Abstract
SnS thin films deposited using nebulizer spray pyrolysis method by changing the precursor solution volume is reported in this work. The prepared films were characterized by X-ray diffraction, scanning electron microscopy, Raman spectrum, atomic force microscopy, EDAX, UV–Vis spectroscopy and PL spectrofluorometer analysis. Electrical studies done using Hall Effect measurement for the film is also reported. Structural and surface morphological analyses showed high crystalline single phase of SnS thin films with relatively low surface roughness. Optical studies done on the films revealed a decrease in band gap from 1.82 to 1.73 eV for the increase of solution volume from 5 to 15 ml. The CdS film prepared by this method showed a maximum of 75% transmittance and band gap of 2.51 eV. Prepared SnS thin film showed p-type conductivity with resistivity 2.01 × 101 Ω cm and carrier concentration 4.71 × 1017/cm3. A FTO/n-CdS/p-SnS heterostructure was also fabricated using the grown film and studied its photoconductivity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In the last 20 years researchers have been paying attention on the renewable energy sources for power generation. Development of new photoactive materials with high mobility is an easy way to fulfill the energy demand with reduced pollution to the environment [1]. Tin sulfide (SnS) having narrow band gap attracts much in recent years and also it exhibits optical activity in the near-infrared (NIR) which is suitable for solar cell applications. Low-cost, plenteous and non hazardous are also the advantages of this material to select for solar cell application. It also possesses enviable properties for solar cell applications such as high hole mobility and high absorption [2]. Sn–S phase diagram has three known stoichiometry compounds, with various ratios of tin and sulfur: SnS, SnS2 and Sn2S3 are most technologically interesting materials. Among these three phases Tin sulfide (SnS) is attracted for solar cell applications as it has narrow, direct and indirect band gap from 1.1 to 2.33 eV [3]. Its enhanced conduction mechanism, optical properties such as high optical absorption (> 10 4cm−1) and Raman spectral shift in the visible region [4] are also suitable for the solar cell applications. In spite of its attractive properties, there are only few reports on the fabrication and characterization of SnS thin film based solar cells. Reddy et al. [5] designed solar cells using spray deposited SnS absorber layer and indium doped CdS window layer with a photovoltaic conversion efficiency of about 1.3%. Avellaneda et al. [6] reported chemically deposited SnS thin film solar cells and obtained conversion efficiency of 0.2%. Ghosh et al. [7] reported an efficiency of 0.003% for SnS/ZnO heterostructure fabricated using electrodeposited ZnO and thermally evaporated SnS thin films. However, theoretical studies on conversion efficiency of SnS based solar cells reported as 24% [8]. This large difference between the experimental and theoretical efficiencies may be due to the presence of defects and impurities resulted from the preparation methods used to make the films. Hence, a special attention is needed to improve the photovoltaic properties of SnS.
As per the literature available on SnS, different techniques such as chemical bath deposition(CBD) [6], atmospheric pressure chemical vapour deposition [6], electrodeposition [7], co-evaporation [8], SILAR [9], atomic layer deposition (ALD) [10], plasma-enhanced chemical vapor deposition (PECVD) [11], metal organic chemical vapor deposition (MOCVD) [12], spray pyrolysis [13] electrodeposition [14] and d.c. magnetron sputtering [15] techniques were used to fabricate SnS thin films. In this work, structural, morphological, optical, and electrical properties of SnS thin films deposited by nebulizer spray using different solution volumes (5, 10 and 15 ml) are reported.
2 Experimental details
2.1 SnS and CdS layer preparation
SnS thin films were deposited on glass substrates using nebulizer spray pyrolysis (NSP) technique by varying the solution volume from 5 to 15 ml. SnCl2·2H2O (0.1 M) and thiourea (0.1 M) were dissolved in deionised water to prepare precursor solution. Some drops of Conc. HCl were added and stirred to produce complete dissolution of the precursors. Compressed air with pressure 0.15 Pascal was used as carrier gas to produce fumes in the nebulizer spray. The distance between spray nozzle and substrate was fixed as 50 mm and the effective substrate area as approximately 2.5 cm2 and keeping the substrate at 325 °C. The chamber was provided with an exhaust fan to continuously remove the solvent vapors and other gaseous by-products. A microprocessor controlled stepper motor system was used to move the nebulizer spray gun in x–y direction and get uniform coatings. After deposition and cooling down to room temperature the films were removed from the heater for further characterization and thickness measurement. Similarly, the CdS films were also sprayed using cadmium chloride and thiourea of each 0.1 M in 5 ml of distilled water keeping the substrate at 320 °C.
2.2 Characterization
The deposited SnS films were characterized to investigate the structural, morphological, compositional, optical and electrical properties using XRD, SEM, AFM and Raman studies. X-ray diffractometer (PANanalytical X’ Pert PRO) with CuKα radiation in the Bragg angle range of 10–80° was used for structural study. Raman analysis was done by STR 500 mm Focal Length Laser Raman Spectrometer to verify the compound formed. EVO 18 Carl ZEISS scanning electron microscope attached with EDAX was used to analyze the morphology and elements presented in the film. A double-beam UV– Vis–NIR Spectrophotometer (Lambda Perkin Elmer) was used in the range 300–1100 nm to obtain transmittance and absorption spectra of the films and to calculate the optical bandgap of the films. The photoluminescence spectra were studied using a spectrofluorometer. Keithley interactive digital source-meter (Model-2450) was used for Hall Effect and current–voltage measurement of SnS and CdS/SnS Heterostructure thin films.
3 Result and discussion
3.1 Structural studies
3.1.1 XRD analysis
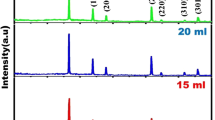
Figure 1 presents the XRD pattern of SnS thin films grown onto glass substrates using different volumes of solution from 5 to 15 ml. The XRD pattern confirmed that the grown films are in polycrystalline nature with crystallites orientation dominant along (210) and (111) directions. All the peaks including the smaller peaks (3 0 1), (2 1 1), and (4 0 2) correspond to JCPDS Card No. (65-2610) of orthorhombic structure. As seen in the figure, the intensity of (111) peak at 31.6° is increased with the increase of solution volume from 5 to 10 ml, showing the enhanced orientation and crystalline quality of the films formed at 10 ml solution. Both the thickness and crystallite size of the films were increased with the increase of solution volume from 5 to 10 ml. These structural details were also in accordance with the reported data of SnS films [16, 17]. For an increase in volume of solution from 10 to 15 ml, the intensity of the peaks is decreased which indicates the crystalline quality of the film is slightly decreased. These results indicate that increasing of solution volume (15 ml) degrades the crystallinity of the film due to the influence of stress arising from the peeling of the SnS film which also reduces the thickness of the film [18]. Solution volume is also found to influence on film thickness and crystallite size of the films. The calculated thickness and its corresponding crystallites sizes for the variation of solution volume are shown in Table 1.
From the observed 2θ values and its corresponding planes the lattice parameter were calculated using Eq. (1) [19].
The calculated lattice constant values of SnS films are tabulated in Table 2, and those calculated values are in agreement with the values of JCPDS card file data (65-2610). The volume of crystal unit cell calculated is also found to be increased with the increase of volume of solution as shown in the Table 2. This increase of crystal volume due to the increase of solution volume may be attributed to stress created by non-hydrostatic pressure [20].
To calculate the crystallite size (D) of SnS films the XRD profile information was used in Scherrer’s equation (2) [3, 19].
where D is average crystallite size, β is full width at half maximum (FWHM) value of XRD peak, λ is the wavelength of X-ray used, K is the Scherrer constant(K = 0.9) and θ is the Bragg angle for the peak. The crystallite size of the films calculated using the plane (111) is given in the Table 1. It shows an increase of crystallite size with the increase of solution volume from 5 to 10 ml. This increase of crystallite size with solution volume increase is also witnessed by XRD studies. Further increase of solution volume from 10 to 15 ml the crystallite size is decreased. So, the higher solution volume reduces the size of crystallites and also thickness of the film.
Dislocation density (δ) of a film can be estimated from crystallite size using Williamson and Smallman’s formula (3) [21].
Microstrain (ɛ) of the SnS films can be calculated using Eq. (4) [21, 22]
Table 1 shows the variation of dislocation density and microstrain with the different solution volume. The calculated dislocation density and micro strain are found to decrease with the increase of solution volume and consequently the thickness of the films is increased with the solution volume from 5 to 10 ml. Further increase of solution volume from 10 to 15 ml the dislocation density and strain are increased. This result attributes the peeling effect of solution volume on nucleation leading to poor crystallinity of the film for 15 ml volume. The smaller values of dislocation density (δ) and microstrain (ε) of the film coated at 10 ml volume indicates a regular and smooth arrangement of crystallites with fewer defects. This perhaps happens because, on keeping the 10 ml volume of solution, more energy is supplied to the dislocations and increases the mobility of the atoms. The dislocations gradually move towards the grain boundaries and get neutralized [23]. Figure 1 also shows the XRD pattern of nebulizer spray deposited CdS film. Observed XRD pattern corresponds to JCPDS Card No. (41-1049) indicating the formation of polycrystalline hexagonal phase CdS.
3.1.2 Raman Analysis
Figure 2 shows room temperature Raman spectra of SnS films obtained in the range 50–500 cm−1. The predominant Raman modes LO and TO are obtained in the region 50–500 cm−1. Raman modes observed at 68, 95 cm, 164 and 217 cm−1 associated to different optical phonons modes of SnS phase, which agrees with the literature [24, 25]. In general, the presences of different modes are caused by the direction of incident and scattering along the axis (a, b, c) and the direction of electrical polarization of incident and scattered photons [26]. The peaks presented at 68 and 95 cm−1 associated to Ag, B2g mode and transverse optical Ag (TO) mode, however mode at 217 cm−1 are assigned to the longitudinal optical Ag (LO) mode. These Ag (TO) and Ag (LO) modes are very close to the Raman modes reported by Srinivasa Reddy et al [25].The Raman peak at 68, 95 cm−1 is increased with solution volume increase from 5 to 10 ml. It confirms that the films prepared at different solution volume are pure without any impurities; SnS2 peak at 312 cm−1 and Sn2S3 at 308 cm−1 were not obtained [25]. Thus the Raman study along with XRD confirms the growth of single phase SnS films in this nebulizer spray pyrolysis method.
3.2 Surface analysis
3.2.1 SEM analysis
Figure 3 exhibit the SEM morphology of SnS thin films prepared at different solution volumes. These SEM pictures clearly demonstrate that all the films are uniform with homogeneous nature covering the substrate without pinholes and cracks. The surface morphology is found to be affected by the solution volume when increased from 5 to 10 ml. It is also apparent from the SEM image shown in Fig. 3a that the sample prepared at 5 ml has spherical like morphology. This similar type of morphology has also been reported by Polivtseva et al. [27] for spray pyrolysis prepared SnS thin films. Figure 3b, c shows the SEM images of the films prepared at 10 and 15 ml, which indicates uniform covering of the substrate with spherical shaped grains. The film shown in Fig. 3b deposited for 10 ml solution volume has a fine spherical shaped grain with smooth surface along with larger agglomeration of grains judge against films deposited for 5 ml solution. Figure 3c the film deposited for 15 ml solution volume the films shows small spherical shaped particles. The grain size is increased as the volumes of solution increases from 5 to 10 ml.
3.2.2 EDAX analysis
The elemental composition of nebulizer spray deposited SnS and FTO/CdS/SnS films was evaluated by EDAX. Figure 3d shows the EDAX spectra of the SnS films deposited using 10 ml of solution. From this figure the atomic ratio (S/Sn) of the film is with nearly stoichiometric ratio [28, 29]. The EDAX spectrum of the FTO/CdS/SnS heterojunction structure is shown in Fig. 3e. It confirms the presence of both SnS and CdS layer showing the peaks Sn, Cd and S. The trace F and Sn and O are from FTO substrate.
3.2.3 AFM analysis
The digital images obtained using this technique helps to quantitatively analyze the surface features like root mean square roughness and grain size of the films. Figure 4 represents the 2D atomic force microscopic (AFM) images of SnS films deposited by nebulizer spray pyrolysis process onto glass substrate with various solution volumes (5 ml – 15 ml). The film surface of area 5 × 5 µm was scanned to get surface morphological features. Figure 4a illustrates the 2D AFM topography of SnS films deposited at 5 ml volume. The surface of the films exhibits spherical shaped particles formed as clusters covering uniformly the full area. Figure 4b shows the 2D visualizations of AFM images of tin sulfide films deposited at 10 ml, which reveals some remarkable change on the nucleation of the film. It shows dense structure with bigger grains obtained by the nucleation of spherical and subsequent coalescence during growth process [29]. It is evident from the micrograph that grain size is increased with the increase of solution volume. The 2D micrograph also reveals the increase of grain size and surface roughness in response to the change in solution volume. The root-mean-square (RMS) roughness of the film varies from 20 to 31 nm for the hike of solution volume from 5 to 10 ml. Further increase of solution volume 15 ml, the roughness of the film is decreased slightly to 27 nm. High volume (10 ml) induces coalescence of particle to form bigger grains [30]. The root-Mean-Square surface roughness and the grains size values are reported in Table 3. Therefore, it is evident that the grain sizes and the surface roughness changes with the solution volume.
3.3 Optical analysis
3.3.1 UV–Vis–NIR spectroscopy
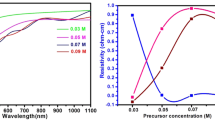
Incident wavelength (λ) dependent change of optical transmittance (T) of SnS thin films prepared using different solution volume is shown in Fig. 5. It is seen that the transmittance of the film is increased with the increase of wavelength, however, it is found to decrease with the increase of solution volumes. In the near-infrared spectral region transmittance is increased showing reduction in reflection due to scattering [31]. The low transmittance at 10 ml solution volume might be due to small losses of sulfur in the film as shown in EDAX [32]. Inset of Fig. 5 shows transmittance spectrum of CdS film, it shows transmittance around 75% in the visible range indicating its suitability for solar cell applications. The absorbance spectra observed in the wavelength region 500 to 1100 nm shown in Fig. 6, displays a, noticeable increase in absorbance value for the increasing the solution volume from 5 to 10 ml. For further increasing the solution volume the absorption of the film is slightly decreased.
Bandgap (Eg) value of the films can be evaluated using Eq. (5) for allowed transitions in direct gap semiconductor [33].
where h, ν, α, Eg, A represent the Planck’s constant, photon frequency, absorption coefficient, energy gap and proportionality constant respectively. Figure 7 shows the plot of hν vs (αhν)2 of the films deposited at different solution volumes. From the extrapolation of straight line portions of the curves the band gaps calculated are 1.82, 1.73 and 1.76 eV for the solution volumes 5, 10 and 15 ml respectively. The calculated energy gap (Eg) is found to decrease from 1.82 to 1.73 eV for the increase of solution volume from 5 to 10 ml, showing the crystallinity improvement with the densely packed atoms. It represents the modulation of localized density states in the band levels due to the change of solution volume. This Eg value of SnS is found to be in excellent agreement with reported values [17]. These calculated band gap values are higher than the theoretical values of SnS, it is likely because of contamination due to sulfur during nebulizer spray pyrolysis [34]. The inset of Fig. 7 shows the direct energy gap of CdS thin film deposited by nebulizer spray technique. The obtained band gap value is 2.51 eV for CdS, perfectly matches with band gap value of earlier report [35].
Optical constants of SnS thin films such as refractive index (n) and extinction coefficient (k) were calculate using the following relations [36].
where
ns, na and n are the refractive indices of substrate, air, and the SnS films, respectively. T is the transmittance value at a particular wavelength
where α, λ and k are the absorption coefficient, wavelength, and the extinction coefficient respectively. Figure 8 show the variation of both n and k of SnS films deposited using different solution volumes. Refractive index is a significant parameter which has to be considered for the selection of optical materials in the development of anti reflection coatings [37]. Refractive index of the films increased from 2.81 to 2.93 at 500 nm for the increase of solution volume from 5 to 10 ml. This behavior can be explained on the basis that by increasing the solution volume more dense packing of particles are produced, which can impede the propagation of light through the film [37]. The extinction coefficient of the films is varied in the range from 0.43 to 0.52 with the increase of solution volume. The maximum extinction coefficient value obtained around wavelength of 500 nm is similar to the value reported in various methods [14, 38].
3.3.2 PL spectra
Photoluminescence (PL) is a technique that experimentally probes the band structure of the solids, from which the band gap of the material can be directly determined. Depending upon the nature of the impurities present in the materials, the line shape, and energy of PL emission can be changed, which may be due to the interaction of light with impurity carriers. Figure 9 displays the PL spectra of SnS films in the wavelength range 500–800 nm excited with laser of wavelength 450 nm. This PL emission of SnS thin film shows a sharp peak centered at 725 nm. It is different from the earlier reports of 700 nm for SnS films [39] and 760 nm for bulk SnS [18]. The observed peak 725 nm for the excitation of 450 nm (~ 1.75 eV) is credited to near band edge emission, is little lower than band gap estimated from UV–Vis data. Ghosh et.al [40] also reported the PL band in SnS (at 450 nm) showing lower energy than band gap. As seen from Fig. 9, the intensity of the peaks obtained for different films deposited at different solution volume is found to be increased without any shift of the emission peak (725 nm), indicating a small amount of imperfection causing radiative recombination in the films deposited at higher solution volume [40].
3.4 Electrical studies
3.4.1 Hall effect measurement
Electric properties of thin films are of great importance in determining the use of films in photovoltaic field. The electric parameters can be determined by studying the Hall Effect of the sample. Electrical resistivity (ρ) of different films deposited at various solution volumes are shown in Table 4. It shows decrease of resistivity from 2.01 × 101 to 0.601 × 101 Ω cm for the increase of solution volume 5–10 ml. The lowest resistivity (0.601 × 101 Ω cm) obtained for the solution volume at 10 ml is due to the improved crystallinity of the film at this solution volume. These results are strictly supported by XRD profiles (Fig. 1) indicating that the decrease in electrical resistivity of the film deposited at 10 ml is due to the increase in grain size leading to the reduction in grain boundary scattering [41]. This difference in electrical conductivity is generally form variations produced in thickness and grain size of the films due to the increase of solution volume. The film prepared at 10 ml showed high conductivity 1.663 × 101 Ω−1 cm−1, higher than the value reported by Devika et al. [30] for thermal evaporated SnS films. Hall measurement done on the sample showed p-type conductivity and increase of carrier concentration from 3.36 × 1017 to 4.71 × 1017 for the increase of solution volume from 5 to 10 ml, it indicates the increase of tin metallic component [42].
3.4.2 I–V characteristics of p-SnS/n-CdS heterostructure
The SnS based photovoltaic (PV) cells were fabricated using 10 ml solution volume. The schematic diagram of the prepared FTO/CdS/SnS heterostructure device is shown in Fig. 10. The I–V characteristics of FTO/CdS/SnS heterostructures in dark and illuminated are shown in Fig. 11. The inset of Fig. 11 shows dark I–V characterization curve. The structures present high rectification properties in the dark and light condition. It also shows very strong PV response under illumination. Due to the availability of minimum amount of carriers at dark the current obtained is less. However, under illumination the current is increased showing the photovoltaic nature of the device producing photo-induced charge carrier. This thin film heterojunction photovoltaic device FTO/n-CdS/p-SnS by nebulizer spray deposited CdS and SnS with active area about 0.5 cm2. Figure 12 depicts the early outcomes on illuminated I–V nature of FTO/n-CdS/p-SnS photovoltaic cell. The energy band diagram of CdS/SnS heterojunction drawn based on Anderson model is shown in Fig. 13. As seen from the figure valance bands separation between p-SnS and n-CdS is large compared to the separation of conduction band edges. Therefore, this heterojunction can separate the hole-electron pairs efficiently causing more photo generated carriers. When energy gap of SnS is decreased with increase of solution volume, the separation of valance bands are increased. This improves further the generation and transport of carriers further increasing the current.
The fill factor (FF) and efficiency (η) are evaluated from the relation [43]
where the Vm and Im are the values of maximum voltage and maximum current. The efficiency η (%) is calculated from the relation.
where Pinput is the input light energy. The fill factor (FF) and efficiency (η) was also evaluated using standard equations. In the present study the short circuit current density of 40 µA, an open circuit voltage of 0.167 V, a fill factor of 0.30 and with conversion efficiency of about 0.081% when illuminated by source of light at 100 mW/cm2. Thus the photovoltaic effects of the CdS/SnS cells are much weaker than the CdS/SnS cells by spray pyrolysis technique [35]. The major criteria for the low efficiency of the device arise due to the various types of loss mechanisms involved during the processing of the film. The prepared SnS film mostly contained of small grain structure which produced more grain boundaries so that the recombination sites for the minority carrier was effective [44].
4 Conclusion
SnS thin films were prepared by cheap nebulizer spray pyrolysis method with device quality properties. Heterojunction diode was also fabricated successfully using SnS and CdS layers prepared by this technique. The grown SnS thin films are in polycrystalline nature with single phase, having the orthorhombic structure with high intense at (210) and (111) peaks as preferred orientation. The crystallite size of the SnS thin films is about 28 nm for the solution volume 10 ml. Raman analysis showed high intense peaks at 68, 95, 164 and 217 cm−1 confirming the formation of SnS thin films. The extinction coefficient of the films varied in the range 0.43–0.54 depending upon solution volume variation. Both optical transmittance and energy band gap decreased with the increase of solution volume. The films showed a band gap in the range of 1.82–1.73 eV for the variation of solution volume. The surface morphological study showed the solution volume variation changes the grain size. AFM images indicate that regular, homogeneous surface and surface roughness was obtained under these conditions. The samples deposited with the solution volume of 10 ml exhibited a low resistivity of 2.01 × 101 Ω cm compared to other samples. From the electrical studies, a solar cell of the type FTO/n-CdS/p-SnS was fabricated and the strong PV response was detected for the 10 ml solution volume.
References
V. Steinmann, R.E. Brandt, R. Chakraborty, R. Jaramillo, M. Young, B.K. Ofori-Okai, C. Yang, A. Polizzotti, K.A. Nelson, R.G. Gordon, T. Buonassisi, Apl Mater. 4, 026103 (2016)
R.E. Abutbul, E. Segev, L. Zeiri, V. Ezersky, G. Makov, Y. Golan, J. R. Soc. Chem. 6, 5848–5855 (2015)
B. Kherchachi, A. Attaf, H. Saidi, A. Bouhdjer, H. Bendjedidi, Y. Benkhetta, R. Azizi, J. Semicond. 37, 032001 (2016)
J. Vijayarajasekaran, K. Vijayakumar, Int. J. Thin Films Sci. Technol. 4, 231–235 (2015)
K.T. Ramakrishna Reddy, N. Koteswara Reddy, R.W. Miles, Sol. Energy Mater. Sol. Cells 90, 3041–3046 (2006)
L.S. Price, I.P. Parkin, A.M.E. Hardy, R.J.H. Clark, Chem. Mater. 11, 1792–1799 (1999)
B. Ghosh, M. Das, P. Banerjee, S. Das, Semicond. Sci. Technol. 24, 025024 (2009)
V. Robles, J.F. Trigo, C. Guillen, J. Herrero, J. Mater. Sci. 48, 3943–3949 (2013)
K.G. Deepa, J. Nagaraju, Mater. Sci. Semicond. Process. 27, 649–653 (2014)
A. Sanchez-Juarez, A. Tiburcio-Silver, A. Ortiz, Thin Solid Films 480–481, 452–456 (2005)
P. Sinsermsuksakul, J. Heo, W. Noh, A.S. Hock, R.G. Gordon, Adv. Energy Mater. 1, 1116–1125 (2011)
J. Park, M. Song, W.M. Jung, W.Y. Lee, J. Lee, H. Kim, I.-W. Shim, Bull. Korean Chem. Soc. 33, 3383 (2012)
S.M. Ahmed, L.A. Latif, A.K.H. Salim, J. Basrah Res. (Sciences). 37, 15 (2011)
S. Cheng, Y. Chen, C. Huang, G. Chen, Thin Solid Films. 500, 96–100 (2006)
K.T. Ramakrishna Reddy, P. Purandhara Reddy, P.K. Datta, R.W. Miles, Thin Solid Films. 403–404, 116–119 (2002)
N. Koteswara Reddy, K.T. Ramakrishna Reddy, Thin Solid Films. 325, 4–6 (1998)
P.K. Nair, A.R. Garcia-Angelmo, M.T.S. Nair, Phys. Status Solidi A 213, 170–177 (2016)
K. Santhosh Kumar, C. Manoharan, S. Dhanapandian, A. Gowri Manohari, T. Mahalingam, Optik. 125, 3996–4000 (2014)
E. Turan, M. Kul, A.S. Aybek, M. Zor, J. Phys. D 42, 245408 (2009)
M. Patel, I. Mukhopadhyay, A. Ray, J. Alloys Compd. 619, 458–463 (2015)
I.B. Kherchachi, H. Saidi, A. Attaf, N. Attaf, A. Bouhdjar, H. Bendjdidi, Y. Benkhetta, R. Azizi, M. Jlassi, Optik. 127, 4043–4046 (2016)
M.S. Arulanantham, S. Valanarasu, K. Jeyadheepan, A. Kathalingam, J. Mater. Sci. Mater. Electron. 28, 18675–18685 (2017)
N. Koteeswara Reddy, K. Ramesh, R. Ganesan, K.T. Ramakrishna Reddy, K.R. Gunasekhar, E.S.R. Gopal, Appl. Phys. A 83, 133–138 (2006)
M.V. Reddy, G. Sreedevi, C. Park, R.W. Miles, K.T.R. Reddy, Curr. Appl. Phys. 15, 588–598 (2015)
T. Srinivasa Reddy, M.C. Santhosh Kumar, RSC Adv. 6, 95680 (2016)
H.R. Chandrasekar, R.G. Humphreys, U. Zwick, M. Cardona, Phys. Rev. B. 15, 2177–2183 (1977)
S. Polivtseva, I. Oja Acik, A. Katerski, A. Mere, V. Mikli, M. Krunks, Energy Procedia 60, 156–165 (2014)
T.H. Sajeesh, A.R. Warrier, C. Sudha Kartha, K.P. Vijayakumar, Thin Solid Films 518, 4370–4374 (2010)
A. Akkari, M. Reghima, C. Guasch, N. Kamoun-Turki, J. Mater. Sci. 47, 1365–1371 (2012)
M. Devika, N.K. Reddy, K. Ramesh, V. Ganesan, E.S.R. Gopal, K.T.R. Reddy, Appl. Surf. Sci. 253, 1673–1676 (2006)
M.S. Selim, M.E. Gouda, M.G. El-Shaarawy, A.M. Salem, W.A. Abd El-Ghany, Thin Solid Films 527 164–169 (2013)
C. Shi, Z. Chen, G. Shi, R. Sun, X. Zhan, X. Shen, Thin Solid Films 520, 4898–4901 (2012)
S. Cheng, Y. He, G. Chen, E.-C. Cho, G. Conibeer, Surf. Coat. Technol. 202, 6070–6074 (2008)
T. Sall, M. Mollar, B. Mari, J. Mater. Sci. 51, 7607 (2016)
N. Koteeswara Reddy, K.T. Ramakrishna Reddy, Mater. Chem. Phys. 102, 13–18 (2007)
K.T. Ramakrishna Reddy, G. Sreedevi, R.W. Miles, J. Mater. Sci. Eng. A 3(3), 182–186 (2013)
S. Gedi, V.R. Minnam Reddy, C. Park, J. Chan-Wook, K.T. Ramakrishna Reddy, Opt. Mater. 42, 468–475 (2015)
E.R. Shaaban, M.S.A. El-Sadek, M. El-Hagary, I.S. Yahia, Phys. Scr. 86(1–6), 015702 (2012)
N.G. Deshpande, A.A. Sagade, Y.G. Gudage, C.D. Lokhande, J. Alloys Compd. 436, 421–426 (2007)
B. Ghosh, M. Das, P. Banerjee, S. Das, Appl. Surf. Sci. 254, 6436–6440 (2008)
E. Guneri, C. Ulutas, F. Kirmizigul, Appl Surf Sci 257, 1189–1195 (2010)
B. Ghosh, R. Bhattacharjee, P. Banerjee, S. Das, Appl. Surf. Sci. 257, 3670–3676 (2011)
M.A. Barote, A.A. Yadav, T.V. Chavan, E.U. Masumdar, J. Nanomater. Biostruct. 6, 979–990 (2011)
B. Ghosh, M. Das, P. Banerjee, S. Das, Fabrication of the SnS/ZnO heterojunction for PV applications using electrodeposited ZnO films. Semicond. Sci. Technol. 24, 025024 (2009)
P.A. Nwofe, K.T. Ramakrishna Reddy, G. Sreedevi, J.K. Tan, R.W. Miles, Jpn. J. Appl. Phys. 51, 10NC36 (2012)
S.P. Zimin, E.S. Gorlachev, I. IAmirov, V. VNaumov, G.A. Dubov, V.F. Gremenok, S.A. Bashkirov, Semicond. Sci. Technol. 29, 015009 (2014)
T. Rath, L. Gury, I. Sanchez-Molina, L. Martinez, S.A. Haque, Chem. Commun. 51, 10198–10201 (2015)
Acknowledgements
The authors wish to express their sincere thanks to the Department of Science and Technology, New Delhi, India for their financial assistance for the work by the project number SB/FTP/PS-131/2013. The authors would like to thank Dr. K. Jeyadheepan, SASTRA University, for recording the Hall Effect measurements data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arulanantham, A.M.S., Valanarasu, S., Kathalingam, A. et al. Solution volume effect on structural, optical and photovoltaic properties of nebulizer spray deposited SnS thin films. J Mater Sci: Mater Electron 29, 12899–12909 (2018). https://doi.org/10.1007/s10854-018-9409-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-018-9409-1