341. https://doi.org/10.1107/S0021889808042726
Frisch MJTGW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF et al (2009) Gaussian 09, Revision D.01 // Gaussian Inc., Wallingford CT
Fuhrmann J, Rurainski A, Lenhof H-P, Neumann D (2010) A new lamarckian genetic algorithm for flexible ligand-receptor docking. J Comp Chem 31:1911–1918. https://doi.org/10.1002/jcc.21478
Gaillard T (2018) Evaluation of AutoDock and AutoDock Vina on the CASF-2013 benchmark. J Chem Inf Model 58:1697–1706. https://doi.org/10.1021/acs.jcim.8b00312
Ghashghaei O, Masdeu C, Alonso C, Palacios F, Lavilla R (2018) Recent advances of the Povarov reaction in medicinal chemistry. Drug Discov Today Technol 29:71–79. https://doi.org/10.1016/j.ddtec.2018.08.004
Glushkov VA, Tolstikov AG (2008) Synthesis of substituted 1,2,3,4-tetrahydroquinones by the Povarov reaction. New potentials of the classical reaction. Russ Chem Rev 77:137–159. https://doi.org/10.1070/rc2008v077n02abeh003749
Goodsell DS, Olson AJ (1990) Automated docking of substrates to proteins by simulated annealing. Proteins Struct Funct Bioinform 8:195–202. https://doi.org/10.1002/prot.340080302
Gosmini R, Nguyen VL, Toum J, Simon C, Brusq J-MG, Krysa G, Mirguet O, Riou-Eymard AM, Boursier EV, Trottet L, Bamborough P, Clark H, Chung C-w, Cutler L, Demont EH, Kaur R, Lewis AJ, Schilling MB, Soden PE, Taylor S, Walker AL, Walker MD, Prinjha RK, Nicodème E (2014) The discovery of I-BET726 (GSK1324726A), a potent tetrahydroquinoline ApoA1 up-regulator and selective BET bromodomain inhibitor. J Med Chem 57:8111–8131. https://doi.org/10.1021/jm5010539
Hanashalshahaby EHA, Unaleroglu C, Can AAK, Ozgun A, Garipcan B (2019) Design, synthesis, and antitumor evaluation of novel methylene moiety-tethered tetrahydroquinoline derivatives. Turk J Chem 43:1552–1569. https://doi.org/10.3906/kim-1907-71
Hou Y, Wang X-L, Saha TT, Roy S, Zhao B, Raikhel AS, Zou Z (2015) Temporal coordination of carbohydrate metabolism during mosquito reproduction. PLoS Genet 11:e1005309. https://doi.org/10.1371/journal.pgen.1005309
Kerner C, Straub S-D, Sun Y, Thiel WR (2016) A rapid and additive-free ruthenium-catalyzed reductive amination of aromatic aldehydes. Eur J Org Chem 2016:3060–3064. https://doi.org/10.1002/ejoc.201600515
Kerzmann A, Fuhrmann J, Kohlbacher O, Neumann D (2008) ChemInform Abstract: BALLDock/SLICK: a new method for protein-carbohydrate docking. ChemInform. https://doi.org/10.1002/chin.200848212
Khairutdinov B, Ermakova E, Sitnitsky A, Stoikov I, Zuev Y (2014) Supramolecular complex formed by DNA oligonucleotide and thiacalix[4]arene. NMR-spectroscopy and molecular docking. J Mol Struct 1074:126–133. https://doi.org/10.1016/j.molstruc.2014.05.073
Kimura T, Suga T, Kameoka M, Ueno M, Inahashi Y, Matsuo H, Iwatsuki M, Shigemura K, Shiomi K, Takahashi Y, Ōmura S, Nakashima T (2019) New tetrahydroquinoline and indoline compounds containing a hydroxy cyclopentenone, Virantmycin B and C, produced by Streptomyces sp. AM-2504. J Antibiot 72:169–173. https://doi.org/10.1038/s41429-018-0117-0
Kitamura S, Harada T, Hiramatsu H, Shimizu R, Miyagawa H, Nakagawa Y (2014) Structural requirement and stereospecificity of tetrahydroquinolines as potent ecdysone agonists. Bioorg Med Chem Lett 24:1715–1718. https://doi.org/10.1016/j.bmcl.2014.02.043
Kumar A, Srivastava S, Gupta G, Chaturvedi V, Sinha S, Srivastava R (2011) Natural product inspired diversity oriented synthesis of tetrahydroquinoline scaffolds as antitubercular agent. ACS Comb Sci 13:65–71. https://doi.org/10.1021/co100022h
Li L-P, Cai X, Xiang Y, Zhang Y, Song J, Yang D-C, Guan Z, He Y-H (2015) The α-chymotrypsin-catalyzed Povarov reaction: one-pot synthesis of tetrahydroquinoline derivatives. Green Chem 17:3148–3156. https://doi.org/10.1039/c4gc01123f
Macrae CF, Sovago I, Cottrell SJ, Galek PTA, McCabe P, Pidcock E, Platings M, Shields GP, Stevens JS, Towler M, Wood PA (2020) Mercury 4.0: from visualization to analysis, design and prediction. J Appl Crystallogr 53:226–235. https://doi.org/10.1107/S1600576719014092
Martínez S, Pavani C, Baptista M, Becerra M, Quevedo M, Ribone S (2019) Identification of the potential biological target of N-benzenesulfonyl-1,2,3,4-tetrahydroquinoline compounds active against Gram-positive and Gram-negative bacteria. J Biomol Struct Dyn 38:1–18. https://doi.org/10.1080/07391102.2019.1633410
Mendes CC, Mirth CK (2016) Stage-specific plasticity in ovary size is regulated by insulin/insulin-like growth factor and ecdysone signaling in Drosophila. Genetics 202:703–719. https://doi.org/10.1534/genetics.115.179960
Mert-Balci F, Imrich H-G, Conrad J, Beifuss U (2013) Influence of guanidinium salts and other ionic liquids on the three-component aza-Diels–Alder reaction. Helv Chim Acta 96:1681–1692. https://doi.org/10.1002/hlca.201200655
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comp Chem 30:2785–2791. https://doi.org/10.1002/jcc.21256
Morris GM, Huey R, Olson AJ (2008) Using AutoDock for ligand-receptor docking. Curr Protoc Bioinform 24:8.14.1-8.14.40. https://doi.org/10.1002/0471250953.bi0814s24
Muthukrishnan I, Sridharan V, Menéndez JC (2019) Progress in the chemistry of tetrahydroquinolines. Chem Rev 119:5057–5191. https://doi.org/10.1021/acs.chemrev.8b00567
Onyedibe KI, Dayal N, Sintim HO (2021) SF5- and SCF3-substituted tetrahydroquinoline compounds as potent bactericidal agents against multidrug-resistant persister Gram-positive bacteria. RSC Med Chem 12:1879–1893. https://doi.org/10.1039/D1MD00211B
Ozaki T, Sugiyama R, Shimomura M, Nishimura S, Asamizu S, Katsuyama Y, Kakeya H, Onaka H (2019) Identification of the common biosynthetic gene cluster for both antimicrobial streptoaminals and antifungal 5-alkyl-1,2,3,4-tetrahydroquinolines. Org Biomol Chem 17:2370–2378. https://doi.org/10.1039/c8ob02846j
Petronijević J (2017) An enolate ion as a synthon in biocatalytic synthesis of 3,4-dihydro-2(1H)-quinoxalinones and 3,4-dihydro-1,4-benzoxazin-2-ones: lemon juice as an alternative to hazardous solvents and catalysts. Green Chem 19:707–715. https://doi.org/10.1039/c6gc02893d
Povarov LS (1967) αβ-Unsaturated ethers and their analogues in reactions of diene synthesis. Russ Chem Rev 36:656. https://doi.org/10.1070/RC1967v036n09ABEH001680
Rogers RD, Seddon KR (2003) Ionic liquids as green solvents. American Chemical Society, Washington
Rurainski A, Hildebrandt A, Lenhof H-P (2009) A consensus line search algorithm for molecular potential energy functions. J Comput Chem 30:1499–1509. https://doi.org/10.1002/jcc.21175
Savchenko RG, Kostyleva SA, Odinokov VN, Akhmetkireeva TT, Benkovskaya GV (2015) Stress-and geroprotective properties of 20-hydroxyecdysone and its derivatives. Adv Gerontol 5:247–251. https://doi.org/10.1134/S2079057015040190
Savchenko RG, Limantceva RM, Khursan SL, Mescheryakova ES, Tolstikov AG, Odinokov VN (2022) Towards understanding the regioselectivity of the one-pot reaction of phenylenediamines with aldehydes and cyclopentadiene (Povarov reaction). Combined experimental and theoretical approaches. J Heterocyclic Chem 59:2025–2036. https://doi.org/10.1002/jhet.4540
Sheldon RA (2005) Green solvents for sustainable organic synthesis: state of the art. Green Chem 7:267–278. https://doi.org/10.1039/b418069k
Sheldrick G (2008) A short history of SHELX. Acta Crystallogr A 64:112–122. https://doi.org/10.1107/S0108767307043930
Tolstikov AG, Savchenko RG, Lukina ES, Limantseva RM, Odinokov VN (2014a) Synthesis of 6-aryl-6,6a,7,9a-tetrahydro-5H-cyclopenta[c] 1,7-and-1,8-phenanthrolines. Russ Chem Bull 63:2077–2080. https://doi.org/10.1007/s11172-014-0704-6
Tolstikov AG, Savchenko RG, Lukina ES, Nedopekin DV, Limantceva RM, Khalilov LM, Mescheryakova ES, Odinokov VN (2014b) Synthesis of 4-aryl-8-fluoro-3a,4,5,9b-tetrahydro-3H-cyclopenta [c] quinolines and their ozonides. Helv Chim Acta 97:1317–1325. https://doi.org/10.1002/hlca.201300456
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comp Chem 31:455–461. https://doi.org/10.1002/jcc.21334
Ueno M, Yokoi T, Nakagawa Y, Miyagawa H (2021) Receptor-binding affinity and larvicidal activity of tetrahydroquinoline-type ecdysone agonists against Aedes albopictus. J Pest Sci 46:101–108. https://doi.org/10.1584/jpestics.D20-089
Van Aken K, Strekowski L, Patiny L (2006) EcoScale, a semi-quantitative tool to select an organic preparation based on economical and ecological parameters. Beilstein J Org Chem 2:3. https://doi.org/10.1186/1860-5397-2-3
Van Voorhis WC, Rivas KL, Bendale P, Nallan L, Hornéy C, Barrett LK, Bauer KD, Smart BP, Ankala S, Hucke O, Verlinde CLMJ, Chakrabarti D, Strickland C, Yokoyama K, Buckner FS, Hamilton AD, Williams DK, Lombardo LJ, Floyd D, Gelb MH (2007) Efficacy, pharmacokinetics, and metabolism of tetrahydroquinoline inhibitors of Plasmodium falciparum protein farnesyltransferase. Antimicrob Agents Chem 51:3659–3671. https://doi.org/10.1128/AAC.00246-07
Yokoi T, Nakagawa Y, Miyagawa H (2019) Asymmetric synthesis of tetrahydroquinoline-type ecdysone agonists and QSAR for their binding affinity against Aedes albopictus ecdysone receptors. Pest Manag Sci 75:115–124. https://doi.org/10.1002/ps.5160
Acknowledgements
The authors thank the Russian Foundation for Basic Research (Grant No. 20-03-00649) for financial support. A part of the studies was carried out in accordance with the Federal Program FMRS-2022-0081.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Savchenko, R.G., Limantseva, R.M., Benkovskaya, G.V. et al. Eco-friendly synthesis of substituted tetrahydroquinolines as potential ecdysone receptor agonists. Chem. Pap. 77, 5495–5506 (2023). https://doi.org/10.1007/s11696-023-02880-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-023-02880-7