Abstract
The ligation behavior of the chalcone ligand namely (E)-3-(4-hydroxy-3-methoxyphenyl) acrylic acid (ferulic acid) (FA) toward the Cu(II) and Zn(II) ions was determined. The structure of the isolated solid complexes was elucidated by elemental analyses, spectral techniques (IR, UV–Vis, 13C– and 1H-NMR spectra) as well as the conductance measurements and thermal analyses. UV–Vis spectra and magnetic moments had suggested square planar and tetrahedral stereochemistry for Cu(II) and Zn(II) complexes, respectively. The kinetic and thermodynamic parameters for some selected decomposition steps have been calculated. Two precise and sensitive spectrophotometric methods were utilized to determine Zn(II) and Cu(II) complexes with ferulic acid using a micellar media of cetylpyridinium bromide (CPB) and sodium lauryl sulfate (SLS) with an absorption maxima of 430 and 465 nm for Zn(II) and Cu(II), respectively. Various analytical conditions, for example, the concentration of the reagent, temperature, the sequence and timing of addition were also looked into. Under optimum conditions, the complexes exhibited good linearity in concentration range of 2.0–70.0 and 4.0–140.0 µg mL−1; molar absorptivities 1.3161 × 104 and 8.826 × 103 L mol−1 cm−1; and Sandell’s sensitivity 0.00496 and 0.00719 µg cm−2 for the proposed methods of Zn(II) and Cu(II), respectively. The complexes ratio was found to be 1:2 [Zn(II):FA or Cu(II):FA] and the stability constants were 2.771 × 105 and 2.826 × 105, respectively. Finally, the newly synthesized complexes were shown potent antimicrobial activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
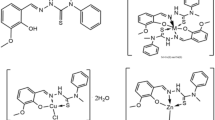
Polyphenols are a diverse class of natural compounds defined by the presence of an aromatic ring containing one or more hydroxyl groups in their chemical skeleton. Polyphenols are divided into many sub-classes depending on their structure including flavonoids, phenolic acids and phenolic alcohols (Genova et al. 2012). Ferulic acid (4-hydroxy-3-methoxycinnamic acid, FA) is a phenolic acid which widely spread in the cell walls of fruits, vegetables and grain plants (Wang et al. 2020). It has a broad range of potential therapeutic effects beneficial in the treatments of lung, diabetes, cancer and cardiovascular diseases, as well as hepatic, neuro and photoprotective effects and anti-inflammatory and antimicrobial activities (Paiva et al. 2013). Ferulic acid (Fig. 1) is a free radical scavenger, but also an inhibitor of enzymes that catalyze free radical generation and an enhancer of scavenger enzyme activity (Zdunska et al. 2018).
Copper is a biologically active element, whose compounds have a strong effect on the vital activities of organisms. Copper also plays an important role in biological operations including metabolism, hemoglobin synthesis, nerve function and bone development (Veitia et al. 2009; Horstkotte et al. 2012; Kannan and Arumugham 2013). It has an effective role in industry as it enter in coin making, wire making, fashioning metal products, alloys, thermal conductivity and transportation industry. The accumulation of copper in human body and the environment through many industrial sources causes serious problems to public health (Alharthi and Al-Saidi 2020; Ahmed and Zannat 2012). In the human body, zinc is the second most abundant transition element. Zinc is found in various foods such as cereals, liver, meat, rice, oysters, cheeses nuts; in several enzymes and DNA-binding proteins. It plays a huge physiological role in human beings like gene transcription, mammalian reproduction, brain function, immune function and pathology (Reddy et al. 2016; Frederickson et al. 2005). Many spectrophotometric methods were reported for the determination of Cu(II) and Zn(II) using different analytical reagents (Alharthi and Al-Saidi 2020; Souza et al. 2016; Babayeva et al. 2017; Jiaa et al. 2020; Rahmani et al. 2015; Reddy et al. 2016; Najim et al. 2020). Among the various analytical techniques suitable for the quantification of Cu(II) and Zn(II), spectrophotometry is the most widely employed one. In this study, Cu(II) and Zn(II) chelates of FA had been prepared in solution as well as isolated as solid. The isolated solid complexes were characterized utilizing elemental, thermal, spectroscopic, spectrophotometric, and antimicrobial methods. Moreover, the developed spectrophotometric methods were applied for the determination of Cu(II) and Zn(II) in various analytical implementations.
Experimental
Equipments
The spectrophotometric experiments were made by using a T80 UV/Vis double beam spectrophotometer with a spectral bandwidth of 2.0 and 10.0 mm matched quartz cells, manufactured by PG Instruments Ltd. in the United Kingdom. The pH readings were taken with a pH meter (Adwa pH meter, Model AD 1030, Romania). The apparatus and its templates used to classify the chemical structures of the synthetic complexes are summarized in Table 1.
Synthesis of (E)-3-(4-hydroxy-3-methoxyphenyl) acrylic acid (FA)
Ferulic acid was prepared by the condensation reaction of vanillin with malonic acid using benzylamine as the catalytic agent, toluene as the solvent and a reaction temperature of 85–95 °C for about 2.0 h (Fig. 2) according to reported method (Shiyi and Kwok 2004). Appearance: white solid; Mp: 169–171 °C; MF: C10H10O4; MWt: 194.18 (Fig. S1); 1HNMR (300 MHz, d6-DMSO): 12.13 (s, 1H), 9.56 (s, 1H), 7.48 (d, J = 15.9 Hz, 1H), 7.28 (d, J = 1.8 Hz, 1H), 7.08 (dd, J = 1.8 Hz, 6.3 Hz, 1H), 6.78 (d, J = 8.1 Hz, 1H), 6.36 (d, J = 15.9 Hz, 1H), 3.81 (s, 3H); 13C NMR (300 MHz, d6-DMSO): 167.98, 149.02, 147.86, 144.49, 125.73, 122.79, 115.57, 115.47, 111.06, 55.61; LC MS (m/z): [M+ H+]—194.19.
Synthesis of complexes
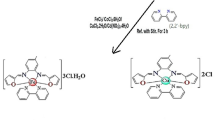
The [Cu(FA)2]SO4.3H2O solid complex was prepared by adding 1.0 mmol (0.2499 g) of CuSO4.5H2O in 20.0 mL acetone drop-wise to a stirred solution of 1.0 mmol (0.194 g) ferulic acid (FA) in 50.0 mL acetone. The reaction mixture was refluxed for 6.0 h; the formed olive green precipitate was filtered off, washed several times with acetone and dried under vacuum over anhydrous CaCl2. The yellowish-white solid complexes, [Zn(FA)2]SO4.H2O, were prepared similarly at 1:2 molar ratio (M:FA).
Materials and solutions
Commercially available chemicals were purchased from commercial suppliers (Sigma Aldrich Chemical Company (St. Louis, MO, USA)) and used as received, without further purification. A standard solution was formed by combining 0.01951 g, (99.50%) of ferulic acid in dimethyl formamide (DMF) and diluting to 100.0 mL in volumetric flask to obtain the final concentration of (1.0 × 10–3 M). A (1.0 × 10–3 M) of Zn(II) standard solution was elaborated by blending 0.01616 g of anhydrous ZnSO4 (99.90%, M. wt. 161.47 g mol−1) in deionized water and diluting to the mark in 100.0 mL volumetric flask. A (1.0 × 10–3 M) of Cu(II) standard solution was drawn up by thawing 0.02499 g of CuSO4.5H2O (99.90%, M. wt. 249.68 g mol−1) in deionized water and diluting to the mark in 100.0 mL volumetric flask. Series of different surfactants including anionic surfactants [sodium lauryl sulfate (SLS), dioctyl sodium sulfosuccinate (aerosol GPG-E)], nonionic surfactants [triton X-100, tween 80] and cationic surfactants [cetyltrimethyl ammonium bromide (CTAB), cetylpyridinium bromide (CPB)] were prepared at concentration (1.0 × 10–2 M) by dissolving the suitable amount of each surfactant in deionized water and diluting to the mark in 100.0 mL volumetric flask. They were applied with no additional purifying. A set of buffer solutions including borate, phosphate and universal buffers in the pH range from 7.0 to 12.0 were prepared by standard methods (Britton 1952; Bower and Bates 1955; Lurie 1978).
Procedures
Spectrophotometric procedures
Aliquots solutions of ZnSO4 (10.0–1000.0 µg mL−1) and CuSO4.5H2O (10.0–1800.0 µg mL−1) were poured into a set of 10.0 mL volumetric vials. A concentration of 2.0 mL (1.0 × 10–3 M) of ferulic acid reagent was applied. Following that, for Zn(II) assays, 5.0 mL borate buffer pH = 10.0 was used, and for Cu(II) samples, 4.5 mL phosphate buffer pH = 8.0 was used. Then, 1.2 mL of CPB and 1.0 mL of SLS surfactants were added to Zn(II) and Cu(II) samples, respectively. The mixtures were diluted to 10.0 mL with deionized water, mixed well, and kept at room temperature for 15.0 min. The absorbance of this solution was measured at 430 and 465 nm in comparison to each blank solution made in the identical way with all additions except metal ion for Zn(II) and Cu(II) complexes, respectively (Elgendy et al. 2020a, b; Abd El-Wahaab et al. 2020; Elgendy et al. 2020a, b).
Applications
Determination of zinc and copper in brass alloy
A brass sample weighing 0.2 g was dissolved in 10.0 mL concentrated HNO3 and 5.0 mL concentrated H2SO4 with heating until practically dry. The residual was re-dissolved in 5.0 mL concentrated HNO3 and diluted with 10.0 mL deionized water after 30.0 min at 80 °C. A solution was frigid to room temperature and blended with deionized water to a constant volume appropriate for the concentration limits. The total concentration of zinc and copper was determined by the general procedures described above. Copper was masked by sodium thiosulfate (Fabre and Reynes 2010); then, the concentration of zinc was determined as described above. The obtained results were compared by the data obtained from the recommended method (Fabre and Reynes 2010).
Determination of zinc and copper in vegetables by digestion method [Spinach and Broccoli]
A dried vegetables weighing 0.5 g were mixed and stirred with 0.5 mL concentrated sulfuric acid, 1.0 mL perchloric acid, and 5.0 mL concentrated nitric acid and heated gently for few minutes by constantly raising the temperature. The blend was digested for 15.0 min on hot until white vapors emerged. The subsequent combination temperature was lowered to 25 °C then adding 10.0 mL of deionized water and the temperature was raised to 70 °C again for another 20.0 min. As previously, the solution was cooled, diluted, and examined.
Determination of zinc and copper in sea water, waste water and tap water
A 1000.0 mL of water sample was concentrated to 20.0 mL by heating for 5.0 h on a hot plate. Then, 5.0 mL of concentrated HNO3 and 2.0 mL of 30% hydrogen peroxide were added to this solution and evaporated to near dryness. The leavings were dissolved in 5.0 mL of hydrochloric acid (5.0%) and 10.0 mL deionized water at 80 °C for 30.0 min. The resulting solution was cooled, diluted and analyzed according to the abovementioned method.
Determination of zinc and copper in blood
A specific volume of blood (10.0 mL) was taken from an adult human body. A 5.0 mL portion of this sample was centrifuged using a tube containing sodium citrate as an anticoagulant to separate plasma, and the remaining 5.0 mL was centrifuged to separate serum. A 0.5 mL serum sample was placed into a beaker containing a (1:3) concentrated (HClO4:HNO3). The combination was gradually heated until the solution was transparent, then cooled and analyzed as previously stated.
Antibacterial potency
The antibacterial activity was assessed utilizing the diffusion process (Beecher and Wong 1994) against Escherichia coli, Citrobacter and Salmonella Typhi as Gram -ve bacteria, Staphylococcus aureus, Bacillus cereus and Listeria monocytogenes as Gram + ve bacteria. Amoxycillin/clavulanic calibration disks were used as positive controls for antibacterial efficacy, whereas DMSO was used as a negative control. Müller–Hinton agar was prepared which was then cooled to 47 °C and planted with checked microorganisms. During solidification, 5.0 mm diameter holes were drilled with a sterile cork-borer. The tested compounds were introduced in holes (only 100.0 μL) after being dissolved in DMSO at 1.0 × 10–3 M. These culture plates were then incubated at 37 °C for 20.0 h. Then, the diameters of the inhibition zones have been recorded.
Results and discussion
The new synthesized chelates of Cu(II) and Zn(II) with ferulic acid (FA) were stable, colored and non-hygroscopic in nature. The Cu(II) and Zn(II) complexes were dissolved in Dimethyl formamide and dimethyl sulfoxide. The results of analytical data for the FA ligand and its prepared complexes are listed in Table 2. The chemical analysis demonstrated the existence of SO42− anions outside the coordination sphere. The molar conductivity for freshly prepared DMSO solutions (1.0 × 10–3 M) of the complexes was measured at room temperature. The data showed the molar conductance value of the complexes Cu(II) and Zn(II) around 95.40 and 99.36 S cm2 mol−1, respectively, indicating these complexes as 1:1 electrolytes, (Geary 1971). The measurements demonstrate that the metal complexes were electrolyte in contrast with FA ligand. The complexes were characterized through their elemental analysis, magnetic properties, melting points, molar conductance, FT-IR, UV–Vis as well as thermogravimetric analyses. The results enable us to characterize the complexes and make an appreciation of the bonding and structure inherent in them. The magnetic susceptibility tests showed that Zn(II) chelate was diamagnetic but Cu(II) complex was paramagnetic (1.65 B.M), which indicated one unpaired electron per Cu(II) ion.
Spectroscopic analysis
FT-IR spectra
The IR spectrum of ferulic acid ligand exhibits a distinct peak of the carbonyl group ν(C=O)COOH and the hydroxyl group ν(O–H)COOH stretching vibrations at 1690 and 3068 cm−1, respectively (Table 3). The infrared spectra of the two complexes were compared with those of the free ligand in order to determine the site of coordination that might be involved in chelation. On coordination to a metal, the ligand bands were shifted to lower or higher frequencies with concomitant variation in intensity (Fig. 3) (Kalinowska et al. 2014). In the spectra of Cu(II) and Zn(II) chelates, the positions and intensities of various aromatic peaks were modified, and the peaks ascribed to carboxylic group vibrations vanished. Two extremely strong peaks were found at 1631, 1642 and 1410, 1423 cm−1 attributed to ν(COO−) asymmetric and symmetric stretches, respectively, with an average Δν value of ~ 200 cm−1 suggesting unidentate coordination mode of the carboxylate group (Zordok et al. 2012). A peak at 3437 cm−1 in the spectra of FA ligand was ascribed to the stretching v(OH)ar vibrations of the substituent in the ring. This peak was pushed toward lower wavenumber in the spectra of metal chelates (3383–3425 cm−1), suggesting that hydroxyl substituents could participate in metal binding. Coordination via oxygen of carboxylate and hydroxyl substituent was confirmed by ν(M–O) peaks at 443 cm−1 for Cu(II) and 567 cm−1 for Zn(II) (Zordok et al. 2012). FA was therefore a bidentate ligand that is coordinated with metal via oxygen atoms in carboxylates and hydroxyl substituents (Scheme 1).
Thermal analysis
The stoichiometry of the resultant volatile decaying components as well as the properties of the complexes was studied employing thermal analyses. The TG decay stages with the temperature maximum and weight loss for the complexes were indexed in (Table 4) and presented graphically in (Fig. S2) TG data for FA manifested two stages of decaying with temperature maxima 303 °C and 493 °C with total weight loss amounted to 99.90% (cal. 99.99%). The thermal decomposition of Cu(II) complex showed first stage at 113 °C with a mass loss of 8.37% (cal. 9.00%) due to removal of three hydrated H2O molecules. The second step occurred at 145 °C with loss of 16.01% (cal. 15.98%) assignable to elimination of sulfate anion. The third step occurred at 256 °C assignable to elimination of partial decomposition of the organic ligand. The last step at 411 and 450 °C with removal of 19.00% (cal. 19.51%) corresponded to further decomposition of the organic ligand with production of CuO + C as final product. For Zn(II) complex, the first step appeared at 90 °C with mass loss 2.98% (cal. 3.18%) was due to removal of hydrated H2O molecule. The second step started at 259 °C and corresponds to removal of sulfate anion with mass loss of 16.55% (cal. 16.98%). The third one step appeared at 537 °C with partial degradation of the organic ligand with mass loss of 37.69% (cal. 37.49%). The last step at 796 °C with weight loss of 4.49% (cal. 4.59%) assigned to further degradation of the organic moiety leaving ZnO + 12C as final residue.
NMR spectroscopy
The 13C and 1H chemical shifts were important indicators in the study of the structure of complexes, including FA. In the 13C NMR spectra of the complexes signals corresponding to the carbonyl groups (C9) were not recorded, which shows the COO− group involvement in the formation of the bonds with metal atoms. The signal of the C4 phenolic groups (147.86 ppm) in the spectrum of Cu(II) and Zn(II) complexes was shifted relative to (146.9, 146.1 ppm), respectively. This showed that the oxygen of the phenolic group was bound with metal atoms in the complexes. The 1H NMR characteristic signals spectra of FA and Zn(II) complex are given in Table 5. 1H NMR spectrum of FA showed singlet signals at 12.13 and 9.56 ppm due to carboxylic (COOH) and phenolic (OH) protons, respectively. For complex Zn(II), 1H NMR spectrum exhibited singlet signal at 8.84 ppm due to phenolic proton (Fig. S4). This shift indicated the participation of the phenolic group in complex formation. On the other hand, the carboxylic proton was absent in the spectrum of complex supporting the participation of the deprotonated COOH group in coordination to the metal center (Reddy et al. 2016).
Spectrophotometric analysis of ferulic acid and its metal complexes in the solution
Electronic absorption spectral study
The assignments of electronic absorption bands of ferulic acid reagent and its metal complexes were measured in alkaline medium within the range of 200–800 nm against the blank solution, (Fig. 4). It was observed that Zn(II) and Cu(II) with ferulic acid reagent formed colored complexes in alkaline medium using borate buffer (pH = 10.0) for Zn(II) complex and using phosphate buffer (pH = 8.0) for Cu(II) complex with maximum absorbance values at 395 and 415 nm, respectively.
Absorption spectra of ferulic acid (2.0 × 10–4 M) against bsuffer blank; Zn(II)-ferulic acid and Cu(II)-ferulic acid complexes against the reagent blank; Where, Zn(II) or Cu(II) = (1.0 × 10–4 M) and ferulic acid (2.0 × 10–4 M). The used excess of ferulic acid decreased the absorbance intensity of the complexes
Optimization of analytical conditions
Various factors have been taken to select the optimal required conditions for the quick formation and stability of the studied complexes.
Effect of pH and buffer solutions
The pH effect on the formation of the complexes was investigated by using 0.1 M NaOH and 0.1 M HCl solutions, and the appropriate pH value was modified using a pH meter within the pH range of 1.0–12.0. It was observed that the suitable pH range for complete formation of the complexes was from pH = 7.0 to 11.0, (Fig. 5). Also, the absorbances were steadily decreased in more acidic or more alkaline solutions which can be attributed to incomplete formation and partial dissociation of the complexes. In order to fix the pH of the media, the effect of different buffer solutions (borate, phosphate and universal) was investigated. It was observed that the maximum absorbance and color intensity of the complexes were achieved using 5.0 mL borate buffer (pH = 10.0) and 4.5 mL phosphate buffer (pH = 8.0) for Zn(II) and Cu(II) complexes, respectively, (Fig. 6).
Effect of reagent concentration
The reagent concentration effect was tested by measuring the absorbance of the solutions containing fixed concentration 1.0 mL of (1.0 × 10–3 M) of metal ion [Zn(II) or Cu(II)] and varying concentrations (0.5–4.0 mL of 1.0 × 10–3 M) of ferulic acid reagent. Additionally, the highest absorbance was observed using 2.0 mL of (1.0 × 10–3 M) of ferulic acid reagent. The excess of ferulic acid up to 2.0 mL of (1.0 × 10–3 M) reduced the absorbance intensity.
Effect of surfactants
In order to increase the sensitivity of the complexes, the influence of various concentrations varying between 0.2 and 2.0 mL (1.0 × 10–2 M) of the abovementioned surfactants was tested. The absorbance values were increased by many surfactants but the maximum values were observed using 1.2 mL (1.0 × 10–2 M) of CPB for Zn(II) complex and by 1.0 mL (1.0 × 10–2 M) of SLS for Cu(II) complex, (Fig. 7).
Other conditions (order of the addition, time and temperature)
The effect of the order of addition on the performance of the spectrophotometric measurements was investigated to choose the most appropriate one for the maximum absorbance values of the formed complexes. It was evident that the order (metal ion-ferulic acid reagent-buffer-surfactant) results in maximum absorbance values. In addition, the influence of temperature on the absorbance measurements was studied from 5.0 to 85.0 °C. It was found that the absorbance values of the complexes were increased between 20.0 and 45.0 °C; then, the decrease in the absorbance was noticed between 45.0 and 85.0 °C, (Fig. 8). Thus, the absorbance measurement at room temperature was fine. Finally, the influence of time on the stability of the formed complexes was tested within the range of 0.0–120.0 min and after 24, 48 h. The results indicated that the complexes were formed spontaneously, but they reached their maximum absorbance values after 15.0 min for Zn(II) and Cu(II) complexes. Also, the complexes remained stable for a long time about 48 h, (Fig. 9).
Stoichiometric ratio of the reaction
Job’s method of continuous variation (Job 1928) was applied to determine the molar ratio between metal ion and ferulic acid reagent. In this method, an equimolar solutions of metal ion [Zn(II) or Cu(II)] and reagent were mixed in different proportions from 0.1 to 0.9 mL (1.0 × 10–3 M) of the same concentration, whereas the total molar concentration was held constant. The absorbance was measured at the recommend wavelengths and plotted against mole fraction, (Fig. 10). The results showed that the ratio was found to be 1:2 for both complexes.
Stability constant of the complexes
The stability constant of the complexes was calculated from Job’s method by the following equation (Tirmizi et al. 2012).
where A1 = absorbance at break point, A2 = actual absorbance, CM = concentration of metal and CL = concentration of ligand. The stability constants of the complexes were found to be 2.771 × 105 and 2.826 × 105 for Zn(II) and Cu(II) complexes, respectively.
Calibration curve and analytical characteristics of the proposed spectrophotometric methods
The calibration graph of the investigated complexes was made from the absorbance measurements which performed using the abovementioned optimized conditions. Good linear relationship was obtained in the concentration range of 2.0–70.0 and 4.0–140.0 µg mL-1 for Zn(II) and Cu(II) complexes, respectively, (Fig. 11). A Ringbom plot was also conducted to select the optimum concentration range for the system that obeyed Beer’s law. It was achieved at intermediate concentration values of (8.0–60.0 µg mL-1) and (10.0–120.0 µg mL-1) for Zn(II) and Cu(II) complexes, respectively. The spectrophotometric methods have good reproducibility for a set of seven measurements of (40.0 µg mL-1) of Zn(II) and (60.0 µg mL-1) of Cu(II) under optimum conditions. Some statistical parameters were calculated to show the high sensitivity, excellent linearity and good accuracy and precision of the proposed spectrophotometric methods as indicated in Table 6.
Effect of foreign ions (Interferences)
The effect of some foreign ions which were added relative to (40.0 µg mL−1) of Zn(II) and (60.0 µg mL−1) of Cu(II) was investigated (Fig. S3). Then, the absorbance was measured according to the abovementioned procedures. The tolerance limit was defined as the amount of foreign species causing < ± 5% error in the absorbance values. The tolerance limits of different foreign ions that were likely to interfere during the spectrophotometric methods are indicated in Table 7. It was found that Al3+, Ba2+, Cd2+, Ca2+, Mn2+, Hg2+, Sn4+, Ce3+, Co2+, Cr3+, La3+, Mg2+, Ni2+, K+, Li+, Na+, Sm3+, As3+, Pb2+, Mo6+, U6+, Nd3+, Pr3+, Gd3+, Y3+, Th4+, Ga3+, Bi3+, Se4+, V5+, Cl−, CO32−, NH4+, SO42−, NO3−, PO43+, S2O32− and CH3COO− did not interfere, while Pd2+, Cu2+, Sr3+, Fe3+ interfered with Zn(II)-ferulic complex. Also, Pd2+, Zn2+, Fe3+, Sn4+, Sr3+ interfered with Cu(II)-ferulic complex. Fe(III) was masked by using urea (Park et al. 2001). The methods were selective and free from most interfering ions. The results indicated the possible use of the proposed methods for the determination of Cu(II) and Zn(II) in diverse samples.
Analytical applications
The proposed spectrophotometric methods were applied for the determination of zinc and copper in industrial sample (brass alloy), vegetables samples (spinach, broccoli), biological sample (human blood) and water samples (waste water, sea water, tap water) under all optimum conditions. The analytical results of these samples are given in Table 8. Good recoveries in the range from 99.70 to 100.50% were obtained for zinc and copper in these samples, demonstrating the accuracy of the proposed methods. The results showed good agreement with that obtained from the recommended method and prove the applicability of the proposed methods. As a result, we can infer that the methods can be widely used for the determination of zinc and copper in a variety of models with excellent precision, selectivity and sensitivity.
Comparative study of the suggested methods with other reported detectability spectrophotometric methods
The performances of the suggested methods were compared with that of the other existing UV–visible spectrophotometric methods (Table 9). It was clear from the table that the suggested methods were simple, selective, not complicated and required less time to complete the analysis more than many other spectrophotometric methods. The other advantages of the proposed methods were their sensitivity and their wider linear ranges. Moreover, the methods were free from many interfering ions.
Microbial applications
To estimate the biological potential of the FA and their metal complexes were tested against different bacterial strains. The ferulic acid ligand (FA) has no effect against Escherichia coli, Citrobacter, Salmonella Typhi, Staphylococcus aureus, Bacillus cereus and Listeria monocytogenes bacteria. The results in Table 10 and Fig. 12 revealed that the newly synthesized complexes have noticeable bactericidal activity and their activities increased when complexed with the metal ions. This indicated that chelating agents inhibit bacterial growth when complexes with many metals. The assessments of biological activity of the newly synthesized complexes were performed compared with FA ligand. Cu(II) complex was found to have the highest activity against all the tested organisms relative to all compounds. The sequence of inhibitory capacities growth was: Cu(II) ˃ Zn(II) ˃ Amoxycillin/Clavulanic ˃ FA (for S. aureus), Cu(II) ˃ Amoxycillin/Clavulanic ˃ Zn(II) ˃ FA (for Listeria), Cu(II) ˃ Amoxycillin/Clavulanic ˃ Zn(II) ˃ FA (for E.coil), Cu(II) ˃ Zn(II) ˃ Amoxycillin/Clavulanic ˃ FA (for Citrobacter), Cu(II) ˃ Zn(II) ˃ Amoxycillin/Clavulanic ˃ FA (for Salm. typhi), Cu(II) ˃ Zn(II) ˃ Amoxycillin/Clavulanic ˃ FA (for B-cereus). We concluded from these results that metal complexes have a remarkable activity against pathogenic bacteria and that explained on the basis of chelation theory the chelation could facilitate the ability of a complex to cross a cell membrane of the pathogens and also ease their diffusion through the lipid layer of spore membrane to the site of action and ultimately killing them (El-Shwiniy et al. 2020; Okulik and Jubert 2005; Zaky and Yousef 2011; Mohamed and Sharaby 2007).
Conclusion
This work studied new spectrophotometric methods for the determination of Zn(II) and Cu(II) by forming colored complexes with ferulic acid. The suggested methods revealed high sensitivity, accuracy, precision and good reproducibility. These methods were successfully applied for the determination of zinc and copper in brass alloy, blood, spinach, broccoli, sea water, waste water and tap water. We introduced the synthesis and characterization of novel Zn(II) and Cu(II) complexes containing FA as ligand. Based on the results of the physicochemical and spectral techniques, the data showed that FA acted as a bidentate with metal ions. The results revealed that Cu(II) complex was the more active. The potential antimicrobial effects of the new metal complexes indicated that they could be used effectively.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Abd El-wahaab B, Elgendy K, El-didamony A (2020) Synthesis and characterization of new azo-dye reagent and using to spectrophotometric determination of samarium(III) in some industrial and blood samples. Chem Pap 74:1439–1448. https://doi.org/10.1007/s11696-019-01000-8
Admasu D, Reddy DN, Mekonnen KN (2016) Trace determination of zinc in soil and vegetable samples by spectrophotometry using pyridoxal thiosemicarbazone and 2-acetyl pyridine thiosemicarbazone. Cogent Chem 2:1249602–1249615. https://doi.org/10.1080/23312009.2016.1249602
Ahmed MJ, Zannat T (2012) A Simple spectrophotometric method for the determination of copper in some real, environmental, biological, food and soil samples using salicylaldehyde benzoyl hydrazone. Pak J Anal Environ Chem 13:22–35
Alabidi HM, Farhan AM, Al-Rufaie MM (2021) Spectrophotometric determination of Zn(II) in pharmaceutical formulation using a new azo reagent as derivative of 2-napthol. Curr Appl Sci Technol 21:176–187
Alharthi SS, Al-Saidi HM (2020) Spectrophotometric determination of trace concentrations of copper in waters using the chromogenic reagent 4-amino-3-mercapto-6-[2-(2-Thienyl)Vinyl]-1,2,4-Triazin-5(4H)-one: synthesis, characterization, and analytical applications. Appl Sci 10:3895–3911. https://doi.org/10.3390/app10113895
Babayeva K, Demir S, Andac M (2017) A novel spectrophotometric method for the determination of copper ion by using a salophen ligand, N, N′ -disalicylidene-2,3-diaminopyridine. J Taibah Univ Sci 11:808–814. https://doi.org/10.1016/j.jtusci.2017.02.001
Beecher DJ, Wong AC (1994) Identification of hemolysin BL-producing Bacillus cereus isolates by a discontinuous hemolytic pattern in blood agar. Appl Environ Microbial 60:1646–1651. https://doi.org/10.1128/aem.60.5.1646-1651.1994
Bower VE, Bates RG (1955) pH values of the Clark and Lubs buffer solutions at 25 °C. J Res Natl Bur Stand 55:197–200
Britton HTS (1952) Hydrogen ions, 4th edn, vol 28. London, Chapman and Hall, pp 359–364
Elgendy K, El-didamony A, Abd El-wahaab B (2020a) Analytical applications using spectrophotometric technique for the determination of uranium(VI), samarium(III) and cerium(III) by new organic reagent. J Iran Chem Soc 17:1317–1327. https://doi.org/10.1007/s13738-020-01856-8
Elgendy K, El-didamony A, Zaky M, Abd El-Wahaab B (2020) Spectrophotometric determination of cerium(III) in some industrial and plant samples using new synthesized azo-dye reagent: synthesis and characterization. Egypt. J. Chem. 63:2405–2417. https://doi.org/10.21608/ejchem.2019.16914.2029
El-Shwiniy WH, Shehab WS, Zordok WA (2020) Spectral, thermal, DFT calculations, anticancer and antimicrobial studies for bivalent manganese complexes of pyrano [2,3-d]pyrimidine derivatives. J Mol Struct 1199:126993–127042. https://doi.org/10.1016/j.molstruc.2019.126993
Fabre PL, Reynes O (2010) Determination of copper and zinc in brass: two basic methods. J Chem Educ 87:836–837. https://doi.org/10.1021/ed100259b
Frederickson CF, Koh JY, Bush AI (2005) The Neurobiology of zinc in health and disease. Nat Rev Neurosci 6:449–453. https://doi.org/10.1038/nrn1671
Geary WJ (1971) The use of conductivity measurements in organic solvents for the characterisation of coordination compounds. Coord Chem Rev 7:81–122. https://doi.org/10.1016/S0010-8545(00)80009-0
Genova G, Iacopini P, Baldi M, Ranieri A, Storchi P, Sebastiani L (2012) Temperature and storage effects on antioxidant activity of juice from red and white grapes. Int J Food Sci Technol 47:13–23. https://doi.org/10.1111/j.1365-2621.2011.02801.x
Horstkotte B, Alexovic M, Maya F, Duarte CM, Andruch V, Cerda V (2012) Automatic determination of copper by in-syringe dispersive liquid–liquid microextraction of its bathocuproine-complex using long path-length spectrophotometric detection. Talanta 99:349–356. https://doi.org/10.1016/j.talanta.2012.05.063
Jiaa H, Liu W, Li N, Wang J, Song Y (2020) Spectrophotometric determination of copper(II) in water based on fluorescein diacetate. J Anal Chem 75:330–342. https://doi.org/10.1134/s1061934820030089
Job P (1928) Formation and stability of inorganic complexes in Solution. Ann Chim 9:113–203
Kalinowska M, Piekut J, Bruss A, Follet C, Sienkiewicz-Gromiuk J, Swisłocka R, Rzaczynska Z, Lewandowski W (2014) Spectroscopic (FT-IR, FT-Raman, 1H, 13C NMR, UV/VIS), thermogravimetric and antimicrobial studies of Ca(II), Mn(II), Cu(II), Zn(II) and Cd(II) complexes of ferulic acid. Spectrochim Acta Part A 122:631–638. https://doi.org/10.1016/j.saa.2013.11.089
Kannan D, Arumugham MN (2013) Synthesis, characterization, DNA-binding studies and antimicrobial activity of copper (II) complex with 1, 10-phenanthroline, L-tyrosine and urea as ligands. Int J Inorg Bioinorg Chem 3:8–15
Lurie JU (1978) Handbook of analytical chemistry, 2nd edn. Mir publishers, Moscow
Lutfullah SS, Rahman N, Azmi SNH, Iqbal B, Amburk MIBB, Al Barwani ZMH (2010) UV spectrophotometric determination of Cu(II) in synthetic mixture and water samples. J Chin Chem Soc 57:622–631
Mohamed GG, Sharaby CM (2007) Metal complexes of Schiff base derived from sulphametrole and o-vanilin: synthesis, spectral, thermal characterization and biological activity. Spectrochim Acta A 66:949–958. https://doi.org/10.1016/j.saa.2006.04.033
Najim SS, Hameed MA, Al-Shakban MA, Fandi TS (2020) Spectrophotometric determination of zinc in pharmaceutical medication samples using 8-Hydroxyquinoline reagent. Int J Chem 12:29–36. https://doi.org/10.5539/ijc.v12n1p29
Okulik N, Jubert AH (2005) Theoretical analysis of the reactive sites of non–steroidal anti–inflammatory drugs. Internet Electron J Mol Des 4:17–30
Paiva LB, Goldbeck R, Santos WD, Squina FM (2013) Ferulic acid and derivatives: molecules with potential application in the pharmaceutical field. Braz J Pharm Sci 49:395–411. https://doi.org/10.1590/S1984-82502013000300002
Park CI, Huang HZ, Cha KW (2001) Spectrophotometric determination of uranium(VI) with pyrocatechol violet in surfactant media. Bull Korean Chem Soc 22:84–86
Raafid E, Al-Da’amy MA, Kadhim SH (2020) Spectrophotometric determination of Cu(II) in analytical sample using a new chromogenic reagent (HPEDN). Indones. J Chem 20:1080–1091. https://doi.org/10.22146/ijc.47894.
Rahmani M, Habibi M, NiAZi A (2015) Simultaneous spectrophotometric determination of Zinc and Copper with 4-(2-thiazolylazo) resorcinol using parallel factor analysis (PARAFAC), partial least squares (PLS) and orthogonal signal correction- partial least squares (OSC-PLS). Sci J 36:1601–1608
Reddy PNK, Reddy GT, Kumar SD, Reddy AVR, Parveen SN, Reddy NCG (2016) Spectrophotometric determination of Zn(II) in food and water samples using 2-hydroxy-N’-(1-(pyridin-2-yl)ethylidene) benzohydrazide as a sensitive and selective analytical reagent. Der Pharm Lett 8:251–259
Shiyi Ou, Kwok K-C (2004) Review Ferulic acid: pharmaceutical functions, preparation and applications in foods. J Sci Food Agric 84:1261–1269. https://doi.org/10.1002/jsfa.1873
Souza JC, Toci AT, Beluomini MA, Eiras SP (2016) Spectrophotometric determination of copper(II) in sugarcane spirit using 1-(2-pyridylazo)-2-naphthol and a homogeneous ternary mixture of the solvents water, ethanol and methyl isobutyl ketone. Rev Virtual Quim 8:687–701. https://doi.org/10.5935/1984-6835.20160052
Tirmizi SA, Wattoo FH, Wattoo MHS, Sarwar S, Memon AN, Ghangro AB (2012) Spectrophotometric study of stability constants of cimetidine–Ni(II) complex at different temperatures. Arab J Chem 5:309–314. https://doi.org/10.1016/j.arabjc.2010.09.009
Veitía MSI, Dumas F, Morgant G, Sorenson JRJ, Frapart Y (2009) Synthesis, structural analysis and anticonvulsant activity of a ternary Cu (II) mononuclear complex containing 1,10-phenanthroline and the leading antiepileptic drug valproic acid. Biochimie 91:1286–1293. https://doi.org/10.1016/j.biochi.2009.06.015
Wang Y, Chen X, Huang Z, Chen D, Yu B, Yu J, Chen H, He J, Luo Y, Zheng P (2020) Dietary ferulic acid supplementation improves antioxidant capacity and lipid metabolism in weaned piglets. Nutrients 12:3811–3821. https://doi.org/10.3390/nu12123811
Zaky RR, Yousef TA (2011) Spectral, magnetic, thermal, molecular modelling, ESR studies and antimicrobial activity of (E)-3-(2-(2-hydroxybenzylidene) hydrazinyl)-3-oxo-n(thiazole-2-yl)propanamide complexes. J Mol Struct 1002:76–85. https://doi.org/10.1016/j.molstruc.2011.06.050
Zdunska K, Dana A, Kolodziejczak A, Rotsztejn H (2018) Antioxidant properties of ferulic acid and its possible application. Skin Pharmacol Physiol 31:332–336. https://doi.org/10.1159/000491755
Zordok WA, Sadeek SA, El-Shwiniy WH (2012) Spectroscopic, thermal analysis, and antimicrobial evaluation of new Y(III), Zr(IV), and U(VI) ibuprofen complexes. J Coord Chem 65:353–369. https://doi.org/10.1080/00958972.2011.654203
Acknowledgements
The authors extend their appreciation to Zagazig University and University of Bisha.
Author information
Authors and Affiliations
Contributions
BAE contributed to conceptualization and funding acquisition; BAE and WSS contributed to investigation; WSS contributed to methodology; BAE, WSS and WHE contributed to project administration, resources, software, and supervision; WHE contributed to validation and visualization; BAE, WHE and WSS contributed to writing—original draft and writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Informed consent
All authors consent to publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Abd El-wahaab, B., Shehab, W.S. & El-Shwiniy, W.H. Synthesis, spectrophotometric, spectroscopic, microbial studies and analytical applications of Cu(II) and Zn(II) complexes of chalcone ligand. Chem. Pap. 76, 931–944 (2022). https://doi.org/10.1007/s11696-021-01916-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-021-01916-0