Abstract
Cotton fabrics were treated with oxygen plasma gas and/or wrinkle-resistant finishing agent with polycarboxylic acid. The results of wicking rate, contact angle and wettability tests revealed that the atmospheric plasma treatment significantly improved hydrophilicity of cotton fiber. Such improvement greatly enhances the effectiveness of post-finishing processes. The study showed that chemical composition of cotton fabric surface changed after plasma and wrinkle-resistant treatment. Chemical composition of surface of treated cotton specimens was evaluated with different characterization methods, namely, FTIR-ATR and EDX. The experimental results are thoroughly discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Apparel products made of cotton fibres have the largest share in the textile market due to their excellent properties, such as breathability, moisture and heat conduction, softness, and hypoallergenic and anti-static properties. However, chemical modification of cellulosic fibres by introducing new functional groups or compounds is still needed in order to improve their performance characteristics which could be a cheaper route to achieve a higher quality and technical performance rather than by using a high cost fibre with inherent built-in performance properties (Holme 2007; Tzanov and Cavaco-Paulo 2006). Wrinkle-resistance of cotton fibres is always unsatisfactory to meet the commercial requirements (easycare properties) of textile materials due to the hydroxyl groups in its chemical structure which are easily deformed during washing. Thus, easycare, durable press and wrinkle-resistant finishes are critical to maintain the image of high performance for apparel textiles (Holme 2007; Tzanov and Cavaco-Paulo 2006).
The ester crosslinking of cotton by the compound BTCA, catalysed with sodium hypophosphite (SHP), has been found to be superior for wrinkle-resistant property of cotton fabric in previous researches (Lam et al. 2010a, b). Using titanium dioxide (TiO2) as a co-catalyst to improve the crease recovery property has been found feasible to enhance the finishing performance with minimum side effects (Yuen et al. 2007; Wang and Chen 2005a, b; Chen and Wang 2006; Lam et al. 2010a, b). On the other hand, low temperature plasma pre-treatment improves the functional finishing by causing a sputtering or etching effect that alters the surface characteristics which include a large variety of chemically active functional groups; it roughens the surface of the materials and increases the surface area for reaction between the finishing agent and the fibre (Hwang and Mccord 2005; Wang et al. 2008; Kaplan 2004; Rajpreet and Gita 2004). In the past, vacuum plasma systems were used for treating textile materials but such systems needed much space and were energy and time consuming processes which were also inadequate for treating the substrate continuously in mass production processes (Karahan and Özdogan 2008). Surface modification of cotton fabrics with atmospheric pressure plasma treatment has been studied widely as it is a continuous and environment friendly process that offers reduction of wet chemicals and energy consumption (Hwang and Mccord 2005; Wang and Qiu 2007).
Previous studies show that wrinkle-resistant and mechanical properties were improved after plasma pre-treatment and wrinkle-resistant treatment (Lam et al. 2010a, b, c). In this paper, the effect of atmospheric plasma treatment on the change of hydrophilicity of cotton fiber is studied. The study has shown that chemical composition of cotton fabric surface varies upon plasma and wrinkle-resistant treatment. Different characterization methods, namely, Fourier Transform Infrared spectroscopy with attenuated total reflection mode (FTIR-ATR) and Energy dispersive X-ray analysis (EDX) were used.
Experimental
Material
100% semi-bleached plain weave cotton fabrics (density: 58 × 58; yarn count: 38 × 40 tex; weight: 175 g/m2) specimens of size of 25 cm × 25 cm were used. The crosslinking agent was 1,2,3,4-butanetetracarboxylic acid (BTCA, 98% purity) supplied by the International Laboratory Ltd. The catalyst used was analytical grade sodium hypophosphite (SHP) supplied by the International Laboratory Ltd. Micro-titanium dioxide (TiO2, 2 μm diameter, 99.5% purity) was obtained from UniChem Ltd. The particle size of TiO2 was further confirmed by a particle size analyzer (LS13320 Beckman Coulter, Beckman Coulter Inc., USA). All other chemicals used in the study were reagent grade.
Plasma pre-treatment
Plasma pre-treatment of cotton fabrics was carried out by an atmospheric pressure plasma jet apparatus manufactured by Surfx Technologies. The apparatus produced a stable discharge of 80 W at atmospheric pressure with radio frequency of 13.56 MHz. The treatment was carried out using a rectangular nozzle which covered an active area of 25.4 mm × 1 mm and was mounted vertically above the substrate. The treatment time was 0.1 s/mm and the jet-to-substrate distance was 4 mm. Helium and oxygen were used as carrier and reactive gases, respectively. The flow rate of helium and oxygen was 15 and 0.1 L/min, respectively.
BTCA two-bath pad-dry-cure treatment
After plasma pre-treatment, cotton fabrics were treated with different concentrations of TiO2 solution mixed with 5% BTCA prepared according to the recipes in Table 1 with a two-bath pad-dry-cure method. In the first bath, the fabrics were dipped and padded with BTCA in the presence or absence of SHP until wet pick up of 80% was achieved at room temperature. The fabrics were then dried completely at 85 °C temperature. In the second bath, dipping and padding processes were performed by using 100 mL TiO2 solution which was dispersed in 10% Matexil DN-VL. Subsequently, the two-bath padded specimens were dried completely at 85 °C temperature and were then cured at 170 °C temperature for 2 min. Finally, the fabrics were conditioned at 21 ± 1 °C temperature and 65 ± 5% relative humidity for 24 h prior to any further treatment.
Contact angle measurement
The contact angle of test specimens was measured by a Contact Angle Meter (Tantec Inc.) at room temperature. After a glycerol drop of size 1 μL was placed on the test specimen’s surface at a distance of 5 mm from the syringe and the test specimen. The value of contact angle was obtained for both front and back side. The contact angle was measured at standard condition: 21 ± 1 °C temperature and 65 ± 5% relative humidity.
Wettability
Wettability of cotton specimens was measured according to the BS4554:1970. A microlitre syringe was placed 5 mm above the substrate and a Glycerol droplet of size 1 μL was dropped on the fabric surface. The time taken for the droplet to be completely absorbed into the fabric was regarded as the water-absorption time. Measurements were taken on both front and back side of each specimen. Wettability was measured at standard conditions: 21 ± 1 °C temperature and 65 ± 5% relative humidity.
Wicking test
A cotton fabric strip of 205 mm × 100 mm was marked at intervals of 10 mm each in both warp and weft directions (total 5 spots) by a marker pen using water-soluble ink. A clamp weighing two grams was attached to the specimens and the specimens were then suspended vertically with the clamped end immersed 20 mm in a distilled water bath. At standard conditions of 21 ± 1 °C temperature and 65 ± 5% relative humidity, the time required for the distilled water to rise along each marked spot was recorded and the average wicking rate in both warp and weft directions was estimated. The experiment was terminated when the water had reached the highest spot.
Fourier transform infrared spectroscopy (FTIR)
Chemical compositions of surfaces of cotton specimens were studied by the Fourier Transform Infrared spectrophotometer (Perkin Elmer Spectrum 100), with scanning range between 4,000 and 700 cm−1 and resolution 4 nm−1, in terms of attenuated total reflection (ATR). The average number of scans was 256; area of the relevant signal in zero-order derivative spectrum was measured.
Energy dispersive X-ray analysis (EDX)
EDX is an analytical technique used to collect elemental information from cotton specimens. It was conducted by the JEOL JSM-6490 Scanning Electron Microscope equipped with a cathode and magnetic lenses, to create and focus a beam of electrons, and elemental analysis capabilities. A detector is used to convert X-ray energy into voltage signals that are sent to a pulse processor which measures the signals and passes the measurements onto an analyzer for data display and analysis.
Results and discussion
Contact angle measurement
Water-repellent properties of cotton fabrics are expressed in terms of contact angle and absorption time. Contact angles of plasma pre-treated cotton fabrics subjected to 0.1 and 0.2% of TiO2 treatment are presented in Fig. 1a. On the other hand, contact angles of plasma pre-treated cotton fabrics subjected to BTCA treatment in the presence of SHP and/or TiO2 are illustrated in Fig. 1b, c. The figures show that plasma pre-treated fabrics had higher contact angles than untreated fabrics irrespective of the BTCA treatment.
After plasma pre-treatment, contact angle of the control fabric was decreased from 65° to 55° (Fig. 1a) because plasma pre-treatment can introduce water compatible functional groups such as hydroxyl groups to the fibres which increase wettability and hence reduce the contact angle subsequently. However, with addition of various amounts of TiO2 to the cotton fibre, TiO2 is physically attached on the fibre surface as shown in the SEM images. The spreading of TiO2 particles on the fibre surface restricts the penetration of water into the fibre and therefore the contact angle increases with increase in the amount of TiO2 used. Although plasma pre-treatment with the use of oxygen introduces hydrophilic groups on the cotton fibre surface (Pandiyaraj and Selvarajan 2008; Hong et al. 2006), the etching effect produces a roughened fibre surface for accommodating the TiO2 particles. Since a dispersing agent was used in preparing the TiO2 solution, the spreading of TiO2 on the cotton fibre surface is even and hence more TiO2 is attached on the fibre surface. This makes the fibre surface more hydrophobic and therefore, the contact angles increased accordingly.
BTCA was applied to produce the crosslinking effect on the cotton fibre (Fig. 1b). It was revealed that all contact angle values with BTCA treatments were higher than the control fabric. The reason is obviously that BTCA is a crosslinking agent and the crosslinkages formed reduce the amorphous region on the fibre surface. As a result, contact angle values were increased. The addition of TiO2 in BTCA treatment serves as a catalyst for forming crosslinkages between cotton fibres. The crosslinkages so formed reduce the absorption property of cotton fibre and thus increase contact angle values. As WRA results reported in a previous study (Lam et al. 2010a) suggest, increasing the amount of TiO2 will improve WRA results, indicating that more crosslinkages will be formed between cotton fibres. The increased amount of crosslinkages will reduce the wetting behaviour of the cotton fibre leading to increased contact angle values. When plasma pre-treatment was conducted, further increase of contact angle values was noted because plasma pre-treatment could improve the wettability of the cotton fibre and therefore, during the application of BTCA with or without TiO2, more chemicals were absorbed. The absorbed chemicals cause crosslinking effects in the subsequent curing process and more crosslinkages are formed. In addition, the etching effect of plasma active species produces a roughened fibre surface for accommodating TiO2 particles. Since the dispersing agent was used in preparing the TiO2 solution, the spreading of TiO2 on the cotton fibre surface is even and hence more TiO2 is attached on the fibre surface. This makes the fibre surface become more hydrophobic and therefore, contact angle values are increased.
Conventional BTCA and SHP treatment were conducted in the presence or absence of TiO2 as catalyst (Fig. 1c). It is clear that addition of SHP greatly increased the contact angle compared with Fig. 1b because SHP is a catalyst for initiating the crosslinking effect between BTCA and cotton fibre. With the help of plasma pre-treatment, the contact angle was increased correspondingly because plasma pre-treatment can improve wettability of cotton fibre leading to higher absorption of BTCA and SHP. Thus, a higher crosslinking effect was achieved. TiO2 serves as co-catalyst along with SHP which means the catalytic effect may be further enhanced and even more crosslinkages will be formed. As a result, contact angle values will be increased further. The effect of co-catalyst induced by TiO2 was further enhanced by plasma pre-treatment. The plasma pre-treatment with oxygen contributed two effects on the cotton fibre surface. Firstly, oxygen plasma chemically modifies the cotton leading to introduction of hydrophilic groups to the surface. Wettability of cotton fibre can be improved consequently. Secondly, plasma active species etch and roughen the fibre surface and cracks and grooves are generated on the fibre surface. Both effects enhance chemicals absorption during BTCA-SHP and BTCA-SHP-TiO2 treatments resulting in more crosslinkages and a more hydrophobic surface. Thus contact angle values increased subsequently.
Absorption and wicking
Water absorption properties of cotton fabrics are expressed not only in terms of contact angle, but also absorption time and wicking rate. Results of absorption time and wicking rate (as well as fabric weight) of different samples are shown in Table 2.
Ideally, for absorption time and wicking rate measurement, water should be the best liquid. However, due to the very good natural water absorption property of cotton, glycerol was used as the liquid for measuring absorption time and water was used for wicking rate. Absorption time and wicking rate can be used as indicators of water transportation in the in-plane and trans-planar direction, respectively.
Generally speaking, short absorption time implies better water absorption ability. Various wrinkle resistant treatments can increase contact angle values differently, as shown in Fig. 1a–c, which indicates that the hydrophobicity of cotton surface was increased to different degrees. As shown in Table 2, the fabric weight decreased in the range of 2.9–4.5% when compared to the control specimen. The absorption time results still further support the increase of cotton surface hydrophobicity. This hydrophobicity is developed through varying degrees of crosslinking effects induced by BTCA and its catalyst. During the crosslinking process, esterification reaction occurs, which converts hydroxyl groups present on cellulosic macromolecules into ester linkages (Li et al. 2008; Yang and Wang 1996). The reduction of hydroxyl groups, which were responsible for attraction of liquid molecules, leads to poorer absorption.
For samples B2 and B3, when different amounts of only TiO2 were added, the TiO2 presence on the fibre surface could serve as a barrier to liquid penetration and the absorption times were slightly increased for such samples, with or without plasma pre-treatment. Although the fabric weight was decreased slightly after plasma pre-treatment, it does not have significant effect on the water absorption ability. The plasma pre-treatment could still significantly lower the absorption time because plasma could introduce hydrophilic groups to cotton fibre surface though TiO2 was present on the fibre surface.
For samples B4–B6, different degrees of crosslinkages were formed on the cotton surface where the crosslinking served as a barrier to liquid penetration. The use of plasma pre-treatment can further increase absorption time because plasma pre-treatment increases absorption of BTCA and TiO2 leading to a higher crosslinking effect. Also the presence of TiO2 on the fibre surface provides a physical barrier to liquid absorption and its effect is proportional to the amount of TiO2 applied.
For samples B7 to B9, the reaction between BTCA and cotton fibre was greatly increased due to the use of SHP and SHP-TiO2 catalysts. Both formation of crosslinkages together with the presence of TiO2 on the fibre surface enhanced the hydrophobicity of cotton fabric’s surface which increased the absorption time significantly compared with the control samples. With the use plasma pre-treatment, absorption time can be further enhanced because plasma pre-treatment increases absorption of BTCA and its catalyst systems leading to more crosslinking effect on the surface.
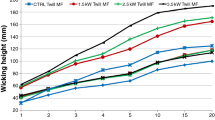
Wicking rate is determined by the distance water travels up on the fabric strip over time. The higher the wicking rate, the better the water absorption of fabrics in the transplanar direction will be. As shown in Table 2, it was noted that wicking rate of fabric was generally reduced after wrinkle resistant treatment compared to the control fabric because the crosslinking reaction consumed the hydroxyl groups in the cotton fibre. However, plasma pre-treatment can increase the wicking rate of fabric with wrinkle resistant treatment because oxygen plasma introduces water compatible functional groups to cotton fibres (Pandiyaraj and Selvarajan 2008; Hong et al. 2006). The wicking effect is the liquid transport phenomenon within the fabric, which is thought to be due to the capillary action, and is governed by fibre surface properties. In addition, the separation between fibres in the yarn also influences the wicking rate. These two factors explain the results in Table 2. For the control sample B1, the plasma pre-treatment introduced hydrophilic functional groups to the fibre surface which may attract more water molecules within the experiment period and thus a faster wicking rate was noted.
For samples B2 and B3, the wicking rate was slower than the control sample; the reduction of wicking rate was proportional to the amount of TiO2 used for wrinkle resistant treatment. In this case, when TiO2 was used, its particles were present not only on the fibre surface but also in the space between fibres and yarns. The presence of TiO2 blocks the inter-fibre and inter-yarn structure which reduces the capillary action. Therefore, the wicking rate was reduced. With plasma pre-treatment, wicking rate was increased because of introduction of hydrophilic groups to the fibre surface. When the amount of TiO2 used was compared, it was noted that wicking rate was reduced with increase in the amount of TiO2 used but it was still faster than plasma untreated samples. Plasma etching provides more space for accommodating the TiO2 on the fibre and the blocking effect of TiO2 in the inter-fibre and inter-yarn structure is increased. The surface effect was now dominated and such that plasma pre-treated B2 and B3 samples had a faster wicking rate than plasma untreated samples.
For samples B4–B6, the wicking rate was further decreased compared with B2 and B3 samples because of presence of the crosslinking agent BTCA. In samples B4, B5 and B6, BTCA and TiO2 were used as crosslinking agent and catalyst, respectively. During the crosslinking process, esterification reaction occurs in which hydroxyl groups present on cellulosic macromolecules are converted into ester linkages (Li et al. 2008; Yang and Wang 1996). The reduction of hydroxyl groups, which were responsible for attraction of water molecules, leads to poorer absorption. In addition, the crosslinking between fibres reduces the inter-fibre and inter-yarn space and therefore, water transportation in the upward direction gets restricted, leading to a slower wicking rate. Also the reduction in wicking rate is proportional to the amount of TiO2 used. Increased amount of TiO2 will not only chemically increase the crosslinking reaction but also it is physically attached in the space between fibres and yarns. Both effects lead to blocking of water transportation during the wicking test and lower the wicking rate. With plasma pre-treatment, the wicking rate was increased because of introduction of hydrophilic groups to the fibre surface. When the amount of TiO2 used was compared, it was noted that the wicking rate was reduced with increase in the amount of TiO2 used but the wicking rate was still faster than samples not plasma pre-treated. Although plasma’s etching effect can provide more space for accommodating TiO2 in the fibre and the blocking effect in the inter-fibre and inter-yarn structure is increased, the surface effect now dominates so that plasma pre-treated B5 and B6 samples have a faster rate than samples not pre-treated with plasma.
Samples B7–B9 were treated with BTCA using different catalysts, SHP or SHP-TiO2. It was clear that the crosslinking effect in these samples was most significant, as reflected from WRA values in Table 2. Because of the significant crosslinking effect, it was not surprising that the wicking rate was slower in these samples compared with samples B1 to B6. The wicking rate of plasma untreated samples B7–B9 was in accordance with the catalysts used. However, when plasma pre-treatment was applied, wicking rate was further lowered if a higher amount of TiO2 was used as a co-catalyst compared with wicking rate obtained from samples on which only SHP catalyst was used. The reason may be the plasma pre-treatment not only increased the fibre’s wettability but also physically introduced cracks and grooves to fibre surface. The increased wettability offers better absorption of BTCA and its catalyst and the surface’s physical structure provides more space for TiO2. The former effect leads to formation of more crosslinkages and the latter effect reduces the space between fibres and yarns. Both effects will finally restrict transportation of water during the wicking test.
Fourier transform infrared spectroscopy (FTIR)
FTIR-ATR is a surface sensitive technique used to characterize the chemical structure of the substrate (Karahan and Özdogan 2008; Chung et al. 2004). FTIR-ATR analysis was performed on the control cotton fabric; the spectrum is illustrated in Fig. 2. Moreover, the spectra of characteristic bands related to their chemical structures were the hydrogen bonded OH stretching centered at 3,300 cm−1, the CO stretching centered at 1,030 cm−1, the CH stretching centered at 2,900 cm−1, and the CH wagging centered at 1,310 cm−1 (Karahan and Özdogan 2008; Chung et al. 2004; Hartzell-Lawson and Hsieh 2000). In addition, a peak around 1,640 cm−1 is corresponding to the absorbed water molecules (Karahan and Özdogan 2008; Chung et al. 2004). All of these intense peaks are associated with the cellulose structure in cotton fibres.
FTIR analyses were performed on fabrics without any plasma pre-treatment and plasma pre-treated fabrics with different parameters (speed, gas flow and nozzle to substrate distance); their corresponding spectra are shown in Figs. 3, 4, 5. The spectra obtained from control and plasma pre-treated cotton species are very similar, but the spectra for plasma pre-treated cotton showed some difference in the peaks of 1,640 and 3,740 cm−1 corresponding to the C=O vibration groups in the carbonyl structure and O–H stretching vibration, respectively (Kan et al. 2009; Zhang et al. 2008; Titov et al. 2005). In addition, a new peak at 1,540 cm−1 which represented COO− stretching vibrations, due to plasma surface oxidation, was observed in Fig. 3c. It is indicated that bombardment of plasma on the cotton fabric enables the initiation of chemical reaction and leads to alteration of chemical composition of the cotton fabric, i.e. increase of hydrophilic groups such as OH, C=O and COO− that can improve hydrophilicity of the fabric (Zhang et al. 2008; Ceach et al. 2002; Pappas et al. 2006). Besides, transmittance intensity of plasma-treated fabric was lower than that of the untreated fabric. This is mainly attributed to the number of polar groups formed and bonded on the fabric polymer in which transmittance intensity is inversely proportional to concentration of the corresponding groups (Pandiyaraj and Selvarajan 2008).
Furthermore, when the fabrics were treated with BTCA (in the presence or absence of catalysts), new peaks, represented by carbonyls band, were formed. Figure 4 illustrate the FTIR-ATR spectra of BTCA-treated cotton fabric in the presence or absence of SHP. On the other hand, Fig. 5 show the FTIR-ATR spectra of BTCA-treated cotton fabric in the presence or absence of SHP and TiO2.
FTIR-ATR was also used to characterize the intermolecular ester crosslinkages in wrinkle-resistant-treated cotton fabric. In general, the esterification reaction is known to proceed in two steps. The first step involves formation of a five-membered cyclic anhydride intermediate through dehydration of two adjacent carboxylic acid groups. In the presence of catalyst, the formed acid anhydrides subsequently undergo esterification reaction with hydroxyl groups of cellulosic macromolecules to form an ester (Yang and Wang 1996; Bhattacharya et al. 1999; Sricharussin et al. 2004; Kim et al. 2000; Yoon et al. 2003). Therefore, when esterification occurs between polycarboxylic acid and cotton cellulose, carbonyls retained in cotton exist in three forms, i.e. intermolecular ester linkage, carboxyl (the acidic form of the free carboxyl) and carboxylate anion (the basic form of the free carboxyl) (Yang 1991; Yang and Bakshi 1996). Figures 4 and 5 showed two strong bands at 1,730 and 1,570 cm−1 which are due to the ester and carboxylate carbonyls, respectively (Yang 1991; Wei and Yang 1999; Choi et al. 1994).
The ester carbonyl band intensity of treated samples was in accordance with the amount of ester formed on the fabric (Wei and Yang 1999). Therefore, ester carbonyl band transmittance was inversely proportional to the amount of ester crosslinkage and the performance of the treated fabric. From Fig. 4, the spectrum showed that the percentage of transmittance of BTCA-treated fabric is similar to the control fabric, except the new peaks formed at 1,730 and 1,570 cm−1. Moreover, SHP would accelerate the formation of a cyclic anhydride from polycarboxylic acid. When the fabric was treated with BTCA in the presence of SHP, the overall transmittance shifted downward implying a more effective crosslinking process as presented in Fig. 4. In addition, Fig. 5 shows that TiO2 acting as co-catalyst can also enhance the effectiveness of wrinkle resistant finishing.
Energy dispersive X-ray analysis (EDX)
EDX was used to collect elemental information of wrinkle-resistant and plasma-treated cotton fabrics. The atomic percentage of carbon (C), oxygen (O), titanium (Ti), sodium (Na) and phosphorus (P) that appeared in wrinkle-resistant-treated fabrics is shown in Table 3.
Table 3 shows that C content dropped by 1% when the fabric was treated with TiO2 and it dropped by 3 and 6.5% when the fabric was treated with BTCA in the absence and presence of SHP, respectively. In general, the esterification reaction involves formation of a five-membered cyclic anhydride intermediate and subsequent formation of an ester (Yang and Wang 1996; Bhattacharya et al. 1999; Sricharussin et al. 2004; Kim et al. 2000; Yoon et al. 2003). Therefore, when esterification occurs between polycarboxylic acid and cotton cellulose, carbonyls retained in cotton exist in three forms, i.e. intermolecular ester linkage, carboxyl (the acidic form of the free carboxyl) and carboxylate anion (the basic form of the free carboxyl) (Yang 1991; Yang and Bakshi 1996). This may be the reason of reduction in C content. In some cases, EDX results also indicate that TiO2 (small amount of Ti recorded) and SHP (small amount of Na and P recorded) were present in the fibre after treatment (Table 3). Atomic percentages of C, O, Ti, Na and P that appeared in plasma pre-treated fabrics are also shown in Table 3.
Due to the chemical effects of plasma species, new functional groups were created which caused various changes in the surface composition (Karahan and Özdogan 2008). The EDX analysis revealed quantitative surface composition changes after the plasma treatment. Table 3 shows that the C atomic percentage decreased obviously while the oxygen atomic percentage increased. The removal of fibre surface material was related to the drop of C content. Also, the O/C ratio of plasma pre-treated fabric, with or without having been subjected to wrinkle-resistant finishing, increased significantly. This is mainly attributed to incorporation of specific oxygen functional groups by the plasma pre-treatment. The increase in O content led to an improvement of hydrophilicity and wickability. The EDX results shown in Table 3 also prove that TiO2 (small amount of Ti recorded) and SHP (small amount of Na and P recorded) were present in the fibre after wrinkle-resistant-treatment.
Conclusion
In the study, the surface chemical composition of treated cotton specimens was evaluated by FTIR-ATR and EDX. The FTIR-ATR spectra for plasma pre-treated cotton showed new peaks about the C=O vibration groups in the carbonyl structure and O–H stretching vibration and COO− stretching vibrations which improved hydrophilicity of the fabric. FTIR-ATR was also used to characterize the formation of two strong bands about ester and carboxylate carbonyls in wrinkle resistant treated cotton fabric. In addition, EDX confirmed that C content dropped when the fabric was treated with wrinkle resistant treatment and the results also indicate that TiO2 (small amount of Ti recorded) and SHP (small amount of Na and P recorded) were present in the fiber after treatment. Moreover, after the plasma pre-treatment, the C atomic percentage decreased obviously while the oxygen atomic percentage increased which may have led to an improvement of hydrophilicity and wickability as further proved in terms of contact angle, wettability and wicking ability.
References
Bhattacharya N, Doshi BA, Sahasrabudhe AS (1999) Cost effective catalyst for polycarboxylic acid finishing. Textile Chemist Colorist 31(6):33–37
Ceach V, Prikryl R, Balkova R, Grycova A, Vanek J (2002) Plasma surface treatment and modification of glass fibres. Compos A 33:1367–1372
Chen CC, Wang CC (2006) Crosslinking of cotton cellulose with succinic acid in the presence of titanium dioxide nano-catalyst under UV irradiation. J Sol Gel Sci Technol 40(1):31–38
Choi HM, Welch CM, Morris NM (1994) Nonphosphorus catalysts for formaldehyde-free DP finishing of cotton with 1, 2, 3, 4-butanetetracarboxylic acid. Textile Res J 64(9):501–507
Chung C, Lee M, Choe E (2004) Characterization of cotton fabric scouring by FTIR ATR spectroscopy. Carbohydr Polym 58:417–420
Hartzell-Lawson MM, Hsieh Y (2000) Characterizing the noncellulosic in developing cotton fibers. Textile Res J 70(9):810–819
Holme I (2007) Innovative technologies for high performance textiles. Color Technol 123:59–73
Hong SM, Kim SH, Kim JH, Hwang HI (2006) Hydrophilic surface modification of PDMS using atmospheric RF plasma. J Phys Conf Ser 34:656–661
Hwang YJ, Mccord MG (2005) Effects of helium atmospheric pressure plasma treatment on low-stress mechanical properties of polypropylene nonwoven fabrics. Textile Res J 75(11):771–778
Kan CW, Yuen CWM, Lam YL, Chan CK (2009) Effect of enzymatic treatment and reactive dyeing on the low stress mechanical properties of linen fabric. Fibers Polym 10(3):325–332
Kaplan S (2004) Plasma processes for wide fabric, film and non-wovens. Surf Coat Technol 186:214–217
Karahan HA, Özdoğan E (2008) Improvements of surface functionality of cotton fibers by atmospheric plasma treatment. Fibers Polym 9(1):21–26
Kim BH, Jang J, Ko SW (2000) Durable press finish of cotton fabric using malic acid as a crosslinker. Fibers Polym 1(2):116–121
Lam YL, Kan CW, Yuen CWM (2010a) Effect of concentration of titanium dioxide acting as catalyst or co-catalyst on the wrinkle-resistant finishing of cotton fabric. Fibers Polym 11(4):551–558
Lam YL, Kan CW, Yuen CWM (2010b) Wrinkle-resistant finishing of cotton fabric with BTCA—the effect of co-catalyst. Textile Res J. doi:10.1177/0040517510380777
Lam YL, Kan CW, Yuen CWM, Au CH (2010c) Effect of plasma pre-treatment on the wrinkle resistant property of cotton fibre treated with BTCA and SHP system with TiO2 as co-catalyst. J Appl Polym Sci (Accepted)
Li WB, Xu XH, Chen SY, Zhou XP, Li L, Chen DJ, Wang XQ (2008) Esterification crosslinking structures of rayon fibers with 1, 2, 3, 4-butanetetracarboxylic acid and their water-responsive properties. Carbohydr Polym 71(4):574–582
Pandiyaraj KN, Selvarajan V (2008) Non-thermal plasma treatment for hydrophilicity improvement of grey cotton fabrics. J Mater Process Technol 199:130–139
Pappas D, Bujanda A, Demaree JD, Hirvonen JK, Kosik W, Jensen R, McKnight S (2006) Surface modification of polyamide fibers and films using atmospheric plasmas. Surf Coat Technol 201(7):4384–4388
Rajpreet KV, Gita NR (2004) Plasma and antimicrobial treatment of nonwoven fabrics for surgical gowns. Textile Res J 74(12):1073–1079
Sricharussin W, Ryo-Aree W, Intasen W, Poungraksakirt S (2004) Effect of boric acid and BTCA on tensile strength loss of finished cotton fabrics. Textile Res J 74(6):475–480
Titov VA, Rybkin VV, Shikova TG, Ageeva TA, Golubchikov OA, Choi HS (2005) Study on the application possibilities of an atmospheric pressure glow discharge with liquid electrolyte cathode for the modification of polymer materials. Surf Coat Technol 199(2–3):231–236
Tzanov T, Cavaco-Paulo A (2006) Surface modification of cellulose fibers with hydrolases and kinases. Modified fibers with medical and specialty applications, pp 159–180
Wang CC, Chen CC (2005a) Physical properties of crosslinked cellulose catalyzed with nano titanium dioxide. J Appl Polym Sci 97(6):2450–2456
Wang CC, Chen CC (2005b) Physical properties of the crosslinked cellulose catalyzed with nanotitanium dioxide under UV irradiation and electronic field. Appl Catal A Gen 293(9):171–179
Wang CX, Qiu YP (2007) Two sided modification of wool fabrics by atmospheric pressure plasma jet—influence of processing parameters on plasma penetration. Surf Coat Technol 201:6273–6277
Wang CX, Liu Y, Xu HL, Ren Y, Qiu YP (2008) Influence of atmospheric pressure plasma treatment time on penetration depth of surface modification into fabric. Appl Surf Sci 254(8):2499–2505
Wei WS, Yang CQ (1999) Predicting the performance of durable press finished cotton fabric with infrared spectroscopy. Textile Res J 69(2):145–151
Yang CQ (1991) Characterizing ester crosslinkages in cotton cellulose with FTIR photoacoustic spectroscopy. Textile Res J 61(5):298–305
Yang CQ, Bakshi GD (1996) Quantitative analysis of the nonformaldehyde durable press finish on cotton fabric: acid-base titration and infrared spectroscopy. Textile Res J 66(6):377–384
Yang CQ, Wang X (1996) Formation of cyclic anhydride intermediates and esterification of cotton cellulose by multifunctional carboxylic acids: an infrared spectroscopy study. Textile Res J 66(9):595–603
Yoon KJ, Woo JH, Seo YS (2003) Formaldehyde free cross-linking agents based on maleic anhydride copolymers. Fibers Polym 4(4):182–187
Yuen CWM, Ku SKA, Kan CW, Cheng YF, Choi PSR, Lam YL (2007) Using nano-TiO2 as co-catalyst for improving wrinkle-resistant of cotton fabric. Surf Rev Lett 14(4):571–575
Zhang HJ, Zhang ZZ, Guo F, Liu WM (2008) The influence of plasma treatment on the tribological properties of hybrid PTFE/cotton fabric/phenolic composites. Polym Compos 30(10):1523–1531
Acknowledgments
This work was supported by a grant from the Research Grant Council of the Hong Kong Special Administrative Region, China, under project PolyU 5192/08E and a financial assistance from The Hong Kong Polytechnic University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lam, Y.L., Kan, C.W. & Yuen, C.W.M. Physical and chemical analysis of plasma-treated cotton fabric subjected to wrinkle-resistant finishing. Cellulose 18, 493–503 (2011). https://doi.org/10.1007/s10570-011-9498-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-011-9498-y