Abstract
Dissolution testing is a very significant tool that adds in vivo relevance to in vitro analytical data, thus providing a realistic in vitro/in vivo correlation. Dissolution profiling serves as a predictor of biological performance since the rate limiting step in the absorption of any drug is the rate of release from its pharmaceutical formulation. Being a routine procedure in quality control laboratories for initial approval process and scaling-up, the development of more and more eco-friendly methods for dissolution monitoring is considered as a worldwide trendy goal. In the present contribution, a comparison was highlighted between the two analytical techniques of utmost importance in acquisition of dissolution profiles: UV-spectrophotometry and HPLC–PDA focusing on the greenness of each one for further consolidation of the biowaiver concept. Both techniques were applied on the recently FDA-approved combination naproxen sodium (NAPR) and diphenhydramine hydrochloride (DIPH) formulated as Aleve pm® tablets. For the first time, this binary mixture was analyzed by three UV-spectrophotometric methods. NAPR was directly determined by zero-order spectrophotometry at 330 nm, where the spectrum of DIPH shows zero contribution. Whereas DIPH was determined by three simple methods exploiting the ratio spectra calculated using the spectrum of 20 µg/ml NAPR as a divisor. The first proposed method was ratio difference (RD), the second was ratio subtraction, and the last one was derivative ratio method. Being the simplest method, RD was the spectrophotometric method of choice that was applied in monitoring the dissolution of DIPH from Aleve pm® tablets. Although RD method has been widely described in many publications dealing with pharmaceutical analysis, it is the first article that opens a new horizon for RD method in the way of being a real-life application rather than only a method for determination. The second technique was a previously published HPLC–PDA, in which dissolution was then monitored by calculating the peak area of each component drug over time. Methods’ validation was performed agreeing with the ICH guidelines. The advantages and challenges of each technique are discussed in a side-by-side comparison giving a key to recognize which one can positively influence environmental well-being.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The international regulatory authorities such as the United States Pharmacopeia (USP), Food and Drug Administration (FDA), and International Conference on Harmonisation (ICH) stated that drug absorption from a solid oral dosage form depends on its release from the drug product (drug dissolution) (ICH 2010; USP 2011; Administration 2015). In vitro dissolution testing has been utilized as a predictor of drug’s in vivo performance in the way that limits the need to costly and time-consuming human studies, giving a key to the biowaiver notion (Anand et al. 2011; Administration 2015; Korany et al. 2018a). Based on this general consideration, in vitro dissolution studies for solid oral dosage forms, such as tablets and capsules, are performed routinely in quality control laboratories to judge the quality of a drug product, also to evaluate the newly developed pharmaceutical formulations (Administration 2015). Recently, the term “green analytical chemistry” (GAC) was raised and gained a growing acceptance originated from the continuous worldwide strive towards a safe and healthy environment (Guardia and Garrigues 2012). The well-known GAC principles were introduced by Anastas and Warner in 1998. They presented a guiding framework applying to all disciplines of chemistry nevertheless analytical chemistry to reach the compromise between increasing quality of the results and raising environmental friendliness (Anastas and Eghbali 2010). In general, GAC prefers instrumental methods; however, they need to be ameliorated via reducing the amounts of reagents consumed and waste generated for increasing operator safety (Gałuszka et al. 2013; Korany et al. 2017a, b, 2018b). Therefore, many pharmaceutical scientists have directed their efforts on the way of greening most of analytical practices particularly those having a routine manner, consequently greening-up the dissolution process would be highly desirable.
USP stated that the standard methods for determining the extent of drug release from tablets or capsules are UV-spectroscopy and high-performance liquid chromatography (HPLC), in which dissolution process is monitored via sampling at definite time intervals (every 5 min for 30 or 45 min) followed by analysis of the active principle (2011). In the present research, the greenness of each technique was investigated using a mixture of NAPR and DIPH present as OTC-tablets Aleve pm®. Naproxen is a significant member of the class of non-steroidal anti-inflammatory drugs, it is used to treat pain and minor body aches (Uysal and Tunçel 2004). Diphenhydramine belongs to first-generation antihistaminic drugs; it is mainly used as a night-time sleep aid due to its sedating effect (Ge et al. 2011). Reviewing the literature carefully revealed that one spectrophotometric method has been reported for the analysis of this mixture yet although it is a widely used OTC drug (Mabrouk et al. 2015). However, other methods such as electrochemical (Abd El-Rahman et al. 2017; Shehata et al. 2017), high-performance thin-layer chromatographic (HPTLC) (Bhole et al. 2015), and HPLC/PDA (Nalluri et al. 2015) have been recently reported for simultaneous determination of both drugs in their binary formulation.
In this work and for the first time, three spectrophotometric methods were developed on this mixture based on ratio spectra manipulation; they are RD, RS, and DD1. Upon application of the proposed methods, RD was thought to be the most simple and reliable method. Plenty of publications have exploited the recently developed RD method in drug assay purposes (Lotfy and Abdel-Monem Hagazy 2012; Darwish et al. 2013; Saad 2015; Abdel-Halim et al. 2016; Abd El-Rahman et al. 2017; Ragab et al. 2018), yet our goal was to expand it beyond determination towards a real application. Thus, RD method was utilized in further dissolution investigations in parallel to a reported HPLC method (Shehata et al. 2017). This study enlightens the opportunities and limitations of the two different methodologies for both determination and dissolution tracking of NAPR and DIPH. The novelty of the current study arises from performing a side-by-side comparison with the aim of evaluating the sustainability of the analytical technique.
Experimental
Instrument
UV–visible spectrophotometer was SHIMADZU dual beam (Kyoto, Japan), model UV-1650 PC. The utilized software was UV-Probe personal spectroscopy software version 2.21 (SHIMADZU). The spectral band was 2 nm and scanning speed was 2800 nm/min with 0.1 nm interval.
Drugs’ dissolution was tested using a VanKel (VK 7000 USA) equipped with standard USP type-I basket.
Samples
Reference samples
Diphenhydramine hydrochloride and naproxen sodium pure standards were purchased from Bayer Co.; the purity of each was certified to be more than 99.99%.
Pharmaceutical formulation
Aleve pm® tablets were formulated by Bayer Pharmaceutical Company, Germany. Batch No. NAA5FPO. Each tablet is labeled to contain 220 mg and 25 mg of NAPR and DIPH, respectively.
Solutions
Naproxen sodium and diphenhydramine hydrochloride stock standard solutions with a concentration of 500 and 100 µg/ml, in distilled water, were prepared in two separate 100-ml volumetric flasks by dissolving 0.05 and 0.01 g of pure NAPR and DIPH, respectively.
Procedure
UV-spectrophotometric methods’ development and calibration
Aliquots (0.4–5.2 ml) of NAPR and (0.4–4 ml) of DIPH standard solutions (500 and 100 μg/ml, respectively), were accurately transferred into two separate series of 10-ml volumetric flasks then completed to volume with distilled water. The prepared series of standard solutions were scanned from 200 to 400 nm and the zero-order (D0) absorption spectrum of each solution was recorded using distilled water as a blank.
NAPR was determined directly by plotting its absorbance in the zero-order spectra (D0) at λmax = 330 nm (where DIPH has no contribution) against its corresponding concentrations then the regression equation was computed. However, for quantitative measurement of DIPH in presence of NAPR, zero-order absorption spectra of DIPH were divided by the spectrum of 20 μg/ml NAPR, subsequently three different spectrophotometric methods were applied on the recorded DIPH ratio spectra.
Ratio difference (RD) spectrophotometric method
The difference in peak amplitudes of the ratio spectra (DIPH/NAPR) at 246 and 256 nm (ΔP256–246) was plotted against the corresponding concentrations of DIPH (μg/ml) then the regression equation was computed.
Ratio subtraction (RS) spectrophotometric method
From the previously obtained ratio spectra, the absorbance of NAPR in the extended region (a constant value) at 275–350 nm was subtracted. The obtained spectra were then multiplied by the spectrum of the divisor (20 µg/ml NAPR). The re-produced spectra (the same as DIPH zero-order spectra) can be directly used for estimation of DIPH at its λmax = 220 nm. Calibration was obtained by plotting the absorbance values versus the corresponding concentrations then the regression equation was computed.
Derivative ratio (DD1) spectrophotometric method
The first derivative of the previously stored ratio spectra was obtained with Δλ of 8 nm and a scaling factor equals to 100. The peak amplitudes of the first derivative of the ratio spectra were measured at 228 and 268 nm. Calibration graphs relating the peak amplitudes of the DD1 curves at 228 and 268 nm to the corresponding concentrations of DIPH were constructed, and the corresponding regression equations were computed.
To prepare laboratory-prepared mixtures containing different ratios of NAPR and DIPH, aliquots from their respective standard solutions (500 and 100 μg/ml) were transferred accurately into a series of 10-ml volumetric flasks and completed to the mark with distilled water. NAPR was determined directly at zero-order by measuring the absorbance of each laboratory-prepared mixture at 330 nm, and then the concentrations of the drug were computed from its corresponding regression equation. For determination of DIPH, zero-order absorption spectrum of each laboratory-prepared mixture was divided by the spectrum of 20 μg/ml of NAPR and then the three proposed spectrophotometric methods were applied on the recorded ratio spectra. The concentration of DIPH in each mixture was obtained from the corresponding regression equation using the same procedure mentioned in the previous section (UV-spectrophotometric methods’ development and calibration).
Assay of NAPR and DIPH content in Aleve pm® tablets
Ten Aleve pm® tablets were separately weighed, powdered, and well mixed. An amount of powder equivalent to one tablet was accurately weighed and transferred into a 100-ml beaker, sonicated for 15 min in 50 ml distilled water. The solution was filtered into 100-ml volumetric flask, and the residue was washed two times each using 5 ml distilled water and the volume was completed to the mark with the same solvent. Afterwards; 1 ml of the prepared solution was transferred into a 10-ml volumetric flask, completed to the mark with water, and analyzed using the proposed spectrophotometric methods. The concentration of each drug was calculated from the corresponding regression equation. The validity of the adopted methods was assessed by applying standard addition technique by spiking the pharmaceutical formulation with known amounts of standard drug powders. The recoveries of the added standards were then calculated after applying the proposed methods.
Dissolution testing
A six-vessel type-I dissolution apparatus containing 900 ml of distilled water, thermostatically controlled at 37 ± 0.5 °C was used, the tablet was introduced into the basket, and the vessel content was agitated using a rate of 75 rpm.
Samples were withdrawn from the dissolution medium at 5-min time intervals, filtered, and the volume was completed with distilled water. NAPR’s concentration was calculated directly by measuring absorbance values of the zero-order spectrum at 330 nm, while RD method was applied to get the concentration of DIPH. Percentage dissolution was then calculated for both drugs, and dissolution curves were plotted.
Results and discussion
Dissolution monitoring is a mandatory routine step for all solid oral dosage forms to ascertain that the active drug(s) is released at the desired rate to exert the required therapeutic effect (Costa and Lobo 2001). Moreover; dissolution testing has been extensively discussed by pharmaceutical scientists as a surrogate for in vivo bioequivalence studies which undoubtedly affect human’s safety and health (Maggio et al. 2013). In this context, dissolution testing has a crucial role in regulatory decision-making and it may be used to waive in vivo bioequivalence study requirements, serving the purpose of biowaiver concept (Karam 2015). Officially stated methods for dissolution monitoring depend mainly on one of the well-established analytical techniques: UV-spectrophotometry and HPLC (2011). Recently, the analytical society has paid considerable attention to the usage of ‘‘Sustainable Analytical Procedures’’ that meet the requirements of GAC (Welch et al. 2010; Gałuszka et al. 2013; Abd El-Rahman et al. 2016). Since the two methodologies are feasible in most of the quality control laboratories worldwide, this research addresses their implementation in the analysis and dissolution testing of Aleve pm® tablets. Additionally, a fair comparison was held between both techniques to get to the greenly recommended one that adheres more closely to the GAC principles.
UV-spectrophotometric methods development and optimization
The zero-order absorption spectra of NAPR and DIPH shows overlapping that hinders direct determination of DIPH; however, NAPR spectrum is more extended in the way that allows its direct determination in presence of DIPH at λmax = 330 nm, where DIPH shows no interference (Fig. 1). Accordingly, three spectrophotometric methods manipulating ratio spectra are adopted in this work for the simultaneous estimation of DIPH in presence of NAPR in their laboratory-prepared mixtures and pharmaceutical formulation. For estimation of NAPR, a linear relationship was plotted between the absorbance at 330 nm and the corresponding concentrations, linearity, regression, and validation parameters are illustrated in Table 1.
Ratio difference (RD) spectrophotometric method
Ratio difference method was newly proposed as a simple method for determination of mixture compounds with overlapping spectra, having the advantage of slight mathematical manipulation of spectral data (Elzanfaly et al. 2012; Eissa 2017). The method includes dividing the spectrum of the analyte by a divisor concentration of the interfering substance, a ratio spectrum will be obtained, and a linear relationship between the difference in amplitudes at any two wavelengths and the corresponding concentrations of the analyte will result. Meanwhile, the ratio spectrum of the interferent will have a constant amplitude parallel to the x-axis, so at any two wavelengths it shows zero difference in amplitudes (Riad et al. 2015) (Fig. 2).
The method includes two critical steps: the first is the selection of the divisor concentration as it should provide minimal noise and maximum sensitivity. Different divisor concentrations of NAPR were tried and 20 µg/ml showed the best results. The second step is the choice of the wavelength pair at which measurements would be recorded. Any two values of wavelength with different amplitudes in the ratio spectrum can be used provided that good linearity is present at each of them. Many wavelength pairs were tried and 246–256 nm showed the best results. Linear correlation was obtained between the differences in peak amplitudes at 246–256 nm and the corresponding DIPH concentrations.
Ratio subtraction (RS) spectrophotometric method
Ratio subtraction method is a successfully applied spectrophotometric method for resolving binary mixtures provided that the spectrum of one component is more extended than the other (Darwish et al. 2011). The method was used for the determination of DIPH in its binary mixture with NAPR that have a more extended spectrum. In this method, the ratio spectra of the mixture can be expressed as DIPH/NAPR + a constant (NAPR/NAPR′). Subtraction of the values of these constants in the extended region (275–350 nm) is followed by multiplication of the obtained spectra by the divisor NAPR’. Finally, the original spectra of DIPH could be obtained as shown in Fig. 3, and DIPH can be directly measured at its λmax = 220 nm. The obtained absorbance values were plotted against the corresponding DIPH concentrations then the regression parameters were computed.
a Zero-order absorption spectra of different NAPR and DIPH lab-mixtures (prepared as mentioned in Table 3). b Ratio spectra of the lab-mixtures using 20 µg/ml NAPR as a divisor. c Ratio spectra of the lab-mixtures after subtraction of the constant. d Re-obtained DIPH spectra after multiplication by the divisor (20 µg/ml NAPR)
Derivative ratio (DD1) method
The method was designed by Salinas (1990), based on derivatization of the ratio spectra for resolving binary or ternary mixtures. The main advantage of this method is that the whole spectrum of interfering substance is canceled, so any wavelength can be for calibration; thus, easy measurements can be done at the wavelength of highest sensitivity either a maximum or a minimum (Abd El-Rahman et al. 2015).
Different ∆λ and scaling factors were tested, where ∆λ = 8 and a scaling factor = 100 were suitable to enlarge the signals of DIPH and to diminish error in readings (Fig. 4). The linearity of the peak amplitudes of the DD1 curve at both 228 and 268 nm was studied. Linear relationships were obtained for DIPH, and the regression parameters were computed.
Validation of the three proposed spectrophotometric methods has been performed according to ICH guidelines (Conference et al. 2005), and the calculated linearity and validation parameters are summarized in Table 1.
Determination of laboratory-prepared mixtures containing different ratios of NAPR and DIPH
The results obtained upon analysis of the laboratory-prepared mixtures containing different ratios of NAPR and DIPH revealed that the proposed spectrophotometric methods can be successfully used for selective determination of NAPR and DIPH in presence of each other (Table 2).
Determination of NAPR and DIPH in Aleve pm® tablets
The proposed spectrophotometric methods were successfully applied for simultaneous determination of NAPR and DIPH contents in their combined dosage form (Aleve pm® tablets), and standard addition technique was performed (Table 3). To examine the validity of the proposed methods, the obtained results were statistically compared to those of the reported HPLC method, and no significant differences were obtained (Table 4).
Dissolution monitoring
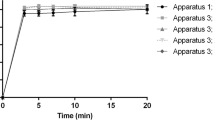
Monitoring of the dissolution of NAPR and DIPH was performed using RD-spectrophotometric method and compared to the reported HPLC method. Typically, dissolution curve is a plotted between the dissolved percentage of the drug and time (Kassem et al. 2014). Among the three proposed spectrophotometric methods, RD was preferably chosen over RS and DD1 methods for further dissolution investigations because it exhibited simpler data manipulation steps and reliable results. To the best of our knowledge; RD method has been reported in many publications, yet this is the first article applying RD method to dissolution monitoring process. Samples were withdrawn from the dissolution medium at 5-min intervals, purified, then the concentrations of NAPR and DIPH by RD and HPLC methods were converted into %dissolution and plotted against time in minutes (Fig. 5). Dissolution profiles by the two methods revealed that NAPR took 35 min while DIPH took 40 min to dissolve completely, that complied successfully with the acceptance criterion stated by the USP which specified that the dissolution curves of immediate-release products typically reach 85–100% at about 30–45 min (2011). According to the Biopharmaceutics Classification System (BCS), NAPR and DIPH could be categorized as BCS class I drugs as they have high solubility and high permeability (Karam 2015). Thus, both drugs are eligible for the biowaiver procedure in which in vitro dissolution testing can be considered as an alternative to in vivo bioequivalence studies eliminating the need to harmful and costly procedure.
Although RD spectrophotometry and HPLC are in good agreements in accurate prediction of the dissolution profiles, the two analytical methodologies differ in number of features particularly from a green analytical point of view. HPLC is the most applicable and versatile analytical technique in dissolution studies. Besides being a separation-based technique, photodiode array detection permits both qualitative and quantitative drug analysis. PDA’s major advantage is related to the prediction of peak purity as the peak shape cannot assure that it corresponds to one or two (or even more) components. In such a case, absorbance at more than a single wavelength is very helpful in deciding whether the peak represents a single compound or not. However, in a case of Aleve pm® tablets, RD method excels as a greener alternative to HPLC. The proposed RD method depends on distilled water as solvent which is considered as green alternative for the organic solvents, since water is environmentally acceptable and recyclable. On the other side, HPLC utilized acetonitrile which is a costly solvent particularly after its prices have been skyrocketed after the so-called “Great acetonitrile crisis” provoked in late 2008. Moreover, acetonitrile is recently considered as a must-replaced solvent due to the debates which rose about its negative environmental impact. Also, the amount of generated waste is decreased dramatically in RD, compared to HPLC which consumes a lot of expensive pure solvents in column washing and conditioning steps. RD spectrophotometry can be considered a “greener” analytical protocol as it exhibits increasing safety of the operator through using environmentally friendly solvents, reduction of the analytical waste, in addition to lower energy consumption and reduced analysis time. To this end, RD spectrophotometry was found to be more closely adhering to the GAC principles, so it is strongly recommended for a greener and environmentally safer dissolution biowaiver studies.
Conclusion
Two different analytical methodologies, UV-spectrophotometry and HPLC, were developed for the simultaneous determination of NAPR and DIPH in their combined dosage form Aleve pm® tablets. The suggested spectrophotometric methods were RD, RS, and DD1 that were used successfully for resolving the spectral overlap between NAPR and DIPH without prior separation based on ratio spectra manipulation. All the developed spectrophotometric and HPLC methods can be used for the determination of NAPR and DIPH in their pure bulk powder and in their combined dosage form with acceptable accuracy and sensitivity. Furthermore, RD method and HPLC were applied successfully to study the dissolution of both drugs from their tablet dosage form; then, the two techniques were compared from a green chemistry perspective. The proposed RD method provided a greener methodology as it utilized safer solvents, less energy consumption, less generated waste, reduced analysis time, and enhanced operator safety.
References
Abd El-Rahman MK, Riad SM, Abdel Gawad SA et al (2015) Stability indicating spectrophotometric and spectrodensitometric methods for the determination of diatrizoate sodium in presence of its degradation product. Spectrochim Acta Part A Mol Biomol Spectrosc. https://doi.org/10.1016/j.saa.2014.10.002
Abd El-Rahman MK, Zaazaa HE, Eldin NB, Moustafa AA (2016) Just-dip-it (potentiometric ion-selective electrode): an innovative way of greening analytical chemistry. ACS Sustain Chem Eng 4:3122–3132. https://doi.org/10.1021/acssuschemeng.6b00138
Abd El-Rahman MK, Riad SM, Fawaz EM, Shehata MA (2017) Three different spectrophotometric methods exploiting ratio spectra for the selective determination of iohexol in presence of its acidic degradate. Curr Pharm Anal. https://doi.org/10.2174/1573412913666171016163544
Abdel-Halim LM, Abd-El Rahman MK, Ramadan NK et al (2016) Comparative study between recent methods manipulating ratio spectra and classical methods based on two-wavelength selection for the determination of binary mixture of antazoline hydrochloride and tetryzoline hydrochloride. Spectrochim Acta Part A Mol Biomol Spectrosc 159:98–105. https://doi.org/10.1016/J.SAA.2016.01.014
Administration F and D (2015) Paving the way for personalized medicine: FDA’s role in a new era of medical product development. US Dep Health, Washington DC
Anand O, Yu LX, Conner DP, Davit BM (2011) Dissolution testing for generic drugs: an FDA perspective. AAPS J 13:328–335. https://doi.org/10.1208/s12248-011-9272-y
Anastas P, Eghbali N (2010) Green chemistry: principles and practice. Chem Soc Rev 39:301–312. https://doi.org/10.1039/B918763B
Anastas PT, Warner JC (1998) Green chemistry. Frontiers
Bhole RP, Shinde SS, Chitlange SS, Wankhede SB (2015) A high-performance thin layer chromatography (HPTLC) method for simultaneous determination of diphenhydramine hydrochloride and naproxen sodium in tablets. Anal Chem Insights 10:47
Conference I, Harmonisation ON, Technical OF et al (2005) ICH harmonised tripartite guideline validation of analytical procedures
Costa P, Lobo J (2001) Modeling and comparison of dissolution profiles. Eur J Pharm Sci 13:123–133
Darwish HW, Hassan SA, Salem MY, El-Zeiny BA (2011) Three different spectrophotometric methods manipulating ratio spectra for determination of binary mixture of Amlodipine and Atorvastatin. Spectrochim Acta Part A Mol Biomol Spectrosc 83:140–148. https://doi.org/10.1016/j.saa.2011.08.005
Darwish HW, Hassan SA, Salem MY, El-Zeany BA (2013) Three different methods for determination of binary mixture of Amlodipine and Atorvastatin using dual wavelength spectrophotometry. Spectrochim Acta Part A Mol Biomol Spectrosc 104:70–76. https://doi.org/10.1016/J.SAA.2012.11.079
Eissa MS (2017) Simultaneous determination of the brand new two-drug combination for the treatment of hepatitis C: sofosbuvir/ledipasvir using smart spectrophotometric methods manipulating ratio spectra. Spectrochim Acta Part A Mol Biomol Spectrosc 183:362–370. https://doi.org/10.1016/j.saa.2017.04.052
El-Rahman MKA, Al-Alamein AMA, Abdel-Moety EM, Fawaz EM (2017) Integrated gold-thiol based potentiometric sensors for in situ dual drug-protein binding studies on naproxen/diphenhydramine salts model. J Electrochem Soc 164:H1013–H1020. https://doi.org/10.1149/2.0531714jes
Elzanfaly ES, Saad AS, Abd-Elaleem A-EB (2012) Simultaneous determination of retinoic acid and hydroquinone in skin ointment using spectrophotometric technique (ratio difference method). Saudi Pharm J 20:249–253. https://doi.org/10.1016/j.jsps.2012.03.004
Gałuszka A, Migaszewski Z, Namieśnik J (2013) The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices. TrAC Trends Anal Chem 50:78–84. https://doi.org/10.1016/j.trac.2013.04.010
Ge Z, Luo Y, Zhao X et al (2011) Simultaneous determination of ibuprofen and diphenhydramine HCl in orally disintegrating tablets and its dissolution by reversed-phase high performance liquid chromatography (RP-HPLC). Afr J Pharm Pharmacol 5:2100–2105
Guardia M, Garrigues S (2012) Handbook of green analytical chemistry. Wiley, New York
ICH (2010) ICH harmonised tripartite guideline evaluation and recommendation of pharmacopoeial texts for use in the ICH regions on dissolution test general chapter Q4B annex 7(R2)
Karam R (2015) Biowaivers: criteria and requirements prepared by Dr. Mazen Kurdi, pp 1–11
Kassem MAA, Elmeshad AN, Fares AR (2014) Enhanced bioavailability of buspirone hydrochloride via cup and core buccal tablets: formulation and in vitro/in vivo evaluation. Int J Pharm 463:68–80. https://doi.org/10.1016/j.ijpharm.2014.01.003
Korany MA, Mahgoub H, Haggag RS et al (2017a) Green chemistry: analytical and chromatography. J Liq Chromatogr Relat Technol 40:839–852. https://doi.org/10.1080/10826076.2017.1373672
Korany MA, Mahgoub H, Haggag RS et al (2017b) Development of a green stability-indicating HPLC–DAD method for the determination of donepezil hydrochloride in the presence of its related substance and degradation products. J Liq Chromatogr Relat Technol 40:930–942. https://doi.org/10.1080/10826076.2017.1386672
Korany MA, Mahgoub H, Haggag RS et al (2018a) Chemometrics-assisted spectrophotometric green method for correcting interferences in biowaiver studies: application to assay and dissolution profiling study of donepezil hydrochloride tablets. Spectrochim Acta Part A Mol Biomol Spectrosc 199:328–339. https://doi.org/10.1016/J.SAA.2018.03.059
Korany MA, Mahgoub H, Haggag RS et al (2018b) Green gas chromatographic stability-indicating method for the determination of Lacosamide in tablets. Application to in vivo human urine profiling. J Chromatogr B 1083:75–85. https://doi.org/10.1016/J.JCHROMB.2018.02.033
Lotfy HM, Abdel-Monem Hagazy M (2012) Comparative study of novel spectrophotometric methods manipulating ratio spectra: an application on pharmaceutical ternary mixture of omeprazole, tinidazole and clarithromycin. Spectrochim Acta Part A Mol Biomol Spectrosc 96:259–270. https://doi.org/10.1016/J.SAA.2012.04.095
Mabrouk MM, Hammad SF, Mansour FR, El-Khateeb BZ (2015) Simultaneous determination of naproxen and diphenhydramine by reversed phase liquid chromatography and derivative spectrophotometry. Der Pharma Chem 7(12):181–191
Maggio RM, Rivero MA, Kaufman TS (2013) Simultaneous acquisition of the dissolution curves of two active ingredients in a binary pharmaceutical association, employing an on-line circulation system and chemometrics-assistance. J Pharm Biomed Anal 72:51–58. https://doi.org/10.1016/j.jpba.2012.09.022
Nalluri BN, Mrudula B, Chitralatha K et al (2015) Development of stability indicating RP-HPLC-PDA method for the simultaneous analysis of naproxen sodium and diphenhydramine hydrochloride in bulk and tablet dosage forms. Indian Drugs 52:9
Ragab MAA, El Yazbi FA, Hassan EM et al (2018) Spectrophotometric analysis of two eye preparations, vial and drops, containing ketorolac tromethamine and phenylephrine hydrochloride binary mixture and their ternary mixture with chlorpheniramine maleate. Bull Fac Pharm Cairo Univ 56:91–100. https://doi.org/10.1016/j.bfopcu.2018.03.004
Riad SM, El-Rahman MKA, Fawaz EM, Shehata MA (2015) A comparative study between three stability indicating spectrophotometric methods for the determination of diatrizoate sodium in presence of its cytotoxic degradation product based on two-wavelength selection. Spectrochim Acta Part A Mol Biomol Spectrosc 145:254–259. https://doi.org/10.1016/j.saa.2015.03.030
Saad AS (2015) Novel spectrophotometric method for selective determination of compounds in ternary mixtures (dual wavelength in ratio spectra). Spectrochim Acta Part A Mol Biomol Spectrosc 147:257–261. https://doi.org/10.1016/J.SAA.2015.03.095
Salinas F (1990) A new spectrophotometric method for quantitative multicomponent analysis resolution of mixtures of salicylic and salicyluric acids. Talanta 37:347–351. https://doi.org/10.1016/0039-9140(90)80065-N
Shehata MA, Fawaz EM, El-Rahman MKA, Abdel-Moety EM (2017) Double-track electrochemical green approach for simultaneous dissolution profiling of naproxen sodium and diphenhydramine hydrochloride. J Pharm Biomed Anal. https://doi.org/10.1016/j.jpba.2017.08.041
USP (2011) The United States Pharmacopeia and National Formulary, the Official Compendia of Standards, 4th edn. The United States Pharmacopeial Convention Inc., Rockville, p 2531
Uysal Ü, Tunçel M (2004) Determination of naproxen in tablets by using first derivative potentiometry. Prostaglandins 1:217–223
Welch CJ, Wu N, Biba M et al (2010) Greening analytical chromatography. TrAC Trends Anal Chem 29:667–680
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abou Al-Alamein, A.M., Abd El-Rahman, M.K., Fawaz, E.M. et al. UV-spectrophotometry versus HPLC–PDA for dual-drug dissolution profiling: which technique provides a closer step towards green biowaiver concept? Novel application on the recent FDA-approved mixture Aleve pm. Chem. Pap. 73, 309–319 (2019). https://doi.org/10.1007/s11696-018-0580-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-018-0580-x