Abstract
A thin film of cobalt ferrite (CoFe2O4) was successfully deposited through nebulizer assisted spray pyrolysis technique. Composition of elements in the thin film plays an important role in determining its magnetic properties. Significant changes in the structural and magnetic properties can be observed in the CoFe2O4 thin films when doped with rare earth elements making it suitable for the applications in magnetic and related devices. Undoped and neodymium-doped CoFe2O4 thin films were prepared through nebulizer assisted spray pyrolysis (NSP) method on bare glass substrates by changing the percentage of Nd doping level from 0 to 5% atomic weight. Standard characterization techniques were used to analyze the deposited films to reveal the effect of doping. Crystallite size is estimated from x-ray diffraction spectra, and is observed to be of the order of 14 nm. The size of the crystallites in the film seems to decrease with the increase in doping concentration. The optical band gap value shows an increase and gets shifted from 1.82 to 1.95 eV on increasing the concentration of Nd doping. The saturation magnetization of the prepared CoFe2O4 films obtained at 0% and 5% show a strong dependence on the Nd doping concentration. This study shows that the Nd-doped CoFe2O4 films can compete with the existing materials for magnetic device applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The architecture of the thin film plays a vital role in most of the electronic devices that use magnetic materials. The magnetic thin films are commonly utilized in technological applications such as magnetic recording and in micro-electromechanical applications (Garcia-Sanchez et al. 2008). Spinel ferrite materials are interesting since they exhibit ferrimagnetic and semiconductor properties that suggest their usage as magnetic media for in numerous applications such as in recording high density data (Khandekar et al. 2011), generation and detection of ultrasonic waves, electronics and telecommunication (Šutka and Mezinskis 2012), gas sensors (Deraz 2010), torque sensors and magnetic hyperthermia (Kim et al. 2008). Its characteristic property such as strong anisotropy, high mechanical hardness and chemical stability paved way for its usage in several industrial applications too. Thus, great efforts have been made for the synthesis and characterization of nano-sized CoFe2O4 materials. The synthesis method decides the distribution of ions in different positions of the spinnel structure which determines the composition as well as microstructure of these films (Tiwari et al. 2020). The larger surface area to volume ratio as the size of the bulk material is reduced to nano material creates distinct physical, chemical, mechanical and especially magnetic properties due to the presence of more number of atoms on the surface creating spin glass behavior and superparamagnetism (Cheng et al. 2005).
Adeela et al. (2015) investigated the influence of manganese substitution on structural and magnetic properties of CoFe2O4 nanoparticles through the co-precipitation technique and found that the saturation magnetization and coercivity values of these thin films increased up to 30% of Mn concentration. Rao et al. (2015) showed that the saturation magnetization and remnant magnetization depend on particle size as well as crystallinity of the nanoparticles. Maaz et al. (2007) observed an increased magnetic moment for particles of smaller size. Houshiar et al. (2014) compared the values of coercivity and saturation magnetization of CoFe2O4 thin films prepared through different routes and reported that the values are better when CoFe2O4 was synthesized through the combustion method rather than using precipitation techniques. Gandha et al. (2015) prepared CoFe2O4 nano particles of size 40 nm with higher coercivity values using hydrothermal method. Gingasu et al. (2016) revealed the antibacterial effect of Ag-doped CoFe2O4 nanoparticles prepared through self-combustion and wet ferritization using aqueous extracts of the leaves and flowers of Hibiscus rosa sinensis. The photocatalytic activity of these nanoparticles on rhodamine B dye was studied by Nguyen et al. (To Loan et al. 2019). The dependence of the superparamagnetic effect on the temperature and applied magnetic field was investigated by Ojha and Kant (2019). The photo degradation property of CoFe2O4/SiO2 nanocomposites using methylene blue dye was investigated by Yakob et al. (2019).
Doping of rare Earth such as Neodymium into cobalt ferrite compounds varies the magnetic anisotropy and results in a large magnetostriction effect even at room temperature (Forester et al. 1978). The magneto optic Kerr effect also gets increased on doping ferrites with rare Earth elements (Avazpour et al. 2016; Cedeño-Mattei et al. 2010) along with an increase in the values of coercivity (Cheng et al. 1999; Karimi et al. 2014), saturation magnetization (Yan et al. 1998) as well as conductivity (Rahman et al. 2014).
Several synthesis techniques were used to prepare Nd-doped films of CoFe2O4 (Abbas et al. 2016; Mounkachi et al. 2017; Xavier et al. 2013). There is no report available on the effect of neodymium doping on the structural, morphological, and magnetic properties of cobalt ferrite thin films deposited through the NSP method. Nebulizer spray pyrolysis is an effective and economic method of depositing various thin films under controlled conditions. It is a common chemical deposition method of depositing oxides. It relies on the production and transport of fine aerosols produced by the nebulizer head toward the hot substrate surface. The characteristics of the deposited films were tailored by varying the deposition conditions such as substrate temperature, spray head and substrate distance, spray rate, solvent, etc. The method is much sought due to its favorable advantages such as ease of its assembly, the capability of doping, control over composition, moderate temperature environment, uniformity in thickness and overall quality of the films. Hence we are focusing on the influence of neodymium doping on the magnetic properties of CoFe2O4 thin films fabricated using the economically feasible nebulizer assisted spray pyrolysis technique.
Experimental details
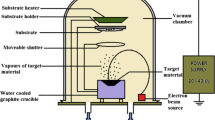
Analytical grade cobalt chloride hexahydrate [CoCl2.6H2O], ferric (III) nitrate (FeN3O9) neodymium (III) acetate (Nd(O2C2H3)3.H2O) were used as the precursors for cobalt, ferrite and neodymium atoms respectively to deposit the CoFe2O4:Nd thin films on bare soda lime glass substrate using nebulizer spray pyrolysis technique. 0.05 M and 0.1 M concentration of cobalt chloride and ferric (III) nitrate was dissolved in de-ionized water (10 ml). The substrate temperature was maintained to be 375 °C (± 5 °C) during the fabrication of all the samples. The compressed air is used as a carrier gas in the nebulizer spray unit to produce fine aerosols of the precursor solution and its flow rate was optimized to be 1.5 megapascal (Mpa). A 40 mm distance was fixed between the head of the spray nozzle and substrate throughout the experiment. However, the percentage of neodymium ion doping concentration was varied as 0%, 3% and 5%.
Characterization
The prepared films were analyzed using standard characterizing techniques to analyze their properties. A PANalytical PW 340/60 x-ray diffractometer was used in the range of 10°to 80° with a source wavelength of 1.5416 Å. An S-3400 N model scanning electron microscope supplied by Hitachi annexed with an EDX spectrometer was utilized to analyze the surface morphology and the elemental composition in the films. The surface morphology was studied further using Digital instruments Nanoscope IV. Perkin Elmer Lambda 35 UV–vis NIR spectrometer was employed to study the optical properties of the samples in the range of 500 nm to 2500 nm. LAKE SHORE 7404 vibrating sample magnetometer was used to study the change in the magnetic properties when Nd ions were doped into the CoFe2O4 lattice.
Result and discussion
Structural characterization
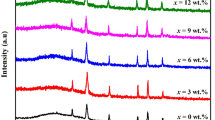
Figure 1 shows the obtained x-ray diffraction patterns of undoped CoFe2O4 and Nd-doped CoFe2O4 thin films prepared with simple nebulizer spray pyrolysis method. The polycrystalline characteristic of the films was confirmed using JCPDS card No. 22-1086 corresponding to cubic structured CoFe2O4 (Yadav et al. 2016). In spite of the variation in the Nd doping concentration, all the samples exhibited a dominant peak along the (311) plane representing the major orientation and phase purity of CoFe2O4. This XRD pattern also confirms the growth of CoFe2O4 polycrystalline thin films with crystallites also oriented along (220) and (511) directions. Similar single phase presence of these polycrystalline films was reported earlier using the spray pyrolysis technique (Zongyan and Xiang 2015) and also in spin coating methodology (Bagade and Rajpure 2015). As observed in Fig. 1, the intensity of the CoFe2O4 films decreases with the increase in the Nd doping concentration from 0 to 5% which might be due to the increase in the values of stress in the deposited films because of the radius of the Nd3+ ion (0.998 Å). No other peak is detected in the XRD studies for any other impurities other than neodymium (Nd). A considerable decrease in the peak intensity is seen in the XRD spectra with the increase in the Nd doping concentration particularly along (311) and (220) planes. This decrease may be related to the structural disorder in the films induced by the introduction of dopant ions (Fitriyanti and Utari 2017).
The crystallite size (D) of thin films was estimated with reference to the plane (311) using Scherrer's formula (Srivastava et al. 1982)
The obtained crystallite size through this equation is associated to the mean minimum dimension of a coherent diffraction domain. It is found that the crystallite size estimated from (311) peak of CoFe2O4 is decreased from 19 to 14 nm when Nd doping concentration was raised from 0 to 5%. When the dopant concentration increases, the number of nucleation site increases and it leads to a decrease in the crystallite size (Garcés Pineda et al. 2015).
The measured crystallite size and additional parameters of the CoFe2O4 films are presented in Table 1.
Dislocation density (δ) and strain (ɛ) was calculated from crystallite size using equations (Tiwari et al. 2020).
The variation of δ and ε of undoped and Nd-doped CoFe2O4 thin films are given in Table 1. It is observed from Table 1 that the dislocation density and micro-strain values show an increase when the Nd doping level is increased. The increment in the values of strain causes a decrement in the size of the crystallites.
Morphological analysis using SEM
In order to understand the structure of grains in the prepared films, SEM micrographs are taken and Fig. 2a–c shows the SEM images of nano grain structured Nd: CoFe2O4 thin films with doping concentrations 0%, 3%, and 5%, respectively.
From the figure, it is observed that all the samples show small nano sized spherical shaped grains uniformly covering the substrate. The grain size gradually decreases as the Nd doping concentration was raised from 0 to 5%. When neodymium ions replace ferric ions in the CoFe2O4 lattice, strains were produced due to the change in lattice parameters and hence a stress is induced in the lattice which hinders the growth of grains. Thus when the doping concentration increases, grain growth gets decreased (Zubair et al. 2017).
Morphological analysis using AFM
An Atomic Force Microscope (AFM) was used for further morphological analysis. The AFM micrographs are shown in Fig. 3.
All the films were found to have good adherence to the substrate. 5% Nd-doped film shows spherical grains with uniform distribution. The grain size for the 5% doped film was measured to be 8 nm. The pure and 3% Nd-doped films exhibit a particle size of 10 and 15 nm, respectively (Nečas and Klapetek 2012). The corresponding roughness of the films was calculated to be 12.6 nm, 13.8 nm and 17.1 nm, respectively.
Compositional analysis
The elemental analysis of pristine and 5% Nd-doped CoFe2O4 thin films was carried out by energy-dispersive x-ray spectroscopic technique.
Figure 4 depicts the EDX spectra of undoped and 5% Nd-doped spinel cobalt ferrite thin films. It is seen from Fig. 4a that the constituent elements Co, Fe and O are present. The presence of rare earth Nd in the deposited film was ascertained by its presence in the EDX spectra as shown in Fig. 4b. The spectra also confirm the fact that the prepared thin films were free from other impurities except for the peaks for Si from the substrate. The corresponding elemental mapping of undoped and 5% Nd-doped CoFe2O4 films are shown in Fig. 5.
Optical studies
The graph of incident wavelength (λ) vs. optical transmittance (T) and absorption spectrum of CoFe2O4 thin films prepared with changing Nd doping concentration is shown in Fig. 6a, b.
It is seen that the transmittance of CoFe2O4 thin film shows an increase with incident wavelength and also with the increases in the Nd doping. It presents a decrementing value in the absorbance of the samples with an increase in the doping concentration of Nd ion. It is also observed that the reflectance of the ferrite film increases when doped with neodymium. Incorporation of Nd increases the reflectance of the deposited film around the wavelength region of 1000 to 2000 nm. Further, the absorption edge shows a blue shift when Nd doping level was increased, which affects the improvement in the band structure.
Optical band gap (Eg) of the prepared films was determined from Tauc formula (Tiwari et al. 2020)
The band gap estimated from Tauc's plot is shown in Fig. 7.
The obtained band gap increases from 1.82 eV to 1.95 eV as the Nd doping concentration was changed from 0 to 5%. The enhancement in the values of bandgap may be attributed to the quantum confinement effect (Arulanantham et al. 2018) and also due to the least values of grain size in the deposited polycrystalline film. It might also be attributed to the Burstein—Moss effect which shifts the Fermi level into the conduction band and hence necessitates larger energy for the transition from the valence band to conduction band resulting in an increased band gap.
Magnetic studies
The magnetic property of undoped and 5% Nd-doped CoFe2O4 thin films was studied by employing a vibrating sample magnetometer (VSM) at room temperature (300 K) under an applied magnetic field up to ± 1.5 T parallel as well as perpendicular to the film surface. Magnetic hysteresis loops of undoped and 5% Nd-doped CoFe2O4 thin films recorded under the application of a parallel magnetic field are shown in Fig. 8.
A typical hysteresis loop is observed for both pure and Nd-doped CoFe2O4 thin films supporting the existence of room temperature ferromagnetism. The value of saturation magnetization for pure CoFe2O4 thin films was observed to be 34.2 emu.gm−1 and when doped with 5% Nd3+, the 40.8 emu.gm−1. The value of saturation magnetization was found to increase with the increase of Nd3+ substitution in cobalt ferrite. These values agree well with the earlier literature (Lee et al. 1998). The Nd ions get substituted in the octahedral site of the CoFe2O4 spinel structure. Neodymium substitution increases the structural stability of the cobalt ferrite film due to which inhomogeneous spin structure gets suppressed and hence an increased value in the saturation magnetization (Yan et al. 2007).
Conclusion
In this work, cobalt ferrite thin films were deposited on the glass substrates with different Nd doping concentrations. The deposited undoped and Nd-doped CoFe2O4 films have excellent adherence and were found to have a good uniformity on the substrate surface. The XRD spectra indicate that the films have a cubic crystal structure. The Raman analysis established peaks corresponding to octahedral as well as tetrahedral vibrations of the lattice. The presence of the rare earth ions as well as the main elements of the ferrite films were affirmed by EDX analysis. The SEM images show a decrease in the size of the nano spherical CoFe2O4 grains with the increase in the doping concentration. The magnetic studies show an enhancement in the magnetic properties of CoFe2O4 thin films with Nd doping. Hence this study reveals that 5% of Nd doping in CoFe2O4 film enhances its structural, morphological, optical as well as magnetic properties and nebulizer assisted spray pyrolysis can be adopted as cheap technique for mass production of Nd-doped CoFe2O4 thin films.
References
Abbas S, Munir A, Zahra F, Rehman MA (2016) Enhanced electrical properties in Nd doped cobalt ferrite nano-particles. IOP Conf Ser Mater Sci Eng 146:12027. https://doi.org/10.1088/1757-899x/146/1/012027
Adeela N, Maaz K, Khan U, Karim S, Nisar A, Ahmad M, Ali G, Han XF, Duan JL, Liu J (2015) Influence of manganese substitution on structural and magnetic properties of CoFe2O4 nanoparticles. J Alloys Compd 639:533–540. https://doi.org/10.1016/j.jallcom.2015.03.203
Arulanantham AMS, Valanarasu S, Kathalingam A, Jeyadheepan K (2018) Solution volume effect on structural, optical and photovoltaic properties of nebulizer spray deposited SnS thin films. J Mater Sci Mater Electron 29:12899–12909. https://doi.org/10.1007/s10854-018-9409-1
Avazpour L, Toroghinejad MR, Shokrollahi H (2016) Enhanced magneto-optical Kerr effect in rare earth substituted nanostructured cobalt ferrite thin film prepared by sol–gel method. Appl Surf Sci 387:869–874. https://doi.org/10.1016/j.apsusc.2016.06.168
Bagade AA, Rajpure K (2015) Development of CoFe2O4 thin films for nitrogen dioxide sensing at moderate operating temperature. J Alloys Compd 657:414–421. https://doi.org/10.1016/j.jallcom.2015.10.115
Cedeño-Mattei Y, Perales-Pérez O, Uwakweh ONC, Xin Y (2010) Colossal room-temperature coercivity in size-selected cobalt ferrite nanocrystals. J Appl Phys 107:09A741. https://doi.org/10.1063/1.3339781
Cheng F, Liao C, Kuang J, Xu Z, Yan C, Chen L, Zhao H, Liu Z (1999) Nanostructure magneto-optical thin films of rare earth (RE=Gd, Tb, Dy) doped cobalt spinel by sol–gel synthesis. J Appl Phys 85:2782–2786. https://doi.org/10.1063/1.369594
Cheng Y, Zheng Y, Wang Y, Bao F, Qin Y (2005) Synthesis and magnetic properties of nickel ferrite nano-octahedra. J Solid State Chem 178:2394–2397. https://doi.org/10.1016/j.jssc.2005.05.006
Deraz NM (2010) Glycine-assisted fabrication of nanocrystalline cobalt ferrite system. J Anal Appl Pyrolysis 88:103–109. https://doi.org/10.1016/j.jaap.2010.03.002
Fitriyanti E, Utari PB (2017) Comparison XRD pattern of CoFe2O4 thin films and nanoparticles. J Phys Conf Ser 909:12010. https://doi.org/10.1088/1742-6596/909/1/012010
Forester DW, Vittoria C, Schelleng J, Lubitz P (1978) Magnetostriction of amorphous TbxFe1−x thin films. J Appl Phys 49:1966–1968. https://doi.org/10.1063/1.324765
Gandha K, Elkins K, Poudyal N, Liu JP (2015) Synthesis and characterization of CoFe2O4 nanoparticles with high coercivity. J Appl Phys 117(1–4):17A736. https://doi.org/10.1063/1.4916544
Garcés Pineda FA, Budini N, Koropecki R, Arce R (2015) Structural analysis of ZnO(:Al, Mg) thin films by X-ray diffraction. Procedia Mater Sci 8:551–560. https://doi.org/10.1016/j.mspro.2015.04.108
Garcia-Sanchez F, Chubykalo-Fesenko O, Mryasov O, Asselin P, Chantrell R (2008) Switching and thermal stability properties of bilayer thin films: single versus multigrain cases. J Appl Phys 103:07F505-07F505. https://doi.org/10.1063/1.2829584
Gingasu D, Mindru I, Patron L, Calderon-Moreno JM, Mocioiu OC, Preda S, Stanica N, Nita S, Dobre N, Popa M, Gradisteanu G, Chifiriuc MC (2016) Green synthesis methods of CoFe2O4 and Ag-CoFe2O4 nanoparticles using hibiscus extracts and their antimicrobial potential. J Nanomater 2016:2106756. https://doi.org/10.1155/2016/2106756
Houshiar M, Zebhi F, Razi ZJ, Alidoust A, Askari Z (2014) Synthesis of cobalt ferrite (CoFe2O4) nanoparticles using combustion, co precipitation, and precipitation methods: A comparison study of size, structural, and magnetic properties. J Magn Magn Mater 371:43–48. https://doi.org/10.1016/j.jmmm.2014.06.059
Karimi Z, Mohammadifar Y, Shokrollahi H, Asl SK, Yousefi G, Karimi L (2014) Magnetic and structural properties of nano sized Dy-doped cobalt ferrite synthesized by co-precipitation. J Magn Magn Mater 361:150–156. https://doi.org/10.1016/j.jmmm.2014.01.016
Khandekar MS, Kambale R, Patil J, Kolekar Y, Suryavanshi SS (2011) Effect of calcination temperature on the structural and electrical properties of cobalt ferrite synthesized by combustion method. J Alloy Compd 509:1861–1865. https://doi.org/10.1016/j.jallcom.2010.10.073
Kim D-H, Nikles D, Johnson D, Brazel C (2008) Heat generation of aqueously dispersed CoFe2O4 nanoparticles as heating agents for magnetically activated drug delivery and hyperthermia. J Magn Magn Mater 320:2390–2396. https://doi.org/10.1016/j.jmmm.2008.05.023
Lee JG, Park JY, Oh YJ, Kim CS (1998) Magnetic properties of CoFe2O4 thin films prepared by a sol-gel method. J Appl Phys 84:2801–2804. https://doi.org/10.1063/1.368393
Maaz K, Mumtaz A, Hasanain SK, Ceylan A (2007) Synthesis and magnetic properties of cobalt ferrite (CoFe2O4) nanoparticles prepared by wet chemical route. J Magn Magn Mater 308:289–295. https://doi.org/10.1016/j.jmmm.2006.06.003
Mounkachi O, Lamouri R, Abraime B, Ez-Zahraouy H, El Kenz A, Hamedoun M, Benyoussef A (2017) Exploring the magnetic and structural properties of Nd-doped Cobalt nano-ferrite for permanent magnet applications. Ceram Int 43:14401–14404. https://doi.org/10.1016/j.ceramint.2017.07.209
Nečas D, Klapetek P (2012) Gwyddion: an open-source software for SPM data analysis. Open Phys 10:181–188. https://doi.org/10.2478/s11534-011-0096-2
Ojha VH, Kant KM (2019) Temperature dependent magnetic properties of superparamagnetic CoFe2O4 nanoparticles. Phys B Condens Matter 567:87–94. https://doi.org/10.1016/j.physb.2019.04.035
Rahman MT, Vargas M, Ramana C V (2014) Structural characteristics, electrical conduction and dielectric properties of gadolinium substituted cobalt ferrite. J Alloys Compd 617:547–562. https://doi.org/10.1016/j.jallcom.2014.07.182
Rao KS, Choudary GSVRK, Rao KH, Sujatha C (2015) Structural and magnetic properties of ultrafine CoFe2O4 nanoparticles. Procedia Mater Sci 10:19–27. https://doi.org/10.1016/j.mspro.2015.06.019
Srivastava CM, Srinivasan C, Aiyar R (1982) Exchange constants in ferrimagnetic garnets. J Appl Phys 53:781–783. https://doi.org/10.1063/1.329990
Šutka A, Mezinskis G (2012) Sol-gel auto-combustion synthesis of spinel-type ferrite nanomaterials. Front Mater Sci 6:128–141. https://doi.org/10.1007/s11706-012-0167-3
Tiwari R, De M, Tewari HS, Ghoshal SK (2020) Structural and magnetic properties of tailored NiFe2O4 nanostructures synthesized using auto-combustion method. Results Phys 16:102916. https://doi.org/10.1016/j.rinp.2019.102916
To Loan TN, Hien Lan TN, Thuy Hang TN, Quang Hai N, Tu Anh TD, Thi Hau V, Van Tan L, Van Tran T (2019) CoFe2O4 nanomaterials: effect of annealing temperature on characterization, magnetic, photocatalytic, and photo-fenton properties. Process 7:885. https://doi.org/10.3390/pr7120885
Xavier S, Thankachan S, Jacob BPME (2013) Effect of neodymium substitution on structural and magnetic properties of cobalt ferrite nanoparticles. J Nanomater Mol Nanotechnol 2:7. https://doi.org/10.4172/2324-8777.1000133
Yadav RS, Havlica J, Masilko J, Kalina L, Wasserbauer J, Hajdúchová M, Enev V, Kuřitka I, Kožáková Z (2016) Impact of Nd3+ in CoFe2O4 spinel ferrite nanoparticles on cation distribution, structural and magnetic properties. J Magn Magn Mater 399:109–117. https://doi.org/10.1016/j.jmmm.2015.09.055
Yakob M, Umar H, Wahyuningsih P, Putra RA (2019) Characterization of microstructural and optical CoFe2O4/SiO2 ferrite nanocomposite for photodegradation of methylene blue. AIMS Mater Sci 6:45–51. https://doi.org/10.3934/matersci.2019.1.45
Yan C, Cheng F, Peng Z, Xu Z, Liao C (1998) Fabrication and magnetic investigation of Y or Gd containing CoFe2O4 nanocrystalline films. J Appl Phys 84:5703–5708. https://doi.org/10.1063/1.368834
Yan Z, Wang KF, Qu JF, Wang Y, Song ZT, Feng SL (2007) Processing and properties of Yb-doped BiFeO3 ceramics. Appl Phys Lett 91:82906. https://doi.org/10.1063/1.2775034
Zongyan Z, Xiang Z (2015) Electronic, optical, and mechanical properties of Cu2ZnSnS4 with four crystal structures. J Semicond 36:1–13. https://doi.org/10.1088/1674-4926/36/8/083004
Zubair A, Ahmad Z, Mahmood A, Cheong W-C, Ali I, Khan MA, Chughtai AH, Ashiq MN (2017) Structural, morphological and magnetic properties of Eu-doped CoFe2O4 nano-ferrites. Results Phys 7:3203–3208. https://doi.org/10.1016/j.rinp.2017.08.035
Acknowledgements
The author from KKU extend his appreciation to the research center for advance materials science (RCAMS), King Khalid University for funding this work under grant number RCAMS/KKU/ G014-21.
Funding
UGC-DAE Consortium for Scientific Research, University Grants Commission, CSR-IC-MSRSR-20/CRS-228/2021-22/634, Gunavathy K V.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare no conflict of interest among the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arulanantham, A.M.S., Gunavathy, K.V., Antony, M. et al. Tuning the magnetic properties of neodymium (Nd)-doped cobalt ferrite thin films through nebulizer spray technique. Chem. Pap. 76, 6349–6358 (2022). https://doi.org/10.1007/s11696-022-02319-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-022-02319-5