Abstract
In this study, the effects and mechanism of pretreatments of three types of chemical reagents combined with Fenton on poplar sawdust were studied and the optimization of enzymatic hydrolysis conditions was conducted using response surface methodology. The results showed that cellulase and hemicellulase had the best hydrolysis effect after NaOH-Fenton pretreatment, which were 63.73% and 29.29%, respectively. The optimal process of poplar substrate was to react in 1% NaOH at 100 ℃ for 1 h, then placed in the Fenton reaction system of 0.2 mmol Fe2+ and 25 mmol H2O2 for 7 h, and finally subjected to enzymatic hydrolysis for 72 h at 52 ℃, with a liquid-to-solid ratio of 33 wt/vol and 15 μL/g of β-glycosidase. Under this condition, the enzymatic hydrolysis rates of cellulase and hemicellulase reached 86.65% and 43.90%, respectively. In conclusion, the combination of NaOH and Fenton pretreatment can effectively promote the enzymatic hydrolysis of poplar sawdust, which has great potential in the production of cellulosic ethanol.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Growing energy demands and depletion of fossil fuels stimulated the search for biomass, thus promoting the production of fuels and chemicals from lignocellulose (Zhang et al. 2017; Shuo et al. 2018). Common lignocellulosic biomass materials, like corn stover (Li et al. 2016), bamboo (Kai et al. 2018), sugarcane bagasse (Rocha et al. 2014), and wheat straw (Farid et al. 2010), have been extensively studied. Lignocellulose mainly consists of cellulose, hemicellulose, and lignin, in which cellulose and hemicellulose can be hydrolyzed into reducing sugars, then converted into biofuels by enzymes or microbes (Galbe and Zacchi 2007). However, lignocellulose is difficult to degrade due to its complex structure and the presence of lignin (Zhou et al. 2014), which affects enzyme activity, fluid permeability, and enzyme accessibility (Jain and Vigneshwaran 2012), hindering the utilization of lignocellulosic materials. Therefore, pretreatment of the lignocellulose is necessary to break the compact structure and assist in the removal of lignin, thus promoting the contact between the cellulose and enzymes or microbes (Jørgensen et al. 2007).
Pretreatment methods are generally divided into four kinds: chemical, physical, physical–chemical, and biological pretreatments are widely used (Mosier et al. 2005). Among the various chemical pretreatments, alkali pretreatment is the most common due to its prominent delignification ability (Silverstein et al. 2007). The NaOH pretreatment could reduce the lignin and hemicellulose contents and significantly improve the enzyme digestibility of the agave stalk (Yang and Pan 2012). Du et al. (Du et al. 2013) pretreated cotton straw with high-pressure-assisted NaOH, resulting in a reducing sugar yield of 0.293 g/g biomass and cellulose conversion of 45.82%. However, single NaOH pretreatment has its disadvantage: The effect of enzymatic hydrolysis became worse with the increase of lignin amount except for the low lignin content (Agbor et al. 2011).
Fenton is an advanced oxidation reagent which is used to serve for treating organic wastewater (Zhang et al. 2019), and the Fenton reaction could been used in lignocellulose pretreatment. After the Fenton pretreatment, cotton was degraded effectively and its susceptibility to cellulase was improved (Prateek and Nadanathangam 2012). The enzymatic saccharification effect of four different biomass feedstocks (miscanthus, switchgrass, corn stover, and wheat straw) after Fenton pretreatment showed an average increase of 212% in comparison with untreated control groups, which demonstrated the capacity of Fenton to improve the accessibility of cellulose to enzymes (Kato et al. 2014). In the reaction mechanism, Fenton reagent generates hydroxyl radical through the reaction of Fe2+ and H2O2, as given in Eq. 1, where Fe2+ act as the catalyst. The consumed Fe2+ is regenerated by the reaction of Fe3+ with H2O2 as shown in Eq. 2. These free radicals could oxidize lignocellulose components (Nidheesh 2015; Yu-Cai et al. 2015). Fenton oxidation conditions are mild, and the treatment of alkali combined with Fenton has high potential in lignocellulose pretreatment. The sequential Fenton and dilute NaOH extraction has been applied to pretreat corn stover which changes the cellulose structure characteristics (porosity, morphology, and crystallinity) of the solid residues and enhances the enzymatic saccharification (Yu-Cai et al. 2015).
Cellulose can be hydrolyzed into glucose and other fermentable sugars after pretreatment. In general, common hydrolysis processes include acid hydrolysis and enzyme hydrolysis, and the latter has been widely used because of high hydrolysis efficiency and low environmental pollution (Kumar et al. 2015). The existing studies on the enzymatic hydrolysis conditions of pretreated biomass materials are mainly focused on the optimization of enzyme addition amount, process time, temperature, and pH. The optimal conditions for two-step alkaline-enzymatic hydrolysis are an NaOH concentration of 9%, enzyme ratio of 0.95, and time of 16 h (Łukajtis et al. 2018). In addition, lignocellulose digestibility is affected by liquid-to-solid ratio, buffer concentration, degree of crystallization, and porosity (Selig et al. 2011). When considering the effects of many factors, response surface methodology (RSM) is an effective tool for optimizing the process (Triveni et al. 2001). It can reduce the number of experiments needed to evaluate multiple parameters, and the experimental methodology forms a mathematical model to describe the whole process, in addition to analyzing the influence of independent variables. At present, RSM has been successfully used to optimize the enzymatic reaction conditions (Guan and Yao 2008).
In this study, poplar sawdust was treated with the combination of Fenton and dilute acid (HCl and H2SO4), dilute alkali (ammonia and NaOH), and oxidants (H2O2 and NaClO), respectively, to explore the differences of three chemical pretreatment methods on the removal ability of cellulose and hemicellulose in poplar sawdust and subsequent enzymatic hydrolysis of cellulose. Considering the enzymatic hydrolysis rates of cellulase and hemicellulase as indicators, the effects of the concentration, temperature, and time of NaOH treatment as well as the H2O2 and Fe2+ additions on the enzymatic hydrolysis of sawdust were investigated to obtain the optimal NaOH-Fenton pretreatment conditions. Finally, the optimal enzymatic hydrolysis conditions under the optimal NaOH-Fenton pretreatment was identified using RSM. This study provides a reference for maximizing the hemicellulose and cellulose in poplar sawdust into fermentable sugars, reducing the cost of ethanol production, and fully improve the comprehensive utilization value of poplar sawdust.
Materials and methods
Materials and chemicals
Poplar sawdust was obtained from a farm in the outskirts of Beijing. The raw material was smashed and sifted through a 20-mesh sieve, then dried to a constant weight. Through two-step acid hydrolysis, the contents of cellulose, hemicellulose, and lignin were determined to be 32.57%, 13.88%, and 32.11%, respectively.
The cellulase (≥ 700 units/g, optimal pH 6), β-glucosidase (≥ 2000 units/g, optimal pH 6), hemicellulase (≥ 2500 units/g, optimal pH 4.5), and cycloheximide were purchased from Sigma (St. Louis, MO, USA). Tetracycline hydrochloride was obtained from Amresco (Solon, OH, USA). The glucose standard, xylose standard, and cellobiose are chromatographically pure, and all supplied from Exin Biotechnology Co., Ltd. (Shanghai, China). The other reagents used in the experiment were of analytical purity and were purchased from Beijing Chemical Works (Beijing, China).
Different pretreatments of poplar sawdust (Zhao et al. 2021 )
Fenton treatment
The tube was first loaded with 1 g of sawdust, 0.1 mmol FeSO4·7 H2O and 15 mmol 30% H2O2, and deionized water was added to 15 mL. 98% sulfuric acid was used to adjust the pH to 4. The tube was kept away from light for 10 h to complete the Fenton reaction. After the reaction, the mixture was filtered through a 200-mesh cell sieve, and the remaining sawdust was washed with 2% oxalic acid and deionized water.
Acid combined with Fenton treatment
Acid treatment was conducted with different concentration (1%、2%、3%、4%、5%) of H2SO4 or HCl at 1:15 wt/vol solid-to-liquid ratio at 80℃ water for 2 h. After filtration, the remaining sawdust were subjected to Fenton reaction according to the above method.
Alkali combined with Fenton treatment
Poplar sawdust and dilute alkali solution of different concentration (NaOH was 1%, 2%, 3%, 4%, 5%, ammonia water was 5%, 10%, 15%, 20%) were reacted in a 75℃ water bath for 2 h at a material-to-liquid ratio of 1:15 wt/vol. After solid–liquid separation, performing the Fenton reaction according to the above conditions.
Oxidants combined with Fenton treatment
H2O2 treatment was to mix poplar sawdust and H2O2 solution (1.5%、2.5%、3.5%、4.5%、5.5%) at a ratio of 1:15 wt/vol and incubated at 30 °C for 2 h. Fenton treatment is performed after filtration. NaClO treatment was conducted with NaClO solution (1.5%、2.5%、3.5%、4.5%、5.5%) at 1:15 solid-to-liquid ratio at 80℃ water for 2 h. After filtration, the sawdust was subjected to Fenton treatment.
Determination of content of glucose and xylose
Refer to two-step acid hydrolysis, untreated poplar sawdust was used as samples (Sluiter et al. 2010). Step 1: 0.300 g sample was accurately weighed and mixed with 3.0 ml of 72% sulfuric acid at 30 ℃ for 1 h and swirled every 5–10 min. Step 2: 84 mL deionized water was added to sulfuric acid to dilute the concentration to 4%. After reacting at 121 ℃ for 1 h, the filtrate obtained by suction filtration was passed through a 0.22 μm filter membrane. The contents of glucose and xylose in filtrate were determined by high-performance liquid chromatography (HPLC, Agilent 1200, Palo Alto, CA, USA) system equipped with a refractive index detector, the corresponding protection column, and a Rezex ROA. The samples were eluted with 0.005 M H2SO4 at a flow rate of 0.6 mL/min and a column temperature of 65 ℃. The retention time of glucose and xylose were 10.550 min and 11.000 min, respectively. Each sample was repeated three times and the average was used as the calculation result.
Calculation of enzymatic hydrolysis conversion Rate
After the pretreatment, the poplar sawdust and 25 μL of hemicellulase were mixed with 30 mL acetate buffer (pH 4.8) in a 50-mL centrifugal tube and placed in a 70℃ water bath for 1 d. Also, 60 μg of cycloheximide and 80 μg of tetracycline hydrochloride were added to avoid pH changes caused by microbial growth. When the reaction was complete, the tube reactor was cooled, and 60 μL cellulase and 10 μL β-glucosidase were added to perform the hydrolysis reaction at 120 rpm and 50℃ for 3 d. Finally, the mixed contents were filtered through a 200-mesh cell sieve, and the enzymatic hydrolysate was collected to determine the contents of glucose and xylose for the calculation of enzymatic conversion rate of cellulose and hemicellulose. The calculation equations were as follows (Zhao et al. 2021).
where C1/C2 was the concentration of glucose/xylose in enzymatic hydrolysate (mg/mL), V was the total volume (mL), 0.90 was the conversion factor for glucose to equivalent cellulose, 0.88 was the conversion factor for xylose to equivalent hemicellulose, m was the quantity of poplar sawdust (mg), and W1/W2 is the content of glucose/xylose in two-step acid hydrolysis.
Optimization of NaOH treatment under fixed Fenton conditions
For the optimization of NaOH treatment temperature-concentration, poplar sawdust was mixed with dilute NaOH (1%, 2%, 3%, 4%, 5%) at 1:15 wt/vol solid-to-liquid ratio at 25 ℃, 50 ℃, 75 ℃, and 100 ℃ for 2 h. Then, the Fenton reaction was performed under the conditions of 0.1 mmol FeSO4·7 H2O and 15 mmol 30% H2O2 for 10 h.
For the optimization of NaOH treatment time, poplar sawdust and NaOH solution were incubated for 0.5 h, 1.0 h, 2.0 h, 3.0 h, and 4.0 h at the optimal NaOH concentration and temperature obtained in the previous experiment. Then, Fenton treatment was carried out according to the above conditions.
For the circulation of alkali pretreatment solution, the liquid waste was collected after NaOH pretreatment, and the residual NaOH was determined by titration. According to the measured results, NaOH was added to the liquid waste to reach the initial concentration. The poplar sawdust was processed, and the liquid waste was collected again. The process was repeated.
Optimization of Fenton treatment under fixed NaOH treatment conditions
The optimization of the Fenton reaction conditions was carried out under the optimal conditions of NaOH treatment obtained in the above experiment. For the optimization of H2O2 addition amount, 1 g of alkali treated sawdust was mixed with 0.1 mmol FeSO4·7 H2O and different amounts of H2O2 (5 mmol, 10 mmol, 15 mmol, 20 mmol, 25 mmol, and 30 mmol). Deionized water was added to adjust the material-to-liquid ratio to 1:15 wt/vol, and sulfuric acid was used to adjust the pH to 4. The tube was kept out of the light for 10 h to perform the Fenton reaction. For the optimization of Fe2+ addition amount, the sawdust was mixed with 25 mmol H2O2 and different amounts of FeSO4·7 H2O (0.05 mmol, 0.1 mmol, 0.2 mmol, 0.3 mmol, and 0.4 mmol), and the other operations were the same as above.
For the optimization of Fenton reaction time, 1 g of sawdust was mixed with the optimal addition amount of FeSO4·7 H2O and H2O2 obtained in the above experiment. Deionized water was added to adjust the material-to-liquid ratio to 1:15 wt/vol. Then, the mixture reacted away from the light for different times (1 h, 2 h, 3 h, 4 h, 5 h, 6 h, 7 h, 8 h, 9 h, 10 h, 11 h, and 12 h).
Response surface optimization of enzymatic hydrolysis conditions
According to the Box–Behnken design and the preliminary test results, the enzymatic hydrolysis temperature, liquid-to-solid ratio, enzyme concentration, and reaction time were selected, and each factor was set at three levels to conduct response surface experiments at a total of 30 tests. The total reducing sugar obtained by enzymatic hydrolysis was taken as the response value to optimize the enzymatic hydrolysis of pretreated poplar sawdust. The reducing sugar content of the sample was determined by DNS method (Hu et al. 2008).
Result and discussion
Comparison analysis of different treatments combined with Fenton on lignocellulose enzymatic hydrolysis at ordinary pressure
Dilute acid treatment is suitable for industrial application because of its weak corrosivity and easy recovery of acid. Figure 1 depicts the effect of pretreatment with different concentrations (1–5%) of H2SO4 or HCl combined with Fenton on the enzymatic hydrolysis of sawdust. The conversion rates of cellulose and hemicellulose increased as the dilute acid concentration increased from 1 to 3%. When the concentration exceeded 3% and 4%, the enzymatic conversion rate of cellulose and hemicellulose slightly decreased. The study of Saha et al. showed that the wheat straw gave maximum sugar yield after 0.75% H2SO4 pretreatment, and the fermentable sugar yield decreased with the increase of acid dose, which is consistent with our observation. This might because that as the concentration of acid increases, the sugars gradually degraded into furfural and HMF which could influence enzymatic hydrolysis of the sawdust, resulting in the decrease of the conversion of cellulose and hemicellulose (Bcs et al. 2005). The enzymatic hydrolysis efficiency of the dilute H2SO4 was higher than that of the dilute HCl. When the concentration of dilute H2SO4 was 3%, the enzymatic hydrolysis rates of cellulose and hemicellulose were 9.50% and 28.60% higher, respectively, than those of dilute HCl at the same concentration. This result shows that dilute HCl pretreatment causes indistinctive delignification with subtle structural changes in the fibers (Cristina and Silvia 2014). However, dilute H2SO4 pretreatment can make hemicellulose more soluble, and the chemical structure in the solid residue changes, making the sawdust more porous and improving the cellulose accessibility of enzymes (Hsu et al. 2010; Alviraet al. 2010). Therefore, the combination of 3% H2SO4 and Fenton is a better pretreatment condition for lignocellulose, and the enzymatic hydrolysis rates of cellulose and hemicellulose reached 53.95% and 30.60%, respectively.
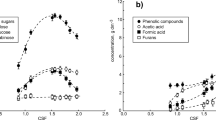
With the increase in oxidant concentration, the cellulose conversion rate gradually increased and tended to be stable at the concentration of 4.5%, while the enzymatic conversion rate of hemicellulose declined after the oxidant concentration exceeded 4.5% (Fig. 2). In addition, the enzymatic hydrolysis rates of cellulose and hemicellulose in the 4.5% H2O2 group were 37.88% and 22.38%, respectively, 3.86% and 18.77% higher than those in the 4.5% NaClO group. Therefore, the pretreatment effect of H2O2 was better than NaClO, which is consistent with previous research (Martelli-Tosi et al. 2017). Oxidants break down the network structure of the materials through peroxidative degradation of lignin, thereby improving the enzymatic hydrolysis efficiency of cellulose and hemicellulose. As the oxidability of HClO produced by NaClO was weaker than H2O2, its ability to oxidize lignin was weak and it is not as effective as H2O2 in promoting enzymatic hydrolysis. In many cases the reaction of oxidant treatment is not selective, resulting in loss of hemicellulose and cellulose and therefore the pretreatment effect is inferior to dilute acid pretreatment (Hendriks and Zeeman 2009). In conclusion, in above pretreatment experiment of poplar sawdust with oxidants combined with Fenton, the pretreatment effect of 4.5% H2O2-Fenton was the best.
When the concentration of NaOH and ammonia was 3–4% and 15–20%, respectively, the hydrolysis rates of cellulose and hemicellulose reached the maximum (Fig. 3). The conversion rates of cellulose and hemicellulose under 15–20% ammonia pretreatment were much lower than that of 3–4% NaOH. However, the enzymatic conversion rates of cellulose and hemicellulose at 4% NaOH did not increase significantly compared with those at 3% NaOH. In consequence, 3% NaOH combined with Fenton reaction was the suitable pretreatment in this experiment, and the enzymatic hydrolysis efficiencies of cellulose and hemicellulose were 63.73% and 29.29%, respectively. Although ammonia treatment promotes lignocellulose enzymatic hydrolysis less than NaOH treatment, the commonly used ammonia explosion pretreatment requires high temperature and high pressure (Cha et al. 2014). In this experiment, the combined pretreatment of ammonia and Fenton was carried out at 75℃, which was more mild and safe.
Among the three types of chemical treatments, the cellulose conversion rate of 3% NaOH combined with Fenton pretreatment was the highest (63.73%), 68.25% and 18.11% higher than that of 4.5% H2O2 and 3% H2SO4, respectively. The hemicellulose conversion rate at 3% NaOH (29.29%) was comparable to that of 3% H2SO4 but 32.75% higher than of 4.5% H2O2. The effect of different kinds of bases on lignocellulosic biomass is the basis of alkali treatments, which may be the reason why diluted NaOH is more effective than ammonia (Mcmillan 1994; Fan et al. 1987). Solvation and saponification are the first reactions to occur in NaOH pretreatment. Solvation reaction can dissolve part of lignin into liquid components. Meanwhile, saponification of intermolecular ester bonds reduces the strength of hydrogen bonds between hemicellulose and lignin and increases porosity and surface area, causing the expansion of the lignocellulosic biomass and makes it easier for enzymes to access (Hendriks and Zeeman 2009; Zhao et al. 2021). NaOH pretreatment improved cellulose digestibility and it was more effective in the solubilization of lignin, exhibiting less dissolution of cellulose and hemicellulose than acid and oxidant processes (Carvalheiro et al. 2008). Moreover, compared with acid method, alkali treatment causes less sugar degradation, and caustic salts such as NaOH can be recovered and/or regenerated (Kumar et al. 2009). NaOH extraction can also change the crystalline state of cellulose thus reducing the degree of polymerization and crystallinity (Gregg and Saddler 1996; Singh et al. 2015). Given the enzymatic hydrolysis efficiency after pretreatments, the combined pretreatment of dilute NaOH and Fenton reaction was determined as the optimal pretreatment process in this study, and the conditions were optimized in the subsequent experiments.
Improvement in alkali pretreatment conditions of lignocellulose under fixed Fenton reaction
Figure 4a, b, c shows the enhancement of the enzymatic hydrolysis of poplar sawdust pretreated by dilute NaOH (0.5% to 4.0%) at 25℃, 50℃, and 75℃ for 2 h, respectively. When the NaOH concentration was increased, the enzymatic hydrolysis efficiency of cellulose gradually increased at 25℃, 50℃, and 75℃ and reached the maximum of 36.70%, 41.50%, and 61.93% at 3% NaOH, respectively, increasing by 31.00%, 19.60%, and 41.06% compared with 0.5% NaOH. The enzymatic conversion rate of cellulose decreased when the NaOH concentration was further increased to 4%. Similar to the cellulase hydrolysis rate, the enzymatic conversion rate of hemicellulase also increased with the increasing NaOH in the low concentration range, reaching the highest value of 27.90%, 28.05%, and 37.40%, respectively, at 1% to 2% NaOH, and then gradually decreased. When the alkali treatment temperature continued to rise to 100℃, cellulose conversion rate was higher than at 25℃, 50℃, and 75℃ (Fig. 4d). The enzymatic hydrolysis rate of cellulose reached a maximum of 66.45% at 1.0% NaOH under 100℃ for 1 h and the further increase of NaOH did not significantly improve the cellulase hydrolysis rate. Hemicellulase hydrolysis rate also rose to the highest value of 33.70% at 1.0% NaOH. Therefore, the optimal concentration of NaOH at 100℃ was 1.0%.
Alkali pretreatment removes lignin, reduces lignin molecular weight, and weakens the inhibition of lignin on cellulose hydrolysis to improve hydrolysis efficiency (Zhang et al. 2020). The cellulose enzymatic conversion rate at 100℃ was much higher than other temperatures. Moreover, the cellulose conversion rate at 1.0% NaOH was 66.40%, which was 85.17%, 60.19%, and 9.05% higher than that at 25℃, 50℃, and 75℃, respectively. This result can be explained by the fact that high temperature alkali pretreatment breaks the fiber, leading to a loose arrangement, thus causing more serious physical structure damage to the substrate (Vijay et al. 2019). The 100℃ NaOH pretreatment provides an efficient removal of lignin (Lloyd and Wyman 2005). In the pretreatment of NaOH combined with Fenton, the optimal condition was 1% NaOH at 100℃.
Figure 5 shows the results of the enzymatic hydrolysis rates of different reaction time with 1.0% NaOH at 100℃. The enzymatic hydrolysis rates of cellulose and hemicellulose reached a maximum of 68.64% and 32.60% at 1 h and 2 h, respectively. The enzymatic hydrolysis rates decreased gradually with the extension of reaction time. In dilute alkali combined with Fenton treatment, the optimum condition was 1.0% NaOH extraction at 100℃ for 1 h.
Alkali pretreatment solution contains phenols produced after lignin degradation and residual NaOH, which is harmful to the environment and could cause the waste of NaOH. The results of the experiment on circulation of waste liquid are shown in Fig. 6. With the increase of cycle times, the enzymatic hydrolysis conversion rates decreased gradually. In the fourth cycle, the enzymatic hydrolysis rates of cellulose and hemicelluloses were 60.59% and 27.61%, respectively, which were 11.73% and 16.60% lower than the first cycle. As the cycle continued, the enzymatic hydrolysis efficiency dropped. Taking production efficiency and cost into consideration, the waste liquid can be recycled for four times to reduce about 31% NaOH consumption.
Improvement in Fenton reaction conditions of lignocellulose under fixed alkali pretreatment
H2O2 is essential in the Fenton reaction because it is converted by Fe2+ to generate hydroxyl radicals (Walling 1975). As shown in Fig. 7, at the fixed concentration of Fe2+, the enzymolysis efficiency of cellulose improved with the increase of H2O2 in the range of 5–25 mmol, which was in accordance with the previous report (Jung et al. 2015). When the H2O2 addition was 25 mmol, the enzymatic conversion rate of cellulose reached the highest value of 72.00%, and as the addition amount of H2O2 continued to increase, it decreased instead. This result was attributed to the fact that excessive H2O2 can oxidize Fe2+ to Fe3+, thus inhibiting the production of hydroxyl radicals and affecting the oxidation capacity of Fenton. The enzymatic hydrolysis rate of hemicellulose increased slightly with the addition of H2O2 and reached the maximum at 15 mmol. The effect of H2O2 addition on the enzymolysis efficiency of hemicellulose was less significant than that of cellulose.
Figure 8 shows the effect of Fe2+ addition on enzymatic hydrolysis of poplar sawdust. Specifically, with the increase of Fe2+, the cellulose conversion rate first rose and then decreased. When the amount of Fe2+ was 0.2 mmol, the enzymatic hydrolysis conversion rate of cellulose reached a maximum of 75.60%, which was 53.90% higher than that at 0.05 mmol. The hemicellulase hydrolysis conversion rate also reached a peak value of 33.70% at 0.2 mmol Fe2+, which was 13.40% higher than that at 0.4 mmol. Therefore, the optimized addition amount of H2O2 and Fe2+ was 25 mmol and 20 mmol, respectively.
As shown in Fig. 9, the enzymatic hydrolysis conversion rates of cellulose and hemicellulose rose with the increase of reaction time in the first 7 h of Fenton, and the hydrolysis rates of cellulose and hemicellulose increased by 76.21% and 79.58% at 7 h, respectively, compared with that at 1 h. With a further extension of the reaction time after 7 h, the enzymatic hydrolysis rates did not ascend anymore and reached a plateau. This trend was consistent with a previous study (Zhang and Zhu 2016). Therefore, the appropriate time for Fenton reaction was 7 h.
Optimization of sawdust enzymatic hydrolysis conditions after pretreatment of NaOH combined with Fenton
On the basis of previous experiments, the system consisted of 30 experiments according to the Box–Behnken center combination design principle. For the investigated variables, it was assumed that the boundary conditions are enzymatic hydrolysis temperature of 45℃, 50℃, and 55℃, liquid-to-solid ratio of 20 wt/vol, 30 wt/vol, and 40 wt/vol, β-glycosidase loading of 5 μL, 10 μL, and 15 μL, and the enzymatic hydrolysis time of 48 h, 60 h, and 72 h. The mass of the obtained reducing sugars was selected as a parameter representing the enzymatic hydrolysis efficiency. Table 1 shows the experimental design and the content of total reducing sugar obtained.
The effects of process parameters (temperature, liquid-to-solid ratio, the β-glycosidase content, and the time of the enzymatic hydrolysis process) on the reducing sugars yield were examined. The relationships among four factors during the enzymatic hydrolysis process were determined by the quadratic multiple regression method. After performing analysis of variance (ANOVA) on the data in Table 1, the quadratic regression equation takes the following form,
where A, B, C, and D represent enzymatic hydrolysis temperature, liquid-to-solid ratio, β-glycosidase content, and the enzymatic hydrolysis time, respectively. Y represents reducing sugar yield.
The analysis of variance for the above model is shown in Table 2. The lack of fit was not significant (P = 0.3453 > 0.05), and the model P value < 0.0001, showing that the model selected in the experiment was highly significant. In addition, the correlation coefficient (R2) was 0.9809, indicating that the correlation between measured and predicted value was very high. The adjusted R2 was calculated for 0.9602, showing that 96.02% of the reducing sugar yield variability was explained by this statistical model, indicating that the model can predict the response. The relatively low coefficient of variation (CV) (3.17%) indicates a good repeatability of the experiments, so it can be used to predict the quality of total reducing sugar obtained by enzymatic hydrolysis.
In Table 2, the F test and its corresponding P values indicate the significance of the regression equation terms on reducing sugar yield. It was concluded from Table 2 that the effects of the primary term A, the interaction term AD, and the quadratic term of A on reducing sugar yield are highly significant (P < 0.01). It is because the essence of cellulase is protein, which needs proper hydrolysis temperature. The effects of enzymatic hydrolysis temperature (A) and time (D) on the reducing sugar yield of poplar sawdust are shown in Fig. 10a. According to the shape of the response surface, the optimum temperature of enzymatic hydrolysis was from 48 to 52℃, and the yield of reducing sugar obtained was lower above or below this range. When the temperature was lower than 47℃, the surface dropped steeply, indicating that the lower temperature had a greater effect on the recovery of reducing sugar. Figure 10a, b, c shows that compared with the duration temperature of the enzymatic hydrolysis process, the trend of reducing sugar yield with liquid-to-solid ratio, β-glycosidase content, or enzymatic hydrolysis time changed slowly.
The optimum conditions of cellulase hydrolysis were further predicted within the test range. A reaction temperature of 51.63℃, liquid-to-solid ratio of 33.00 wt/vol, β-glycosidase content of 14.56 μL/g substrates, and a reaction time of 71.97 h were the optimal conditions. Considering the experimental operation, the conditions were set to a reaction temperature of 52℃, a liquid-to-solid ratio of 33 wt/vol, a β-glucosidase content of 15 μL/g substrate, and a reaction time of 72 h. Under this condition, the predicted reducing sugar yield is 302.49 mg/g substrates after enzymatic hydrolysis. After three confirmatory tests, the total reducing sugar measured was 301.47 mg/g substrates, which was very close to the predicted value. The conversion rates of cellulose and hemicellulose were 86.65% and 43.90% respectively, which were higher than those before optimization. Therefore, this model is effective and practical for the enzymatic hydrolysis of lignocellulose after the pretreatment of NaOH combined with Fenton.
Conclusion
In order to realize the effective conversion of poplar sawdust to fuel ethanol, the effect and mechanism of different chemical pretreatments of poplar sawdust were studied, and the pretreatment and enzymatic hydrolysis conditions were optimized. The enzymatic hydrolysis effects after various pretreatments are that NaOH-Fenton > H2SO4-Fenton > HCl-Fenton > ammonia-Fenton > H2O2-Fenton > NaClO-Fenton, and the cellulase and hemicellulase hydrolysis rates of poplar sawdust treated with dilute NaOH-Fenton were 63.73% and 29.29% before optimization, respectively. The results showed that dilute alkali treatment had less cellulose loss and lower degradation degree of reducing sugar compared with diluted acid treatment and oxidant treatment. Moreover, the alkali liquor can be recycled, which has high environmental benefit. The optimum pretreatment conditions of NaOH combined with Fenton were as follows: 1% NaOH extraction at 100℃ for 1 h and subsequent 25 mmol H2O2 and 0.2 mmol Fe2+ for a Fenton reaction period of 7 h. The optimized enzymatic hydrolysis was performed at a reaction temperature of 52℃, a β-glycosidase content of 15 μL/g substrates, a liquid-to-solid ratio of 33 wt/vol and a reaction time of 72 h after the NaOH-Fenton pretreatment. The cellulose and hemicellulose conversion rates were 86.65% and 43.90%, respectively, significantly higher than those before optimization.
This study explored a suitable pretreatment method for poplar sawdust to promote the degradation of lignocellulose in biomass feedstock. Optimization of the conditions of pretreatment and enzymatic hydrolysis effectively improves the utilization of lignocellulose, which lays the foundation for subsequent research on its conversion to biofuels. The structural characterization of pretreated poplar sawdust needs to study to further clarify its mechanism, and the recycling of alkali black liquor will also be considered in the future.
Data Availability
All authors declare that all data and materials support their published claims and comply with field standards.
Code availability
Not applicable.
References
Agbor VB, Cicek N, Sparling R, Berlin A, Levin DB (2011) Biomass pretreatment: Fundamentals toward application. Biotechnol Adv 29:675–685. https://doi.org/10.1016/j.biotechadv.2011.05.005
Alvira P, Tomas-Pejo E, Ballesteros M, Negro MJ (2010) Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour Technol 101:4851–4861. https://doi.org/10.1016/j.biortech.2009.11.093
Bcs A, Lbi A, Mac A, Yvw B (2005) Dilute acid pretreatment, enzymatic saccharification and fermentation of wheat straw to ethanol. Process Biochem 40:3693–3700. https://doi.org/10.1016/j.procbio.2005.04.006
Carvalheiro F, Duarte LC, Gírio FM (2008) Hemicellulose biorefineries: a review on biomass pretreatments. J Sci Ind Res 67:849–864. https://doi.org/10.1088/0960-1317/18/11/115030
Cha YL, Yang J, Ahn JW, Moon YH, Yoon YM, Yu GD, An GH, Choi IH (2014) The optimized CO2-added ammonia explosion pretreatment for bioethanol production from rice straw. Bioprocess Biosyst Eng 37:1907–1915. https://doi.org/10.1007/s00449-014-1165-x
Cristina T, Silvia B (2014) Effect of four pretreatments on enzymatic hydrolysis and ethanol fermentation of wheat straw. Influence of inhibitors and washing. Bioresour Technol 157:68–76. https://doi.org/10.1016/j.biortech.2014.01.090
Du SK, Zhu XN, Wang H, Zhou DY, Yang WH, Xu H (2013) High pressure assist-alkali pretreatment of cotton stalk and physiochemical characterization of biomass. Bioresour Technol 148:494–500. https://doi.org/10.1016/j.biortech.2013.09.020
Fan LT, Gharpuray MM, Lee YH (1987) Cellulose Hydrolysis. Springer, Berlin, Heidelberg
Farid T, Dimitar K, Irini A (2010) Production of bioethanol from wheat straw: An overview on pretreatment, hydrolysis and fermentation. Bioresour Technol 101:4744–4753. https://doi.org/10.1016/j.biortech.2009.11.080
Galbe M, Zacchi G (2007) Pretreatment of lignocellulosic materials for efficient bioethanol production. Adv Biochem Eng Biotechnol 108:41–65. https://doi.org/10.1007/10_2007_070
Gregg D, Saddler JN (1996) A techno-economic assessment of the pretreatment and fractionation steps of a biomass-to-ethanol process. Appl Biochem Biotechnol 57–58:711–727. https://doi.org/10.1007/BF02941753
Guan X, Yao HY (2008) Optimization of Viscozyme L-assisted extraction of oat bran protein using response surface methodology. Food Chem 106:345–351. https://doi.org/10.1016/j.foodchem.2007.05.041
Hendriks A, Zeeman G (2009) Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour Technol 100:10–18. https://doi.org/10.1016/j.biortech.2008.05.027
Hsu TC, Guo GL, Chen WH, Hwang WS (2010) Effect of dilute acid pretreatment of rice straw on structural properties and enzymatic hydrolysis. Bioresour Technol 101:4907–4913. https://doi.org/10.1016/j.biortech.2009.10.009
Hu RF, Lu L, Liu TJ, Ouyang PK, He BH, Liu SJ (2008) Reducing Sugar Content in Hemicellulose Hydrolysate by DNS Method: A Revisit. J Biobased Mater Bioenergy 2:156–161. https://doi.org/10.1166/jbmb.2008.306
Jain P, Vigneshwaran N (2012) Effect of Fenton’s pretreatment on cotton cellulosic substrates to enhance its enzymatic hydrolysis response. Bioresour Technol 103:219–226. https://doi.org/10.1016/j.biortech.2011.09.110
Jørgensen H, Kristensen JB, Felby C (2007) Enzymatic conversion of lignocellulose into fermentable sugars: Challenges and opportunities. Biofuels Bioprod Biorefining 1:119–134. https://doi.org/10.1002/bbb.4
Jung YH, Kim HK, Park HM, Park Y, Park K, Seo J, Kim KH (2015) Mimicking the Fenton reaction-induced wood decay by fungi for pretreatment of lignocellulose. Bioresour Technol 179:467–472. https://doi.org/10.1016/j.biortech.2014.12.069
Kai W, Ying W, Zheng JS, Yang H, Jing Y (2018) Fenton reaction-oxidized bamboo lignin surface and structural modification to reduce nonproductive cellulase binding and improve enzyme digestion of cellulose. ACS Sustain Chem Eng 6:3853–3861. https://doi.org/10.1021/acssuschemeng.7b04191
Kato DM, Elía N, Flythe M, Lynn BC (2014) Pretreatment of lignocellulosic biomass using Fenton chemistry. Bioresour Technol 162:273–278. https://doi.org/10.1016/j.biortech.2011.09.110
Kumar P, Barrett DM, De Lwiche MJ, Stroeve P (2009) Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind Eng Chem Res 48:3713–3729. https://doi.org/10.1021/ie801542g
Kumar G, Bakonyi P, Periyasamy S, Kim SH, Nemestóthy N, Bélafi-Bakó K (2015) Lignocellulose biohydrogen: Practical challenges and recent progress. Renew Sust Energ Rev 44:728–737. https://doi.org/10.1016/j.rser.2015.01.042
Li W, Liu Q, Ma Q, Zhang T, Ma L, Jameel H, Chang H-m (2016) A two-stage pretreatment process using dilute hydrochloric acid followed by Fenton oxidation to improve sugar recovery from corn stover. Bioresour Technol 219:753–756. https://doi.org/10.1016/j.biortech.2016.08.025
Lloyd TA, Wyman CE (2005) Combined sugar yields for dilute sulfuric acid pretreatment of corn stover followed by enzymatic hydrolysis of the remaining solids. Bioresour Technol 96:1967–1977. https://doi.org/10.1016/j.biortech.2005.01.011
Łukajtis R, Kucharska K, Hołowacz I, Rybarczyk P, Wychodnik K, Słupek E, Nowak P, Kamiński M (2018) Comparison and optimization of saccharification conditions of alkaline pre-treated triticale straw for acid and enzymatic hydrolysis followed by ethanol fermentation. Energies 11:639. https://doi.org/10.3390/en11030639
Martelli-Tosi M, Assis OBG, Silva NC, Esposto BS, Martins MA, Tapia-Blacido DR (2017) Chemical treatment and characterization of soybean straw and soybean protein isolate/straw composite films. Carbohydr Polym 157:512–520. https://doi.org/10.1016/j.carbpol.2016.10.013
Mcmillan JD (1994) Pretreatment of lignocellulosic biomass. Humana, Totowa, NJ
Mosier N, Wyman C, Dale B, Elander R, Lee YY, Holtzapple M, Ladisch M (2005) Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol 96:673–686. https://doi.org/10.1016/j.biortech.2004.06.025
Nidheesh PV (2015) Heterogeneous Fenton catalysts for the abatement of organic pollutants from aqueous solution: A review. RSC Adv 5:40552–40577. https://doi.org/10.1039/c5ra02023a
Prateek J, Nadanathangam V (2012) Effect of Fenton’s pretreatment on cotton cellulosic substrates to enhance its enzymatic hydrolysis response. Bioresour Technol 103:219–226. https://doi.org/10.1016/j.biortech.2011.09.110
Rocha GJM, Nascimento VM, da Silva VFN, Corso DLS, Goncalves AR (2014) Contributing to the environmental sustainability of the second generation ethanol production: Delignification of sugarcane bagasse with sodium hydroxide recycling. Ind Crop Prod 59:63–68. https://doi.org/10.1016/j.indcrop.2014.05.002
Selig MJ, Tucker MP, Law C, Doeppke C, Himmel ME, Decker SR (2011) High throughput determination of glucan and xylan fractions in lignocelluloses. Biotechnol Lett 33:961–967. https://doi.org/10.1007/s10529-011-0526-7
Shuo F, Wenhui W, Shisheng T, Chunyan Z, Ping L (2018) Evaluation of the effects of isolated lignin on cellulose enzymatic hydrolysis of corn stover pretreatment by NaOH combined with ozone. Molecules 23:1495. https://doi.org/10.3390/molecules23061495
Silverstein RA, Chen Y, Sharma-Shivappa RR, Boyette MD, Osborne J (2007) A comparison of chemical pretreatment methods for improving saccharification of cotton stalks. Bioresour Technol 98:3000–3011. https://doi.org/10.1016/j.biortech.2006.10.022
Singh J, Suhag M, Dhaka A (2015) Augmented digestion of lignocellulose by steam explosion, acid and alkaline pretreatment methods: A review. Carbohydr Polym 117:624–631. https://doi.org/10.1016/j.carbpol.2014.10.012
Sluiter BHA, Hames B, Ruliz-Peinadd R (2010) Determination of structural carbohydrates and lignin in biomass. Laboratory Analytical Procedure 1617:1–16
Triveni R, Shamala TR, Rastogi NK (2001) Optimised production and utilisation of exopolysaccharide from Agrobacterium radiobacter. Process Biochem 36:787–795. https://doi.org/10.1016/S0032-9592(00)00279-X
Vijay R, Singaravelu DL, Vinod A, Sanjay MR, Siengchin S, Jawaid M, Khan A, Parameswaranpillai J (2019) Characterization of raw and alkali treated new natural cellulosic fibers from Tridax procumbens. Int J Biol Macromol 125:99–108. https://doi.org/10.1016/j.ijbiomac.2018.12.056
Walling C (1975) Fenton’s Reagent Revisited Accounts Chem Res 8:125–131. https://doi.org/10.1021/ar50088a003
Yang Q, Pan XJ (2012) Pretreatment of Agave americana stalk for enzymatic saccharification. Bioresour Technol 126:336–340. https://doi.org/10.1016/j.biortech.2012.10.018
Yu-Cai H, Yun D, Yu-Feng X, Bin Y, Feng L, Cheng W, Zheng-Zhong Z, Qing Q, Hao W, Cheng Z, Zhi-Cheng T, Dan-Ping Z (2015) Enhancement of enzymatic saccharification of corn stover with sequential Fenton pretreatment and dilute NaOH extraction. Bioresour Technol 193:324–330. https://doi.org/10.1016/j.biortech.2015.06.088
Zhang T, Zhu M-J (2016) Enhancing enzymolysis and fermentation efficiency of sugarcane bagasse by synergistic pretreatment of Fenton reaction and sodium hydroxide extraction. Bioresour Technol 214:769–777. https://doi.org/10.1016/j.biortech.2016.05.032
Zhang H, Wu S, Xie J (2017) Evaluation of the effects of isolated lignin on enzymatic hydrolysis of cellulose. Enzyme Microb Technol 101:44–50. https://doi.org/10.1016/j.enzmictec.2017.03.001
Zhang M-h, Dong H, Zhao L, Wang D-x, Meng D (2019) A review on Fenton process for organic wastewater treatment based on optimization perspective. Sci Total Environ 670:110–121. https://doi.org/10.1016/j.scitotenv.2019.03.180
Zhang JH, Qin YJ, Li KN, Wang JY, Wang ZW (2020) Structure characterization and inhibitory effect of lignin from the outer and inner layers of bamboo after alkali pretreatment. Cellulose 27:5677–5688. https://doi.org/10.1007/s10570-020-03205-7
Zhao Z, Zhang J, Li Y, Li F, Liu P (2021) Effects and Mechanisms of Alkali Recycling and Ozone Recycling on Enzymatic Conversion in Alkali Combined with Ozone Pretreatment of Corn Stover. Appl Biochem Biotechnol. https://doi.org/10.1007/s12010-020-03425-4
Zhou W, Yang MH, Wang CX, Liu JF, Xing JM (2014) Changes in plant cell-wall structure of corn stover due to hot compressed water pretreatment and enhanced enzymatic hydrolysis. World J Microbiol Biotechnol 30:2325–2333. https://doi.org/10.1007/s11274-014-1651-y
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were completed by FL, XL, and YL. The first draft was written by LF PL contributed to the experimental design. XL, YL, XZ, and ZZ reviewed and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, F., Lu, X., Li, Y. et al. Effect and optimization of NaOH combined with Fenton pretreatment conditions on enzymatic hydrolysis of poplar sawdust. Chem. Pap. 76, 533–544 (2022). https://doi.org/10.1007/s11696-021-01887-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-021-01887-2