Abstract
The phase-out of long-chain C8-based perfluorochemicals necessitates the textile industry to find the best alternatives to these durable oil- and water-repellent chemistries. Finding out the alternatives has resulted in a textile market where both fluorine-based and fluorine-free durable water repellent (DWRs) are available. These DWR substitutes depending upon their chemistries are categorized into four major groups: long-chain fluorinated polymers, hydrocarbons including the long-chain fatty acids, silicones, and inorganic nanoparticles. This paper discusses various DWRs regarding their structure, properties, performance, loss, and degradation processes and their effect on the habitat and human health. The alternative DWRs lack performance compared to fluorinated finishes and, most importantly, oil repellence. Degradation products from all DWRs alternatives diffuse to the environment. Research shows that hydrocarbon-based DWRs are the most environment friendly (including long-chain fatty acids), followed by silicone-based DWRs and fluorinated polymer-based DWR finishes. Industrial commitments will play an important role in reducing impurities from available DWR chemistries. Before approaching environment-friendly alternatives of required performance, it is better to select DWR finishes on a specific requirement and always counter the benefits of enhanced performance against the potential threats to the ecology and human beings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nature is the key commander to human beings in inventing different technologies. The development in science and technology is remarkable with the onset of the twenty-first century. However, they have come with a price of increased global warming that led to sudden changes in climate. To sustain and be effective in a certain environment, we need appropriate apparel for working in such a harsh environment. As humans constantly search for excellence, the traditional textile paradigm shifted to multipurpose and functional textiles. Oil and water-repellent fabrics are engineered to save the wearer from outdoor weather conditions. The demand for modern functional textiles has increased daily, leading to the need for new technologies and materials (Saratale et al. 2020; Fahmy et al. 2017).
Cotton fabric is soft, comfortable, easy to handle, inexpensive, and biodegradable (Gao et al. 2016; Pan et al. 2019; Biltekin and Ayça 2019). Due to these specular properties, it has become the most promising and widely used material in the textile and cosmetic industry (Berendjchi et al. 2011; Bhuiyan et al. 2017; Mohsin et al. 2016a). However, it lacks oil and water repellency is one of the biggest drawbacks limiting its usage (Rabia et al. 2020). To make cotton fabric oil and water repellent, efforts have been continued by modifying cotton fabric chemically from the 1980s (Singh and Singh 2017; Han and Min 2020). However, these modifications should meet the criterion of green chemistry without disturbing its remarkable performance (Ferrero et al. 2017). Oil and water repellency (Xiong et al. 2012; Onar et al. 2015; Sharif et al. 2022a), ultraviolet protection (Stan et al. 2019), flame retardancy (Faheem et al. 2019) (Yang and Chen 2019; Bentis et al. 2020), antibacterial properties, self-cleaning (Chauhan et al. 2019; Vasileva-Tonkova et al. 2019), and wrinkle resistance are the other major functional properties desired for cotton fabric. These pivotal functional properties prodigiously increase their utilization in rainwear, sportswear, industrial end-products, medical equipment, and household applications (Schellenberger et al. 2019; Suryaprabha and Sethuraman 2017). Hydrophobic and oleophobic fabric can be obtained by applying paraffin repellents (Bashari and A. H. Salehi K, and N. Salamatipour 2020), silicone-based repellents (Khattab et al. 2020), acrylate backbone repellents, stearic acid–melamine repellents and fluorocarbon-based repellents, mainly C4-, C6-, and C8-based chemistries (Fahmy et al. 2017). A huge variety of traditional oil and water-repellent finishes are available in the market, as shown in Fig. 1. However, most are costly, toxic, hazardous to the environment, non-durable, or inefficient. The most promising, durable, and performance-efficient oil and water repellent are C8-based perfluorinated compounds. However, their use is under immense pressure as PFC’s (perfluorocarbons) compounds release PFOA (perfluorooctanoic acid) and PFOS (perfluorooctanesulfonic acid) on degradation. They are toxic and carcinogenic for humans and the environment (Zhao et al. 2012, 2016; Gargoubi et al. 2020). This review paper will provide a road map for the industry and the researchers to find possible ways forward.
Market share of repellent finishes
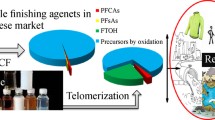
Among all chemicals produced worldwide, the share of the textile sector is almost 25% (Muthu 2015). The textile industry requires about 40% of the total chemicals as a finishing agents for clothing and apparel, as shown in Fig. 2. The size and global market share of textile finishing agents were around USD 9.2 billion in 2021 and are expected to get value to around USD 12.4 billion by 2026. Furthermore, it is envisioned to increase with a CAGR (compound annual growth rate) of 7.5% from 2021–2026. Figure 3 (MMR 2021) depicts the global market share of repellent finishes in 2021. Moreover, the usage of repellent finishes in Asia was the topmost, with a market share of 50%, as calculated in 2018.
With the increased usage of textile goods and chemicals worldwide, manufacturers and industries have come under severe scrutiny as they have adverse and hazardous effects on the environment and ecology (Senthil Kumar and Gunasundari 2018). Durable water and oil repellents (DWRs) are popular finishes coated onto the fabric to protect it from the soil, water, and oil. They also increase the life of the fabric. DWR finishes are mainly fluorinated C8-based polymers that exhibit water repellency and excellent oil repellency (Schellenberger et al. 2018; Liu et al. 2019). However, they degrade into long-chain PFOA and PFOS, which were brought to major concerns due to their adverse and toxic effects on the environment and human beings (Atav and Bariş 2016; Veen et al. 2016; Ma et al. 2018). Numerous efforts were made to eliminate these fluorine-based compounds (Onar and Mete 2016). Hence, many countries banned using these long-chain C8 fluorocarbons (Zhou et al. 2011).
In 2011, a commitment was made by Zero Discharge of Hazardous Chemicals (ZDHC) group member brands to eliminate fluorinated DWR until 2020. Moreover, ZDHC enlisted fluorinated chemicals in the 11 priority chemicals list banned in process industries and international textile goods (ZDHC 2022). ZDHC also approached chemical manufacturers, industries, and regulatory agencies to find efficient, durable, and environment-friendly alternative short-chain fluorocarbons and fluorine-free DWR technology. As society's needs and demand for satisfaction rise higher every day, the functional performance of finishes is not the only interest nowadays.
People are also concerned about environmental and health issues along with outstanding and durable easy-care properties of the finishes. Thus, future textile industry trends are developing green products that do not harm human beings or the environment. In this pathway, famous worldwide brands started working to make their product ‘green,’ such as Levi Strauss & Co. was using PFCs to make their products water-resistant. However, from January 2016 onward, they eliminated the use of PFCs from their products and achieved water repellency as they started working on a safer alternative for humans and the environment. Another famous American brand, Zara, eliminated zero discharge of hazardous chemicals from its production chain in 2020 and moved toward product sustainability before the end of 2025. Moreover, Adidas also discontinued the usage of PFCs in their outdoor product in December 2017. Other well-known brands such as H&M, Nike, and Patagonia have provided PFC’s free end-user products since 2020 (Patra and Pariti 2022).
Research now focuses on short-chain C4-based fluorocarbons free from PFOA and PFOS. They are a comparatively safer alternative to conventional C6- and C8-based fluorocarbons (Atav 2018; Mehta 2018). However, these short-chain fluoropolymers are less performance efficient, do not degrade easily, and are still hazardous for humans and the environment (Shabbir 2019). So, the requirement is fluorine-free chemistries to figure out the concerns. Some non-fluorinated water repellents are aluminum and zirconium compounds (Abidi 2018), paraffin waxes (Abo-Shosha et al. 2008), silicon repellents (Kasapgil et al. 2019), and polyurethane-based repellents (Bhuiyan et al. 2019). However, either they are toxic, non-durable, or less efficient. The long alkyl chain is a possible alternative to fluorocarbons (Liu et al. 2019). Long-chain fatty acids such as stearic acid (Zhang et al. 2019) and palmitic acid (Lee et al. 2014; Sharif et al. 2022b) are bio-based. They can impart water repellency by modifying the surface of cotton fabric, but their effects are much lower than fluorocarbons.
Mechanism of oil and water repellency
When a liquid droplet spreads on the textile substrate, the wetting phenomenon occurs due to the intermolecular interactions between the fabric surface and the liquid droplet. The various finishes with low surface energy or surface tension increase the oil and water repellency (Zhao et al. 2020; Ren and Zhao 2010). These finish coatings prevent the spreading of liquid droplets on the fabric surface (Zisman 1964; Schindler and Hauser 2004; Jung et al. 2020). The critical surface tension of the fabric surface should be less than the surface tension of the oil or water to repel them. The surface tension of water is 72 mN/m (millinewtons per meter) at 20 ℃, whereas the surface tension of oil ranges between 15 and 30 mN/m. Therefore, repellent finishes should lower the surface tension of treated cotton fabric below these limits for respective oil and water repellency. The long-chain hydrocarbon-based repellent coatings lower the surface energy of the treated cotton fabric to around 50 mN/m. Therefore, they can impart only appreciable water repellency with zero oil repellency. Fluorochemical finishes lower the surface energy of the coated fabric to below 30 mN/m and can exhibit sufficient oil and water repellency (Kissa 2001; Rastogi et al. 2013; Dekanić et al. 2018). Various application methods apply oil and water-repellent coatings onto the fabric, such as mechanical applications, chemical reactions, and coating films. Water- and oil-repellent finishes decrease the wettability of the fabric surface. Wettability can be obtained by evaluating the contact angle between a liquid droplet and a fabric surface at their interface. A contact angle of > 90° shows that the substrate is hydrophobic, and a contact angle of < 90° shows that the substrate is hydrophilic, as shown in Fig. 4.
The connection between wettability and contact angle (Gomes et al. 2013)
Water-repellent finishes
Various textile commodities like raincoats, bags, kitchen covers, and athletic wears require a water-repellent finish to make them easy to wear and durable. This can be achieved by using durable water-repellent (DWR) finishes. Among DWR, the most remarkable are fluorocarbon finishes. DWR finishes are long-chain perfluorinated chemistries having oil-, water-, and soil-repellent characteristics. These include chemicals plasma, perfluorooctanoic acid (C8), perfluorohexanoic acid (C6), fatty acids, dendrimers, silicone, paraffin waxes, and nanomaterials. The DWR finishes from textiles coated with DWR finishes diffuse to the water through leaching, wear, and tear and during the laundry and drying process. DWR chemicals also diffuse into the air through evaporation (Holmquist et al. 2016). The loss mechanism during the use of fabric is represented in Fig. 5. Some water- and oil-repellent finishes are given in Table 1.
Release of DWR chemicals during a usage phase of the garment (Holmquist et al. 2016)
Nanotechnology approaches in finishing methods
The nanotechnology finishing methods can be a good method to improve the oil and water repellency and many other characteristics of cotton fabrics through various nanomaterials (Soane et al. 2003). Silanol-based ZnO nanoparticles were applied on cellulose activated via copolymerization of glycidyl methacrylate and acrylic acid (Mohamed et al. 2014; Shabbir and Mohammad 2017). The drop test resulted in greater than 30 min, thus inducing super-hydrophobicity to the cotton fabric. Ghasemi et al. (2018) prepared the superhydrophobic and antimicrobial cotton fabric by combining ZnO nanoparticles and octadecanethiol. The presence of a newly developed finish was confirmed by SEM analysis. Hydrophobicity imparted by the applied finish onto the cotton fabric was measured by the contact angle greater than 161˚. Furthermore, the reduced surface energy of the fabric was also responsible for the reduction in bacterial attacks.
Multifunctional cotton fabric such as hydrophobic, photochromic, crease-resistant, oleophobic antibacterial, and ultraviolet protection was made by coating it with a mixture of silica nanoparticles and spirooxazine followed by alkylsilane compounds. The homogenous coating application onto the cotton fabric was affirmed by scanning electron microscope and X-rays mapping (Ayazi-Yazdi et al. 2017). The finished cotton fabric depicted substantial hydrophobicity with a contact angle of 141°. The finished cotton fabric also indicates antimicrobial activity and ultraviolet blocking properties.
The cotton fabric was cationized using diallyl dimethyl ammonium chloride (DADMAC). Then, the cationized cotton fabric was treated with an aqueous solution of BTCA (butanetetracarboxylic acid), SHP (sodium hypophosphite), 0.5% TiO2, and 0.5% of SiO2. The fabric was then finished with stearic acid to further reduce the surface tension of the cotton fabric. Later, the treated fabric was assessed for hydrophobicity, air permeability, tensile strength, UV protection, and antibacterial activity, while SEM and XRD (Soane et al. 2003) characterized coated fabric.
Despite having the potential for oil and water repellency, nanotechnology is under scrutiny as it can pose risks to ecology, human safety, and health (Almeida and Ramos 2017). Moreover, nanomaterials are costly, can threaten mammals, and trigger breathing issues (Siegfried and Som 2007), as shown in Fig. 6. The above-mentioned issues restricted the use of nanotechnology in oil and water repellency (Jia et al. 2017).
Effects of nanomaterials on the human body (Saleem and Zaidi 2020)
Fluorocarbons finishes
The fluorocarbon finishes started in 1960 and became the most popular in the last decade of the twentieth century. The rise in consumer need for multifunctional textiles (e.g., water-, oil-, and soil-repellent textiles) stimulated the growth of fluorocarbon finishes (Moilliet 1963; Audenaert et al. 1999). These finishes provide outstanding oil, water, and soil repellency (Liu et al. 2019). A surface energy lower than 18 mN/m can be achieved by applying these finishes (Ma et al. 2018). The unique characteristics of fluorocarbons make them suitable for various applications (Sharif et al. 2022b; Almeida and Ramos 2017; Siegfried and Som 2007) and the most effective oil and water-repellent finish for textiles (Luo et al. 2020; Mazrouei-Sebdani and Khoddami 2011; Türk et al. 2015). In 1953, fluorocarbons as oil and water repellent, namely Scotchgard, had invented accidentally when few drops of fluorocarbon spill over Joan Mullin a 3 M Technician’s tennis shoes (Audenaert et al. 1999). Till now Scotchgard is one of the best oil and water repellent.
An 8–10 perfluorinated carbon chain and CF3 as a terminal group are enough to achieve good oil and water repellency (Schindler and Hauser 2004). Uniform distribution of repellent, appropriate orientation, chemical structure, chain length of fluorocarbon, amount of applied finish, origin, and geometry of cotton fabric will determine the repellency rating of fluorocarbon finishes. Generally, perfluorinated acrylate having an acrylic backbone is an effective and durable oil and water repellent, as shown in Fig. 7 (Mohsin et al. 2013). There are several commercially available fluorocarbon products in the market, namely Scotchgard, Teflon, Nuva, and Asahigaurd by the companies 3 M, Teflon, Clariant, and Asahi, respectively.
Acrylic backbone of perfluorinated compounds (Mohsin et al. 2016b)
Highly fluorinated finishes provide a higher water and oil-repellency rating (Ferrero and Periolatto 2013). A group of researchers achieved outstanding water repellency with a water contact angle greater than 150° by using fluorinated polymers on cotton fabrics. The well-known fluorinated water and oil-repelling agents are fluorocarbon finishes that reduce the cotton fabrics' surface energy (Shi et al. 2013). However, the long-chain perfluorocarbon having carbons atoms greater than seven, such as perfluorooctanoic acid, has serious health related issues (Zhao et al. 2012, 2016; Ye et al. 2013). Hence, these long-chain finishes (e.g., C8 fluorocarbons) are restricted in many countries due to customer awareness and pressure from several social organizations (Zhou et al. 2011).
PFCs are mostly applied onto the fabric by the pad-dry-cure method. Curing treatments are responsible for the orientation of the fluorocarbon chain onto the fabric, which is most important in attaining optimum repellency. Washing of fabric changes the orientation of fluorocarbons applied onto the cotton fabric, but re-orientation can be achieved after ironing. Furthermore, there are finishes available in the market that can be re-oriented in air drying.
The performance of fluorocarbon finishes reduced remarkably after successive washing. However, washing durability can be increased by adding cross-linkers to the recipe (Mohsin et al. 2013). N-methylol agents were used as an effective and economical cross-linker for fluorocarbon finishes. Despite this, they release formaldehyde which limits their use (Yang and Chen 2019). Therefore, several formaldehyde-free cross-linkers are part of past and current research. Polycarboxylic acids are the most promising and potential replacement for formaldehyde-based cross-linkers (Xiao et al. 2018).
Environmental aspects of fluorocarbon finishes
The literature has reported that fluorocarbons based on C8 chemistry are excellent textile finishes to impart exceptional oil and water repellency. However, these finishes harm humans and the environment (Sunderland et al. 2019; Mazzon et al. 2019). They are carcinogenic and toxic to the reproduction system (Lei et al. 2017) and cause many other diseases, such as immune toxicity, chronic kidney disease, liver tumor, and behavioral disorders (Jian et al. 2017). Therefore, C8 chemistry and its harbingers have been withdrawn completely from the textile industry since 2015. Moreover, C8- and C6-based fluorocarbons are part of 11 priority chemicals that ZDHC banned in the textile industry (ZDHC). Accordingly, scientists and commercial manufacturers are interested in finding non-bio-accumulative environment- and human-friendly short-chain fluorocarbons.
Existing substitutes to C8-based fluorocarbons are less hazardous short-chain C6-based fluorocarbons (Atav and Bariş 2016). However, they lack in durability and oil and water repellency. Also, the research is now focusing on developing C2 or C4-based oil and water-repellent finishes for the textiles. A company, “3 M, has developed new C4-based fluorocarbons”. Even though the short-chain alternatives seem to replace the long-chain repellents effectively, they are less efficient than long-chain perfluorinated finishes (Gargoubi et al. 2020). Though the short-chain fluorocarbons (e.g., C6 or C4 fluorocarbon) are less dangerous to the ecology, the reduction in the carbon chain made them less effective (Atav 2018; Mehta 2018). This short-chain fluorocarbon finishes exhibit poor performance and durability.
Moreover, they are persistent, do not degrade easily, and remain a potential threat to humans and the ecology (Mehta 2018; Shabbir 2019). So novel research is on the roadmap to find the best alternative for these fluorinated chemistries that should be fluorine-free, eco, and human-friendly finishes as oil and water repellents for textiles. So, researchers are endeavoring to prepare hydrophobic and oleophobic cotton surfaces with multifunction properties.
Fatty acids—stearic acid and the palmitic acid
Fatty acids such as stearic and palmitic acid could be used as an alternatives to fluorinated finishes (Suryaprabha and Sethuraman 2017; Xue et al. 2010; Zahid et al. 2017; Cai et al. 2018). The fatty acid undergoes an esterification reaction with cellulose without degradation (Vaca-Garcia et al. 1998; Yidong and N. A. N., 2016). Stearic acid and palmitic acid can improve the water repellency of cotton fabric (Pan et al. 2019; Sharif et al. 2021; Patil and Netravali 2019; Bashiri Rezaie et al. 2019). These fatty acids are bio-based and can be used as water-repelling agents (Sharif et al. 2022a; Zhang et al. 2019; Lee et al. 2014). Stearic acid (octadecanoic acid) is a saturated fatty acid with eighteen carbon atoms. Its chemical formula is C18H36O2 or C17H35CO2H. Palmitic acid (hexadecanoic acid) is a saturated fatty acid with sixteen carbon atoms. Its chemical formula is C16H32O2 or CH3 (CH2)14COOH. The structure of both fatty acids is provided in Fig. 8. Fatty acids are derived easily from various origins, such as plants, animals, and dairy products (Agrawal et al. 2017). They are classified as safe (Caba et al. 2012). They are a favorable water repellent for cotton fabrics and other materials (Agrawal et al. 2017; Jiang et al. 2018).
Green products for water repellency on textiles
The concept of green chemistry is to produce green finishes and their manufacturing processes to decrease or eliminate lethal chemicals' usage and production, as shown in Table 2 (Anastas and Eghbali 2010). The outset of green chemistries started in the 1990s as countries like the USA, UK, and Italy (Tang et al. 2008) took the initiative of environment-friendly chemicals. The restriction was placed on the companies by the EU’s REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) and the USA TSCA (Toxic Substances Control Act) to get permission from the legislative agencies before the manufacturing of any new and novel formulation, thus assuring only the essential chemistries introduced in society. Since November 2020, the European Union has outlawed the usage of thirty-three chemical compounds in the textiles industry, labeling them as mutagens, carcinogens, hazardous, and toxic for the reproductive system (Christie 2007).
According to the green chemistry theory, durable water repellent (DWR) should be free from toxic fluorocarbons. In this regard, the biomimicry principle is the basis of new developments in the design and production of finishes taken from nature’s best biological entities and used to cope with ecological issues. The technological giants are on the roadmap to find sustainable water-repellent chemistries. Zelan™ R3, based on renewable resources produced by Huntsman and Chemours, is a fluorocarbon-free and more durable water-repellent than existing non-fluorine finishes (Corporation, n.d.). Another fluorine-free water-repellent compound is Altopel™ F3, formulated by Bolger & O’Hearn Inc (Yip 2020). The polymer is also used to produce greener water-repellent garments. INSQIN®, developed by Covestro, is based on a waterborne polyurethane (PU). It is free from solvent and dimethylformamide toxin and only consumes almost 5% of water (Martin and Martin 2016). Rudolph group produced a novel oil and water-repellent finish called Bionic Finish Eco®, a dendrimers derivative (Failed 2019).
Contact angle greater than 150˚ is achieved by lycopodium and natural wax, plant-based filler materials, resulting in superhydrophobic finishes, as shown in Fig. 9. These coatings are mostly natural substances and do not include nanoparticles or other heat treatments (Morrissette et al. 2018). Another water-repellent finish is obtained by applying Carnauba wax to the fabric applied by a layer-by-layer technique. The wetting contact angles for cotton and nylon samples were 131.9˚ and 131.4˚ that reduced to 101.7˚ and 102.4˚ upon successive washings (Bashari and A. H. Salehi K, and N. Salamatipour 2020). Figure 9 represents the green chemistry used in formulations of water-repellent finishes.
Green products in water-repellent finishes (Raj et al. 2022)
Addition of cross-linkers in the water and oil repellents
The researchers added various substances in the C6 and C4 based fluorocarbons to increase repellency and durability. Modifying vegetable oils with trifluoroethanol and tetrafluoro propanol imparts appreciable water repellency (Khattab et al. 2020; Schellenberger et al. 2018), combining dendrimers with fluorocarbons to reduce their amount and acquire remarkable oil and water repellency.
In past, formaldehyde-based cross-linkers (e.g., dimethylol dihydroxy ethylene urea) were mostly used with fluorocarbons. For instance, N-methylol agents have been used as an effective and economical cross-linker for fluorocarbon finishes. However, they release formaldehyde which limits their use (Yang and Chen 2019). Among formaldehyde-based cross-linkers, DMDHEU (1, 3-dimethylol-4,5- dihydroxy ethylene urea) was the most attractive as it is an economical and efficient cross-linker (Haule et al. 2012; Chowdhury et al. 2018). However, formaldehyde can cause skin allergy, eye and respiratory tract irritation, headaches, and breathing issues. Moreover, it is toxic, a nuisance, and carcinogenic to humans (Mommer et al. 2020; Barbosa et al. 2019). Formaldehyde-based cross-linkers are one of the most effective cross-linkers. However, these substances are very lethal to humans. Certain cross-linkers (e.g., butanetetracarboxylic acid) are non-formaldehyde but are too expensive. Hence, formaldehyde-free and economical cross-linkers were employed with fluorinated repelling agents to enhance oil and water repellency (Mohsin et al. 2016a). There are many formaldehyde-free cross-linkers (Fig. 10). The most effective alternatives of formaldehyde-based cross-linkers are polycarboxylic acids (Xiao et al. 2018; Hashem et al. 2011; Dehabadi et al. 2013; Min and Choi 2018). Polycarboxylic acids with more carboxyl groups (e.g., 4–6 carboxyl groups) provide more efficient cross-linking.
The esterification reaction between polycarboxylic acids and cotton fabric can be accomplished in two steps. Firstly, the cyclic anhydride intermediate forms through the dehydration of two adjoining carboxylic acid groups (Dehabadi et al. 2012; Trask-Morrell and Andrews 1991; Lin et al. 2016). Then, this intermediate acid anhydride undergoes a reaction with a hydroxyl group of cellulose to form an ester, as shown in Fig. 11.
Citric acid is a carboxylic acid that is bio-based, less costly, easily available, and generally used cross-linker (Ahmed et al. 2021; Qutab et al. 2021; Taherkhani and Hasanzadeh 2018). The literature reported citric acid as an excellent finishing chemical for cotton fabrics (Mohsin and Sardar 2020). For instance, researchers (Mohsin et al. 2013) added citric acid to Genguard L-19296 (e.g., fluorocarbon-based acrylate polymer). It achieved a water repellency rate at 6 and an oil repellency rate at 1 even after 20 washes. In contrast, when Genguard was used alone, the water repellency was 3, and oil repellency was zero after only five washes. Hence, formaldehyde-free cross-linkers improved the oil and water repellency when used with fluorinated water and oil repellents. The performance of fluorocarbon finishes reduced remarkably after successive washing. In addition to citric acid, another carboxylic acid, maleic acid, has also been reported as a good cross-linking agent (Sharif et al. 2022a; Sarwar et al. 2019). The chemical structures of some carboxylic acids are shown in Fig. 12. For instance, it incorporated maleic acid in a commercial C6-based fluorocarbon repellent and attained excellent oil and water repellency and durability (Mohsin et al. 2016b). Other carboxylic acids include itaconic acid (Gupta et al. 2008; Yang et al. 2000), acrylic acid (Udomkichdecha et al. 2003), and succinic acid (Karimi et al. 2010; Afinjuomo et al. 2019).
Conclusions and recommendations
Many oil and water-repellent finishes are available in the market nowadays. This review paper categorizes them as fluorocarbon finishes, silicone-based water repellents, paraffin waxes, long-chain fatty acids, and nanoparticles. The most effective, unique, and durable repellents finish for textiles is perfluorinated polymer, as they can resist oil/stain, water, and soil. The silicone-based water repellents with PDMS backbone [–Si (CH3)2O–] exhibit high water repellency and softness in finished fabrics. Paraffin waxes are less air permeable and less durable to washing. The oleophobic and hydrophobic performance of non-fluorinated, biodegradables, and environment-friendly DWRs is comparable with C6- and C4-based fluoropolymers and slightly less than C8-based fluoropolymers. Long-chain fatty acids such as stearic and palmitic acids are bio-based and can be used as a hydrophobic finish. However, they lack durability; only repel water, and higher concentrations are needed for required repellency. Polymerization of long-chain fatty acids with polycarboxylic acids resulted in durable water- and oil-repellent finishes. Stain/soil repellency is not always a priority for outdoor apparel. However, non-fluorinated DWRs show little resistance to stains obtained from liquids with high to intermediate surface tension, whereas they have zero repellency from low surface tension liquids.
Developments in new DWR technologies are continued as the long-chain fluorinated polymers are being weeded out due to their environmental and health constraints. With the ambition to improve the ecology and human health, DWR finished textile products phased out fluorinated compounds completely in 2020 as they are persistent in the environment and hazardous. PFASs, highly non-biodegradable chemicals, when released into the environment, will result in nearly irreversible exposures on local (e.g., contaminated groundwater) and planetary scales. Short-chain fluorinated polymers based on hydrocarbons and silicones are improved compared to the C8-based long-chain fluoropolymers. However, these three groups of DWR substitutes are not completely concern-free. Although C6- and C4-based fluorocarbons are less persistent and bio-accumulative than C8 Fluorocarbons, they are still a threat if released into the environment. Silicone-based DWRs are also a red alert to human health, ecology, and fate endpoints. However, they are less nagging as compared to PFASs. Paraffin waxes are currently attaining market attention despite their possible hazards. Several studies confirmed that nanotechnologies have toxic effects on human health and the environment.
Sustainable practices are based on green chemistry. Principles of green chemistry will be adopted in the textile industry after knowledge of the life cycle of hazardous substances and the inefficiencies of the currently available manufacturing processes, usage, and disposal. So, green chemistry techniques are thus a win to win for the industry and society, improving cost-effectiveness and cooperation over compromise. Ethical trading, a net zero carbon footprint, zero-waste production, and healthier lifestyles are valuable benefits of adding green chemistries to the textile industry. DWRs should be further improved to achieve the required market need without disturbing nature and the environment. Based on the hazardous evaluation of DWRs, C8-based side chain perfluorinated polymer DWRs should be used only when oil/stain repellency is necessary. Otherwise, fluorine-free DWRs, particularly hydrocarbon DWRs, should be preferred for daily use where only hydrophobicity is required as they are less harmful to ecology. Silicone-based DWRs can be less lethal if residues of cyclic methyl siloxanes are further decreased. Till environmentally safe DWRs are available in the market, producers of hydrophobic garments should be careful in considering DWR, with the target to reduce the release of dangerous chemicals.
References
Abidi N (2018) Chemical properties of cotton fiber and chemical modification: cotton fiber––physics chemistry and biology. Springer, pp 95–115
Abo-Shosha M, El-Hilw Z, Aly A, Amr A, Nagdy ASIE (2008) Paraffin wax emulsion as water repellent for cotton/polyester blended fabric. J Ind Text 37:315–325
Afinjuomo F, Barclay TG, Song Y, Parikh A, Petrovsky N, Garg S (2019) Synthesis and characterization of a novel inulin hydrogel crosslinked with pyromellitic dianhydride. React Funct Polym 134:104–111
Agrawal N, Munjal S, Ansari Mohd Z, Khare N (2017) Superhydrophobic palmitic acid modified ZnO nanoparticles. Ceramics International 43:14271–14276
Ahmed M, Sukumar N, Gideon RK (2021) Crease resistance finishing optimization of citric acid and fibroin solution for cotton fabrics. J Nat Fibers 18:297–307
Almeida L, Ramos D (2017) "Health and safety concerns of textiles with nanomaterials," In: IOP conference series: materials science and engineerin, p. 102002.
Anastas P, Eghbali N (2010) Green chemistry: principles and practice. Chem Soc Rev 39:301–312
Atav R (2018) Dendritic molecules and their use in water repellency treatments of textile materials: in waterproof and water repellent textiles and clothing. Elsevier, pp 191–214
Atav R, Bariş B (2016) Dendrimer technology for water and oil repellent cotton textiles. AATCC J Res 3:16–24
Audenaert F, Lens H, Rolly D, Vander Elst P (1999) Fluorochemical textile repellents—synthesis and applications: A 3M perspective. J Text Inst 90:76–94
Ayazi-Yazdi S, Karimi L, Mirjalili M, Karimnejad M (2017) Fabrication of photochromic, hydrophobic, antibacterial, and ultraviolet-blocking cotton fabric using silica nanoparticles functionalized with a photochromic dye. J Text Inst 108:856–863
Barbosa E, dos Santos ALA, Peteffi GP, Schneider A, Müller D, Rovaris D et al (2019) Increase of global DNA methylation patterns in beauty salon workers exposed to low levels of formaldehyde. Environ Sci Pollut Res 26:1304–1314
Bashari A, Salehi AHK, Salamatipour N (2020) Bioinspired and green water repellent finishing of textiles using carnauba wax and layer-by-layer technique. J Text Inst 111:1148–1158
Bashiri Rezaie A, Montazer M, Mahmoudi Rad M (2019) Low toxic antibacterial application with hydrophobic properties on polyester through facile and clean fabrication of nano copper with fatty acid. Mater Sci Eng C. 97:177–187
Bentis A, Boukhriss A, Gmouh S (2020) Flame-retardant and water-repellent coating on cotton fabric by titania–boron sol–gel method. J Sol Gel Sci Technol 94:719–730
Berendjchi A, Khajavi R, Yazdanshenas ME (2011) Fabrication of superhydrophobic and antibacterial surface on cotton fabric by doped silica-based sols with nanoparticles of copper. Nanoscale Res Lett 6:1–8
Bhuiyan R, Hossain M, Zakaria M, Islam M, Zulhash Uddin M (2017) Chitosan coated cotton fiber: physical and antimicrobial properties for apparel use. J Polym Environ 25:334–342
Bhuiyan MR, Wang L, Shaid A, Shanks RA, Ding J (2019) Polyurethane-aerogel incorporated coating on cotton fabric for chemical protection. Prog Org Coat 131:100–110
Biltekin S, Ayça A (2019) Investigation of the effect of cotton knitted fabric structure of babywear on moisture management properties. Ind Text 70:495–501
Boukhriss A, Gmouh S, Hannach H, Roblin J-P, Cherkaoui O, Boyer D (2016) Treatment of cotton fabrics by ionic liquid with PF6− anion for enhancing their flame retardancy and water repellency. Cellulose 23:3355–3364
Cai R, Glinel K, De Smet D, Vanneste M, Mannu N, Kartheuser B et al (2018) Environmentally friendly super-water-repellent fabrics prepared from water-based suspensions. ACS Appl Mater Interfaces 10:15346–15351
Chauhan P, Kumar A, Bhushan B (2019) Self-cleaning, stain-resistant and anti-bacterial superhydrophobic cotton fabric prepared by simple immersion technique. J Colloid Interface Sci 535:66–74
Choudhury AKR (2017) Principles of textile finishing. Woodhead Publishing
Chowdhury KP, Chowdhury S, Hosain MA, Al Mamun A, Alahi N, Rahman MS (2018) Comparative enactment of fluorocarbon-free and fluorocarbon-based water repellent finishes on cotton single jersey fabrics. Int J Curr Eng Technol 8:1–7
Christie R (2007) Environmental Aspects of Textile Dyeing. Elsevier Science
De P, Sankhe M, Chaudhari S, Mathur M (2005) UV-resist, water-repellent breathable fabric as protective textiles. J Ind Text 34:209–222
de la Caba K, Peña C, Ciannamea EM, Stefani PM, Mondragon I, Ruseckaite RA (2012) Characterization of soybean protein concentrate—stearic acid/palmitic acid blend edible films. J Appl Polym Sci 124:1796–1807
Dehabadi VA, Buschmann H-J, Gutmann JS (2012) Durable press finishing of cotton fabrics with polyamino carboxylic acids. Carbohyd Polym 89:558–563
Dehabadi VA, Buschmann H-J, Gutmann JS (2013) Durable press finishing of cotton fabrics: An overview. Text Res J 83:1974–1995
Dekanić T, Tarbuk A, Flinčec Grgac S (2018) The liquid moisture management properties of low-temperature cured water-repellent cotton fabrics Tekstil. J Text Cloth Technol 67(7):189–200
Edward NW, Goswami P (2018) Plasma-based treatments of textiles for water repellency: waterproof and water repellent textiles and clothing. Elsevier, pp 215–232
Emam HE (2019) Generic strategies for functionalization of cellulosic textiles with metal salts. Cellulose 26:1431–1447
Faheem S, Baheti V, Tunak M, Wiener J, Militky J (2019) Comparative performance of flame retardancy, physiological comfort, and durability of cotton textiles treated with alkaline and acidic casein suspension. J Ind Text 48:969–991
Fahmy H, Hassabo A (2022) Synthesis and application of new silicone based water repellents. Egypt J Chem 65:1–2
Fahmy H, Aly A, Amr A, Sayed SM, Rabie A (2016) SA/TDI/PEG adducts as water repellent finishes for cotton/polyester blended fabric. Prog Org Coat 99:166–172
Fahmy H, Aly A, Sayed SM (2017) Graft copolymerization of N-vinylpyrrolidone onto stearyl alcohol to impart water repellency and antibacterial properties for cotton/polyester fabric. Prog Org Coat 105:176–182
Ferrero F, Periolatto M (2013) Application of fluorinated compounds to cotton fabrics via sol–gel. Appl Surf Sci 275:201–207
Ferrero F, Periolatto M, Tempestini L (2017) Water and oil repellent finishing of textiles by UV curing: evaluation of the influence of scaled-up process parameters. Coatings 7:60
Gao Q, Hu J, Li R, Pang L, Xing Z, Xu L et al (2016) Preparation and characterization of superhydrophobic organic-inorganic hybrid cotton fabrics via γ-radiation-induced graft polymerization. Carbohyd Polym 149:308–316
Gargoubi S, Baffoun A, Harzallah OA, Hamdi M, Boudokhane C (2020) Water repellent treatment for cotton fabrics with long-chain fluoropolymer and its short-chain eco-friendly alternative. J Text Inst 111:835–845
Ghasemi N, Seyfi J, Asadollahzadeh MJ (2018) Imparting superhydrophobic and antibacterial properties onto the cotton fabrics: synergistic effect of zinc oxide nanoparticles and octadecanethiol. Cellulose 25:4211–4222
Gomes DJ, de Souza NC, Silva JR (2013) Using a monocular optical microscope to assemble a wetting contact angle analyser. Measurement 46:3623–3627
Gupta P, Bajpai M, Bajpai S (2008) Development of cotton fabric with antibacterial properties: part I: preparation of poly (acrylamide-co-itaconic acid) grafted cotton fabric and its water uptake analysis. J Macromol Sci Part A Pure Appl Chem 45:179–185
Han CH, Min BG (2020) Superhydrophobic and antibacterial properties of cotton fabrics coated with copper nanoparticles through sonochemical process. Fibers Polymers 21:785–791
Hashem M, Elshakankery M, Abd El-Aziz S, Fouda MM, Fahmy H (2011) Improving easy care properties of cotton fabric via dual effect of ester and ionic crosslinking. Carbohydr Polym. 86:1692–1698
Haule LV, Rigout M, Carr C, Jones C (2012) Surface and bulk chemical analysis of the durability of an easy care finish on cotton. Cellulose 19:1023–1030
Holmquist H, Schellenberger S, van der Veen I, Peters GM, Leonards PEG, Cousins IT (2016) Properties, performance and associated hazards of state-of-the-art durable water repellent (DWR) chemistry for textile finishing. Environ Int 91:251–264
Islam MT and Asaduzzaman S (2019) Environmentally‐Friendly Textile Finishing. Textiles and Clothing, pp 101–129
Jeong SA, Kang TJ (2017) Superhydrophobic and transparent surfaces on cotton fabrics coated with silica nanoparticles for hierarchical roughness. Text Res J 87:552–560
Ji B, Zhao C, Yan K, Sun G (2016) Effects of acid diffusibility and affinity to cellulose on strength loss of polycarboxylic acid crosslinked fabrics. Carbohydrate Polymers 144:282–288
Jia Y, Lu Y, Zhang G, Liang Y, Zhang F (2017) Facile synthesis of an eco-friendly nitrogen–phosphorus ammonium salt to enhance the durability and flame retardancy of cotton. J Mater Chem A 5:9970–9981
Jian J-M, Guo Y, Zeng L, Liang-Ying L, Lu X, Wang F et al (2017) Global distribution of perfluorochemicals (PFCs) in potential human exposure source–a review. Environ Int 108:51–62
Jiang B, Chen Z, Sun Y, Yang H, Zhang H, Dou H et al (2018) Fabrication of superhydrophobic cotton fabrics using crosslinking polymerization method. Appl Surface Sci 441:554–563
Jung H, Kim M-K, Jang S (2020) Liquid-repellent textile surfaces using zirconium (Zr)-based porous materials and a polyhedral oligomeric silsesquioxane coating. J Colloid Interface Sci 563:363–369
Karimi L, Mirjalili M, Yazdanshenas ME, Nazari A (2010) Effect of nano TiO2 on self-cleaning property of cross-linking cotton fabric with succinic acid under UV irradiation. Photochem Photobiol 86:1030–1037
Kasapgil E, Anac I, Erbil HY (2019) Transparent, fluorine-free, heat-resistant, water repellent coating by infusing slippery silicone oil on polysiloxane nanofilament layers prepared by gas phase reaction of n-propyltrichlorosilane and methyltrichlorosilane. Colloids Surf, A 560:223–232
Khattab TA, Mohamed AL, Hassabo AG (2020) Development of durable superhydrophobic cotton fabrics coated with silicone/stearic acid using different cross-linkers. Mater Chem Phys 249:122981
Kim T, Kang H, Yoon N (2017) Synthesis of non-fluorinated paraffinic water repellents and application properties on textile fabrics. Fibers Polymers 18:285–289
Kissa E (2001) Fluorinated surfactants and repellents. CRC Press
Kissa E (2018) Repellent finishes. Handbook of fiber science and technology: chemical processing of fibers and fabrics. Routledge, pp 143–210
Lam Y, Kan C, Yuen C (2011) Wrinkle-resistant finishing of cotton fabric with BTCA - the effect of co-catalyst. Text Res J 81:482–493
Lam YL, Kan CW, Yuen CWM (2011) Physical and chemical analysis of plasma-treated cotton fabric subjected to wrinkle-resistant finishing. Cellulose 18:493–503
Lee K, Hwang J, Ahn Y (2014) Fabrication of superhydrophobic surface on a cellulose-based material via chemical modification. Bull Korean Chem Soc 35:1545–1548
Lei H, Xiong M, Xiao J, Zheng L, Zhu Y, Li X et al (2017) Fluorine-free low surface energy organic coating for anti-stain applications. Prog Org Coat 103:182–192
Lewin M (1984) Handbook of fiber science and technology chemical processing of fibers and fabrics, fundamentals and preparation part B vol 1 Bleaching of cellulosic and synthetic fabric. Marcel Deckker, New York
Lin L, Zhou X, Xing Z, Wu Y (2016) Synthesis of 2, 3-dibromo-succinic anhydride and application on cotton, polyester and polyester/cotton blended fabric. Text Res J 86:1585–1596
Liu L, Huang Z, Pan Y, Wang X, Song L, Hu Y (2018) Finishing of cotton fabrics by multi-layered coatings to improve their flame retardancy and water repellency. Cellulose 25:4791–4803
Liu X, Yang G, Lipik V (2019) Permanent water repellent chemical modification of cotton fabric with reagents containing aromatic rings. Fibers Polym 20:51–56
Lu Z, Liu J, Dong C, Zhang Z, Wei D (2019) Durable multifunctional antibacterial and hydrophobic cotton fabrics modified with linear fluorinated pyridinium polysiloxane. Cellulose 26:7483–7494
Luo S, Peng X-X, Zhang Y-F, Fu P, Du F-P (2020) Oil-repellent and antifog coatings based on poly (vinyl alcohol)/hydrolyzed poly (styrene-co-maleic anhydride)/fluorocarbon surfactant. Prog Org Coat 141:105560
Ma Y, Zhu D, Si Y, Sun G (2018) Fabricating durable, fluoride-free, water repellency cotton fabrics with CPDMS. J Appl Polym Sci 135:46396
Macbeth B (2019) Don’t fear the water. TCBL Journal. 1
Martin M (2016) Fix Textile and Garment Supply Chains. In: Martin Ed (ed) Building the Impact Economy: Our Future, Yea or Nay. Springer International Publishing, Cham, pp 43–60
Mazrouei-Sebdani Z, Khoddami A (2011) Alkaline hydrolysis: a facile method to manufacture superhydrophobic polyester fabric by fluorocarbon coating. Prog Org Coat 72:638–646
Mazzon G, Zahid M, Heredia-Guerrero JA, Balliana E, Zendri E, Athanassiou A et al (2019) Hydrophobic treatment of woven cotton fabrics with polyurethane modified aminosilicone emulsions. Appl Surf Sci 490:331–342
Mehta S (2018) Optimization of fluorochemical finish concentration for liquid repellency treatment of 100% cotton fabric and resulting physical properties. AATCC J Res 5:15–22
Min J-Y, Choi H-M (2018) Preparation and characterization of amphoteric cotton by N-containing reagent through polycarboxylic acid interconnecting linkage. Cellul Chem Technol 52:891–901
MMR. (2021, 17–10–2022). Global Textile Finishing Chemicals Market: Industry Analysis and Forecast (2020–2026) – By Type, Process, and Region. Available: https://www.maximizemarketresearch.com/market-report/global-textile-finishing-chemicals-market/90605/#details
Mohamed AL, El-Sheikh MA, Waly AI (2014) Enhancement of flame retardancy and water repellency properties of cotton fabrics using silanol based nano composites. Carbohyd Polym 102:727–737
Mohsin M, Sardar S (2020) Development of sustainable and cost efficient textile foam-finishing and its comparison with conventional padding. Cellulose 27:4091–4107
Mohsin M, Carr C, Rigout M (2013) Novel one bath application of oil and water repellent finish with environment friendly cross-linker for cotton. Fibers Polymers 14:724–728
Mohsin M, Sarwar N, Ahmad S, Rasheed A, Ahmad F, Afzal A et al (2016a) Maleic acid crosslinking of C-6 fluorocarbon as oil and water repellent finish on cellulosic fabrics. J Clean Prod 112:3525–3530
Mohsin M, Farooq A, Abbas N, Noreen U, Sarwar N, Khan A (2016) Environment friendly finishing for the development of oil and water repellent cotton fabric. J Nat Fibers 13:261–267
Moilliet JL (1963) Waterproofing and water-repellency. Elsevier Publishing Company
Moiz A, Vijayan A, Padhye R, Wang X (2016) Chemical and water protective surface on cotton fabric by pad-knife-pad coating of WPU-PDMS-TMS. Cellulose 23:3377–3388
Mommer S, Kurniadi J, Keul H, Möller M (2020) Formaldehyde-free curing of cotton cellulose fabrics in anhydrous media. J Appl Polym Sci 137:48371
Morrissette JM, Carroll PJ, Bayer IS, Qin J, Waldroup D, Megaridis CM (2018) A methodology to produce eco-friendly superhydrophobic coatings produced from all-water-processed plant-based filler materials. Green Chem 20:5169–5178
Muresan EI, Balan G, Popescu V (2013) Durable hydrophobic treatment of cotton fabrics with glycidyl stearate. Ind Eng Chem Res 52:6270–6276
Muthu SS (2015) Handbook of sustainable apparel production. CRC Press
Onar N, Mete G (2016) Development of water-, oil-repellent and flame-retardant cotton fabrics by organic-inorganic hybrid materials. J Text Inst 107:1463–1477
Onar N, Mete G, Aksit A, Kutlu B, Celik E (2015) Water-and oil-repellency properties of cotton fabric treated with Silane, Zr, Ti based nanosols. Int J Text Sci 4:84–96
Pan G, Xiao X, Ye Z (2019) Fabrication of stable superhydrophobic coating on fabric with mechanical durability, UV resistance and high oil-water separation efficiency. Surface Coat Technol 360:318–328
Patil NV, Netravali AN (2019) Direct assembly of silica nanospheres on halloysite nanotubes for “green” ultrahydrophobic cotton fabrics. Adv Sustain Syst 3:1900009
Patra AK, Pariti SRK (2022) Restricted substances for textiles. Text Progress. 54:1–101
Qutab HG, Mohsin M, Ramzan N, Ahmad SW, Sardar S (2021) Synthesis and application of a formaldehyde-free flame-retardant for cotton fabrics by polymerization between diammonium hydrogen phosphate and citric acid. Journal of Natural Fibers 18:1913–1923
Rabia S, Muhammad M, Naveed R, Waqas AS, Qutab HG (2020) Development of free fluorine and formaldehyde oil and water repellent finishes for cotton fabrics through polymerization of bio-based stearic acid with carboxyllc acids. Ind Text 71:145–155
Raj A, Chowdhury A, Ali SW (2022) Green chemistry: its opportunities and challenges in colouration and chemical finishing of textiles. Sustain Chem Pharma 27:100689
Rastogi D, Breja K, Goyal N, Jassal M, and Agrawal AK (2013) Comparative Analysis of Selected Fluorocarbon-Based Oil and Water-Repellent Finishes on Textiles. Res J Text Apparel
Ren Q, Zhao T (2010) Synthesis and application of modified vegetable oils in water-repellent finishing of cotton fabrics. Carbohyd Polym 80:381–386
Rovira J, Domingo JL (2019) Human health risks due to exposure to inorganic and organic chemicals from textiles: a review. Environ Res 168:62–69
Saleem H, Zaidi SJ (2020) Sustainable use of nanomaterials in textiles and their environmental impact. Materials 13:5134
Saratale RG, Rajesh Banu J, Shin H-S, Bharagava RN, Saratale GD (2020) Textile industry wastewaters as major sources of environmental contamination: bioremediation approaches for its degradation and detoxification. Bioremediation of industrial waste for environmental safety. Springer, pp 135–167
Sarwar N, Ashraf M, Mohsin M, Rehman A, Younus A, Javid A et al (2019) Multifunctional formaldehyde free finishing of cotton by using metal oxide nanoparticles and ecofriendly cross-linkers. Fibers and Polymers 20:2326–2333
Schellenberger S, Gillgard P, Stare A, Hanning A, Levenstam O, Roos S et al (2018) Facing the rain after the phase out: performance evaluation of alternative fluorinated and non-fluorinated durable water repellents for outdoor fabrics. Chemosphere 193:675–684
Schellenberger S, Hill PJ, Levenstam O, Gillgard P, Cousins IT, Taylor M et al (2019) Highly fluorinated chemicals in functional textiles can be replaced by re-evaluating liquid repellency and end-user requirements. J Clean Prod 217:134–143
Schindler WD, Hauser PJ (2004) Chemical finishing of textiles. Elsevier
Senthil Kumar P, Gunasundari E (2018) Green chemistry in textiles. Sustainable innovations in textile chemistry and dyes. Springer, USA, pp 53–73
Shabbir M (2019) Textiles and clothing: environmental concerns and solutions. Wiley
Shabbir M, Mohammad F (2017) "Insights into the functional finishing of textile materials using nanotechnology: textiles and clothing sustainability. Springer, pp 97–115
Shao J, Sheng W, Wang C, Ye Y (2021) Solvent-free fabrication of tough self-crosslinkable short-fluorinated copolymer nanocoatings for ultradurable superhydrophobic fabrics. Chem Eng J 416:128043
Sharif R, Mohsin M, Ramzan N, Sardar S, Ahmad SW, Ahtisham W (2021) Development of bio and non-fluorinated palmitic acid based water repellent for cotton fabric. J Nat Fibers. 19(13):1–14
Sharif R, Mohsin M, Ramzan N, Ahmad SW, Qutab HG (2022a) Synthesis and application of fluorine-free environment-friendly stearic acid-based oil and water repellent for cotton fabric. J Nat Fibers 19:1632–1647
Sharif R, Mohsin M, Ramzan N, Sardar S, Anam W (2022) Synthesis of bio-based non-fluorinated oil and water repellent finishes for cotton fabric by using palmitic acid succinic acid, and maleic acid. J Nat Fibers 19(16):1–12
Shi Z, Wyman I, Liu G, Hu H, Zou H, Hu J (2013) Preparation of water-repellent cotton fabrics from fluorinated diblock copolymers and evaluation of their durability. Polymer 54:6406–6414
Siegfried B, Som C (2007) "NanoTextiles: Functions, nanoparticles and commercial applications," Semester Thesis in the frame of the “Nanosafe-Textiles project TVS Textilverband Schweiz and Empa
Singh AK, Singh JK (2017) Fabrication of durable super-repellent surfaces on cotton fabric with liquids of varying surface tension: low surface energy and high roughness. Appl Surf Sci 416:639–648
Soane DS, Offord DA, Linford MR, Millward DB, Ware W, Erskine L et al. (2003) "Nanoparticle-based permanent treatments for textiles," ed: Google Patents.
Stan MS, Nica IC, Popa M, Chifiriuc MC, Iordache O, Dumitrescu I et al (2019) Reduced graphene oxide/TiO2 nanocomposites coating of cotton fabrics with antibacterial and self-cleaning properties. J Ind Text 49:277–293
Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG (2019) A review of the pathways of human exposure to poly-and perfluoroalkyl substances (PFASs) and present understanding of health effects. J Eposure Sci Environ Epidemiol 29:131–147
Suryaprabha T, Sethuraman MG (2017) Fabrication of copper-based superhydrophobic self-cleaning antibacterial coating over cotton fabric. Cellulose 24:395–407
Taherkhani A, Hasanzadeh M (2018) Durable flame retardant finishing of cotton fabrics with poly (amidoamine) dendrimer using citric acid. Mater Chem Phys 219:425–432
Tang SY, Bourne RA, Smith RL, Poliakoff M (2008) The 24 principles of green engineering and green chemistry: “improvements productively.” Green Chem 10:268–269
Trask-Morrell B, Andrews BK (1991) Thermoanalytical characteristics of polycarboxylic acids investigated as durable press agents for cotton textiles. J Appl Polym Sci 42:511–521
Türk M, Ehrmann A, Mahltig B (2015) Water-, oil-, and soil-repellent treatment of textiles, artificial leather, and leather. J Text Inst 106:611–620
Udomkichdecha W, Kittinaovarat S, Thanasoonthornroek U, Potiyaraj P, Likitbanakorn P (2003) Acrylic and maleic acids in nonformaldehyde durable press finishing of cotton fabric. Text Res J 73:401–406
Vaca-Garcia C, Thiebaud S, Borredon ME, Gozzelino G (1998) Cellulose esterification with fatty acids and acetic anhydride in lithium chloride/N, N-dimethylacetamide medium. J Am Oil Chem Soc 75:315–319
van der Veen I, Weiss JM, Hanning A-C, de Boer J, Leonards PE (2016) Development and validation of a method for the quantification of extractable perfluoroalkyl acids (PFAAs) and perfluorooctane sulfonamide (FOSA) in textiles. Talanta 147:8–15
Vasileva-Tonkova E, Staneva D, Medel S, Bosch P, Grozdanov P, Nikolova I et al (2019) Antimicrobial, antibiofilm and cytotoxicity activity of a new acridine hyperbranched polymer in solution and on cotton fabric. Fibers Polymers 20:19–24
Whittaker MH, Heine L (2018) Toxicological and environmental issues associated with waterproofing and water repellent formulations: Waterproof and water repellent textiles and clothing. Elsevier, pp 89–120
Xiao H, Yan K, Ji B (2018) Improvement of anti-wrinkle properties of cotton fabrics treated with additives of neutral salts. Fibers and Polymers 19:1576–1583
Xiong D, Liu G, Duncan ES (2012) Diblock-copolymer-coated water-and oil-repellent cotton fabrics. Langmuir 28:6911–6918
Xue C-H, Jia S-T, Zhang J, Ma J-Z (2010) Large-area fabrication of superhydrophobic surfaces for practical applications: an overview. Sci Technol Adv Mater 11:033002
Yang CQ, Chen Q (2019) Flame retardant finishing of the polyester/cotton blend fabric using a cross-linkable hydroxy-functional organophosphorus oligomer. Fire Mater 43:283–293
Yang CQ, Wang X, Lu Y (2000) Infrared spectroscopy studies of cyclic anhydrides as intermediates for ester crosslinking of cotton cellulose by polycarboxylic acids. IV. In situ free radical copolymerization of maleic acid and itaconic acid on cotton. J Appl Polym Sci 75:327–336
Ye H, Li Z, Chen G (2013) Synthesis and application properties of fluorinated aromatic copolymers. J Appl Polym Sci 130:4410–4418
Yidong Z, Netravali AN (2016) ‘Green’ surface treatment for water-repellent cotton fabrics. Surface Innovations. 4:3–13
Yip J (ed) (2020) Latest material and technological developments for activewear. Woodhead Publishing
Zahid M, Heredia-Guerrero JA, Athanassiou A, Bayer IS (2017) Robust water repellent treatment for woven cotton fabrics with eco-friendly polymers. Chem Eng J 319:321–332
ZDHC. (2022). Roadmap to Zero Programme. Available: https://www.roadmaptozero.com/
Zhang Y, Wang X, Wang C, Zhai H, Liu B, Zhao X et al (2019) Facile preparation of flexible and stable superhydrophobic non-woven fabric for efficient oily wastewater treatment. Surf Coat Technol 357:526–534
Zhao T, Zheng J, Sun G (2012) Synthesis and applications of vegetable oil-based fluorocarbon water repellent agents on cotton fabrics. Carbohyd Polym 89:193–198
Zhao Q, Wu LYL, Huang H, Liu Y (2016) Ambient-curable superhydrophobic fabric coating prepared by water-based non-fluorinated formulation. Mater Des 92:541–545
Zhao D, Pan M, Yuan J, Liu H, Song S, Zhu L (2020) A waterborne coating for robust superamphiphobic surfaces. Prog Org Coat 138:105368
Zhou SQ, Cai JH, Dong SW, Chen GQ (2011) Synthesis and performance analysis of new-style fluorine-containing oil-repellent and water-repellent finishing agent with short chain. Adv Mater Res 332:1457–1461
Zisman WA (1964) "Relation of the equilibrium contact angle to liquid and solid constitution: contact angle, wettability, and adhesion. American Chemical Society, pp 1–51
Acknowledgements
The authors cordially thanks the University of Engineering and Technology, Lahore, for supporting this work [ORIC/100-ASRB/1990].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharif, R., Mohsin, M., Qutab, H.G. et al. Durable water and oil repellents along with green chemistries: an overview. Chem. Pap. 77, 3547–3560 (2023). https://doi.org/10.1007/s11696-023-02763-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-023-02763-x