Abstract
Bi2O3/BiFeO3 composite was successfully fabricated by a conventional sol–gel method and structural properties were characterized based on X-ray diffractometer, scanning electron microscope, transmission electron microscope, energy-dispersive X-ray analyzer, nitrogen adsorption–desorption measurement, and UV–visible diffuse reflectance spectroscopy. Bi2O3/BiFeO3 had a good absorption for visible light, which was benefit to photocatalytic activity. The highest degradation efficiency was obtained when the content of Bi2O3 in Bi2O3/BiFeO3 was 63.9%. Effect of experimental conditions was investigated, and the highest photocatalytic activity of Bi2O3/BiFeO3 was observed at photocatalyst dosage of 0.5 g/L, initial BPA concentration of 10 mg/L, and solution pH of 6.3. Bi2O3/BiFeO3 photocatalyst exhibited enhanced photocatalytic activity for BPA, and the reaction rate constant over Bi2O3/BiFeO3 composite was 2.23, 3.65, and 8.71 times higher than that of BiFeO3, Bi2O3 and commercial TiO2 (P25), respectively. Bi2O3/BiFeO3 showed high photocatalytic activity after three cycles, suggesting that it was a stable photocatalyst. The possible photocatalytic mechanism has been discussed on the basis of the theoretical calculation and the experimental results. The hydroxyl and superoxide radicals together with photogenerated holes played significant roles in the photocatalytic reaction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Owing to its extensive usage in industry (Chen et al. 2016), bisphenol A (BPA) has been widely distributed in the environment (Falconer et al. 2006; Vom Saal and Welshons. 2014; Manfo et al. 2014). It is a kind of endocrine disrupting compounds (EDCs), which can cause adverse effects on humans and animals through the interactions with the endocrine system (Paulose et al. 2015; Jandegian et al. 2015; Li et al. 2010). BPA is stable in environment, hardly degraded and tends to bio-accumulate, which makes it very urgent and important for us to develop a sustainable, effective, and economical method to remove BPA in water.
Various methods have been developed to remove BPA from water, such as biological method (Takamiya et al. 2008), chemical oxidation method (Keykavoos et al. 2013), adsorption method (Son and Takaomi 2011), and photocatalysis method (Chen et al. 2015). Among them, the photocatalysis method is the most promising technology for the degradation of BPA because of its high degradation and mineralization efficiency, low cost, low toxicity, and its operating ability under ambient conditions. TiO2 is a typical photocatalyst for its good chemical stability, non-toxicity, low cost, and significant photocatalytic activity (Kumar and Rao. 2017; Lin et al. 2012; Sun et al. 2016). However, pure TiO2 absorbs only ultraviolet light which accounts for only 5% of sunlight. The rapid recombination of photogenerated electron–hole pairs limits the practical application of TiO2 (Yap et al. 2010). Therefore, the study has been a hot topic for developing visible-light-driven photocatalysts. Recently, bismuth-based photocatalysts have attracted much interest because of their narrow bandgaps such as Bi2O3 (Yan et al. 2014a, b), Bi2S3 (Gao et al. 2015), Bi2WO6 (Kumar and Rao. 2015; Liu et al. 2015), and BiVO4 (Li et al. 2015).
Bi2O3 is an important inorganic functional material (Yan et al. 2014a, b). It has got much attention due to its unique optical and electrical properties, which lead to its extensive usage in catalysis, optical coatings, microelectronics, solid fuel cells, gas sensors and glass manufacturing, etc. In addition, it is a good photocatalyst with a narrow bandgap ranging from 2.0 to 2.9 eV. However, there are still some problems for its practical application. The rapid recombination of photogenerated electron–hole pairs leads to the relatively low photocatalytic activity. BiFeO3 is also a promising photocatalyst for its high chemical stability, narrow bandgap (2.0–2.8 eV), and special ferroelectric and ferromagnetic properties (Humayun et al. 2016; Gao et al. 2016). However, the photocatalytic activity of pure BiFeO3 is not satisfying because of its low conduction band position and small surface areas (Lam et al. 2017). It is expected that the combination of Bi2O3 with BiFeO3 would inhibit the recombination of photogenerated electron–hole pairs and enhance the photocatalytic activity.
Recently, sol–gel method was used in the synthesis of BiFeO3, which has great advantages on the purity of BiFeO3 and the nanoparticle morphology (Majid et al. 2015; Kim et al. 2005). In this work, the Bi2O3/BiFeO3 composite photocatalysts were prepared by a novel sol–gel method which is rarely investigated in the literature. The Bi2O3/BiFeO3 composite was fabricated in a single step and the content of Bi2O3 in the heterostructure was controlled by varying the initial molar ratio of Bi/Fe in the synthesis step. The composite photocatalyst was characterized and its photocatalytic activity for BPA was also investigated. Meanwhile, the effects of experimental conditions on the BPA degradation efficiency were discussed. A possible photocatalytic mechanism was tentatively proposed on the basis of the theoretical calculation and the experimental results.
Experimental
Preparation of the photocatalyst
All chemicals were of analytical purity and were used as received without further purification. Ultrapure water was used throughout this study. In a typical approach to obtain the composite, 7.28 g Bi(NO3)3·5H2O and 2.02 g Fe(NO3)3·9H2O (molar ratio of Fe/Bi = 1:3) were dissolved in 36 mL ethylene glycol in a 100 mL glass beaker under magnetic stirring, and 5 mL glacial acetic acid was added. This mixture was then heated at 80 °C for 6 h in SZCL-2 magnetic stirrer (Yuhua, China), resulting in a dark yellow sol. The sol was heated at 100 °C for 24 h in a watch-glass to get the yellow gel, and was then calcinated in a muffle furnace at 500 °C (ramping rate of furnace was 2 °C/min) for 2 h to produce Bi2O3/BiFeO3 composite. For comparison, pure Bi2O3 was obtained without adding Fe(NO3)3·9H2O, and pure BiFeO3 was obtained with an initial Fe/Bi molar ratio of 1:1 by sol–gel method.
Characterization
The crystal phases of the sample powders were characterized by TTRIII X-ray diffractometer (XRD, Rigaku, Japan) with Cu Kα radiation in the 2θ range from 10° to 80°. The surface morphology was observed on FEI QUANTA200 scanning electron microscope (SEM, USA) and JEM-2100 transmission electron microscope (TEM, JEOL, Japan). The elemental composition was analyzed using an energy-dispersive X-ray (EDAX) analyzer attached to the TEM. Nitrogen (N2) adsorption–desorption measurement at 77 K was conducted using TriStar II 3020 (Micromeritics Inc., USA). The surface area of samples was obtained using the Brunauer–Emmett–Teller (BET) plot of N2 adsorption isotherm. The ultraviolet–visible diffuse reflectance spectra (UV–Vis DRS) were recorded at room temperature on a UV-240IPC (JEOL, Japan) spectrophotometer using BaSO4 as a reference.
Photocatalytic activity
Photocatalytic activity of the prepared photocatalysts was investigated through degradation of BPA. The photocatalysis was conducted in an XPA-7 photochemical reactor (Xujiang Electromechanical Plant, Nanjing, China). A 500 W Xe lamp and a filter (λ > 420 nm, Xujiang Electromechanical Plant, Nanjing, China) were used to get visible light. In each test, the photocatalyst was added into a quartz tube with 10.0 mL of BPA solution. Before the photocatalytic degradation experiment, the catalyst and BPA solution were mixed and stirred for 30 min in dark to reach adsorption equilibrium. Then, under the visible-light irradiation, the quartz tube was taken out at given time intervals.
BPA concentration was detected by high-performance liquid chromatography (HPLC, Agilent Technologies 1200 series) at the detection wavelength of 226.16 nm. A Symmetry C18 column (5 μm, 4.6 × 250 mm) was used as separation column. The mobile phase used for HPLC experiments was a mixture of acetonitrile and water (65/35, v/v). The flow rate was set as 1.0 mL/min, the column temperature was 35 °C, and the injection volume was 20 μL.
The degradation efficiency of BPA was calculated by the following equation:
C0 and C t represent the initial concentration of BPA before irradiation and the concentration of BPA remaining in the solution at irradiation time of t, respectively.
A pseudo-first-order kinetic model was used to evaluate the photodegradation efficiency and the degradation kinetics constant k was obtained as
Results and discussion
Characterization
XRD analysis
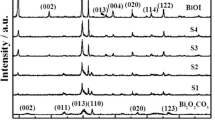
The chemical composition and phase structure of the fabricated samples were confirmed with powder X-ray diffraction (XRD). As shown in Fig. 1, all diffraction peaks of the samples could be unambiguously assigned to rhombohedral phase BiFeO3 (JCPDS No. 71-2494) (Fan et al. 2015) and monoclinic Bi2O3 (JCPDS No. 41-1449) (Cheng and Kang 2014), respectively. The sharp diffraction peaks indicated that the samples were well crystallized. No impurity peaks were observed, indicating a high purity of the products. The Bi2O3/BiFeO3 composite exhibited a co-existence of both Bi2O3 and BiFeO3 phases without significant change in the peak positions, indicating that the combination of the two materials did not change the crystal structure of Bi2O3 and BiFeO3.
SEM analysis
The morphologies and surface structures of fabricated samples were studied by SEM. Figure 2a displays the morphology of pure BiFeO3. Pure BiFeO3 was composed of spherical nanoparticles. The particle size was relatively uniform, and some particles were contacted with each other. The morphology of pure Bi2O3 is shown in Fig. 2b. Pure Bi2O3 displayed mainly nanosheet shape with a thickness in 40–65 nm. From Fig. 2c, it was clearly seen that Bi2O3 nanosheets were surrounded by lots of BiFeO3 nanoparticles, indicating that the two materials were well integrated at the structural level.
TEM and EDS analysis
The morphologies of photocatalysts were further studied by TEM. The TEM image shown in Fig. 4a confirmed that the structure of individual BiFeO3 was nanoparticles. The diameter of the spherical particles was 50–100 nm. Figure 4b shows that the structure of individual Bi2O3 was nanosheets. Figure 4c, d shows that the Bi2O3/BiFeO3 composite was formed from the combination of Bi2O3 nanosheets with BiFeO3 nanoparticles. Moreover, the element composition of the fabricated Bi2O3/BiFeO3 composite was determined by energy-dispersive X-ray spectroscopy (EDS). Figure 3e, f shows taken from the corresponding EDS spectrum of rectangular region A from a nanosheet and region B from a nanoparticle in Fig. 3c, respectively. The Cu-element interference peaks shown in Fig. 3e, f were from the copper mesh which was used as the base. The spectrum showed characteristic peaks of Bi and O in Fig. 3e, while the peaks corresponded to Bi, O, and Fe elements can be detected in Fig. 3f. The peaks further indicated that the nanosheets were consisted of Bi2O3 and nanoparticles of BiFeO3 and the Bi2O3/BiFeO3 composite indeed formed from the two materials.
BET analysis
Figure 4 shows the N2 adsorption–desorption isotherms of Bi2O3/BiFeO3, Bi2O3, and BiFeO3. The isotherm of Bi2O3/BiFeO3 was belong to type V with an H3 hysteresis loop, indicating that the composite exhibited a porous structure and the slit pores were formed by the accumulation of nanosheets and nanoparticles. Such porous structure was extremely useful in photocatalysis as it would provide communicable channels for the diffusion of reactant molecules and products. The BET-specific surface area of Bi2O3/BiFeO3 was observed as 4.98 m2/g, while the BET surface areas of Bi2O3 and BiFeO3 were estimated to be 2.15 and 9.09 m2/g, respectively. The result showed that a large specific surface area was not essential for the high performance of composite in the present study.
UV–Vis DRS analysis
The UV–Vis diffuse reflectance spectra (DRS) of the samples were investigated with the aim to probing their optical properties. As shown in Fig. 5a, commercial TiO2 (P25) absorbed only ultraviolet light (λ < 420 nm). Pure Bi2O3 and BiFeO3 showed absorption edges at about 455 and 570 nm, respectively, indicating that Bi2O3 and BiFeO3 could absorb light with wavelength from UV to visible regions. Bi2O3 has stronger absorbance in UV region, while BiFeO3 in visible region. Bi2O3/BiFeO3 composite had an absorption edge at about 560 nm, which was between the absorption edge of Bi2O3 and BiFeO3.
The optical absorption performance of a semiconductor is evaluated based on band-gap energy (Eg). The band-gap energy (Eg) was evaluated using the following equation (Xiao et al. 2013):
In this equation, α, h, ν, k, and Eg represent absorption coefficient, Planck constant, light frequency, a constant, and band-gap energy, respectively. In addition, the absorbance (A) of the photocatalyst is proportional to its absorption coefficient (α). The value of n depends on the characteristic of the transition in a semiconductor; it is 1 for Bi2O3 and 4 for BiFeO3 (Xiao et al. 2013; Tong et al. 2016). The band-gap energy of BiFeO3 was calculated to be 1.70 eV, and the band-gap energy of Bi2O3 was 2.83 eV.
The conduction band (CB) and valence band (VB) positions of Bi2O3 and BiFeO3 were speculated by a theoretical method. The CB (ECB) and VB (EVB) positions of a semiconductor can be calculated by the following equations (Feng et al. 2015):
where X is the absolute electronegativity of the semiconductor. Ee is the energy of free electrons on the hydrogen scale (about 4.5 eV). Eg is the band-gap energy of the semiconductor. The results are shown in Table 1.
Effect of initial Fe/Bi molar ratio
To investigate the effect of the initial Fe/Bi molar ratio, a series of Bi2O3/BiFeO3 samples with different initial Fe/Bi molar ratios (1:1, 1:2, 1:3, and 1:4) were fabricated. The XRD patterns of the samples are shown in Fig. S1. When the initial Fe/Bi molar ratio was 1:1, pure BiFeO3 was fabricated. In addition, when the initial Fe/Bi molar ratio was 1:2, 1:3, and 1:4, monoclinic Bi2O3 was produced and the diffraction peaks of Bi2O3 gradually increased in intensity when decreasing the initial molar ratio of Fe/Bi. The mass fraction of Bi2O3 in Bi2O3/BiFeO3 composites was calculated by MDI Jade 6.0 software and the results are shown in Table 2.
It was shown that the theoretical content of Bi2O3 and the actual content of Bi2O3 could be regarded as the same when increasing the initial molar ratio of Fe/Bi to 1:2. Excess Bi2O3 was obtained when the initial molar ratio of Fe/Bi was low. We can get the required composition according to the theoretical arithmetic and experimental results.
Figure S2 illustrates the time courses of BPA degradation efficiency over Bi2O3/BiFeO3 composites with different initial Fe/Bi molar ratios. The degradation efficiency increased from 69.8 to 86.5% after 300 min of visible-light irradiation when the initial molar ratio of Fe/Bi decreased from 1:1 to 1:3. However, when further decreased the initial Fe/Bi molar ratio in Bi2O3/BiFeO3 composites from 1:3 to 1:4, the degradation efficiency of BPA decreased to 70.6%. The highest degradation efficiency was obtained when the content of Bi2O3 in Bi2O3/BiFeO3 was 63.9%. This result implied that a suitable content of Bi2O3 would help to enhance the photocatalytic activity, and the optimal initial Fe/Bi molar ratio was 1:3.
Photocatalytic degradation of BPA
Effect of photocatalyst dosage
To investigate the effect of photocatalyst dosages on the photocatalytic degradation efficiencies of BPA, various photocatalyst dosages (0.2, 0.5, 0.8, and 1.0 g/L) were used on the degradation efficiency of BPA at the initial BPA concentration of 10 mg/L, irradiation time of 300 min, and solution pH of 6.3. The results are provided in Fig. 6. The photocatalytic degradation efficiency enhanced from 57.1 to 100% in 300 min when increasing of catalyst dosage from 0.2 to 0.5 g/L. With increasing the catalyst dosage to 0.8 g/L, the degradation efficiency was still 100% after 300 min of visible-light irradiation. Further increasing the catalyst dosage to 1.0 g/L, the degradation efficiency decreased weakly to 97.4%. It was reported that lower catalytic activity was observed when a smaller amount of catalyst was used, because that insufficient catalytic active sites were applied (Chang et al. 2010). However, if the photocatalyst dosage is too high, the light penetration decreased and light scattering effect occurred (Sood et al. 2015). In addition, the number of catalytic surface active sites would decrease, which was possibly caused by the agglomeration and sedimentation of the photocatalyst particles at too high dosage (Puangpetch et al. 2010). Therefore, 0.5 g/L catalyst dosage was selected as the optimal dosage of photocatalysts for the sequential experiment.
Effect of initial BPA concentration
To investigate the effect of the initial BPA concentration on the photocatalytic degradation efficiencies of BPA, various initial BPA concentrations (10, 15, 20, and 30 mg/L) were used on the degradation efficiency of BPA at catalyst dosage of 0.5 g/L, solution pH of 6.3, and irradiation time of 300 min. Results are provided in Fig. 7. The results showed that the degradation efficiencies decreased with the increase of BPA concentration. With the initial BPA concentration 10, 15, 20, and 30 mg/L, the photocatalytic degradation efficiencies at 300 min were 100, 72.0, 62.6, and 39.9%, respectively. Similar results had been reported by others using different catalysts (Chang et al. 2010). With the BPA concentration ranged from 10 to 30 mg/L, organic pollutant molecules accumulated on the surface of photocatalyst and the active sites were covered by high concentration of BPA, which resulted in the decrease of photocatalytic degradation efficiencies. Another possible reason was that more degradation intermediates were produced at higher BPA concentration, which would lead to the competitive adsorption between degradation intermediates and target pollutant (BPA), and finally caused the decrease of degradation efficiencies.
Effect of solution pH
To investigate the effect of solution pH on the degradation of BPA, BPA degradation experiments were performed in the pH range of 3.0–11.0 with catalyst dosage of 0.5 g/L, initial BPA concentration of 10 mg/L, and irradiation time of 300 min. The pH value of BPA solution in natural condition was 6.3. In addition, the other pH values were adjusted by NaOH (0.01 mol/L) and HCl (0.01 mol/L) solution. From Fig. 8, we could see that the highest degradation efficiency was obtained at natural condition (pH 6.3), and all BPA were degraded after 300 min of visible-light irradiation. The photocatalytic degradation efficiencies at solution pH of 3.0, 5.0, 7.0, 9.0, and 11.0 were 15.2, 72.3, 84.2, 59.8, and 40.4% in 300 min, respectively. It could be seen that lower photocatalytic activities were observed with the pH value deviated from natural condition. As well known, hydroxyl radicals (·OH) were generated by oxidizing hydroxide ions and they could react with much organic pollutants. It was reported that ·OH was easier to be generated by oxidizing more hydroxide ions available on photocatalyst surface in alkaline solutions (Konstantinou and Albanis 2004). Therefore, the possible cause induced low degradation efficiencies at strong acid conditions was that less hydroxyl radicals (·OH) were generated because of lower OH− concentration, which would greatly effect the photocatalytic activities. On the other hand, BPA existed in the form of anions at alkaline condition, which may repel the negative charged surface of photocatalyst (Konstantinou and Albanis 2004) and hydroxyl radicals (·OH). And then, the photodegradation efficiencies decreased. The results indicated that the degradation efficiency of BPA over Bi2O3/BiFeO3 composite was most favorable at natural condition.
Photocatalytic activity evaluation
To evaluate the photocatalytic activity towards BPA of prepared Bi2O3/BiFeO3 composite, a series of experiments were carried out at photocatalyst dosage of 0.5 g/L, initial BPA concentration of 10 mg/L, irradiation time of 300 min, and solution pH of 6.3. The visible-light-driven photocatalytic properties of fabricated Bi2O3/BiFeO3 composite, Bi2O3, BiFeO3, and P25 are shown in Fig. 9a. Under visible-light irradiation, the self-degradation of BPA could be negligible. Bi2O3/BiFeO3 composite showed an enhanced photocatalytic activity compared to Bi2O3 and BiFeO3. For instance, the degradation efficiency of the Bi2O3/BiFeO3 composite could reach nearly 100% within 300 min, while 49.2, 69.9, and 22.3% of degradation efficiencies were obtained for pure Bi2O3, pure BiFeO3. and P25, respectively. The result indicated that the combination of Bi2O3 and BiFeO3 could inhibit the recombination of photogenerated electron–hole pairs and enhance the photocatalytic activity efficiently.
Time courses of BPA degradation efficiency over Bi2O3/BiFeO3 composite, Bi2O3, BiFeO3 and P25 (a) and the corresponding fitted plots of pseudo-first-order kinetics (b) (experimental conditions: photocatalyst dosages of 0.5 g/L, initial BPA concentration of 10 mg/L, solution pH of 6.3, and irradiation time of 300 min)
To further investigate the photocatalysis, the degradation kinetics of BPA using fabricated Bi2O3/BiFeO3 composite, Bi2O3, BiFeO3, and P25 were investigated by fitting the experimental data to the pseudo-first-order kinetic model. The results shown in Fig. 9b illustrated that the reaction kinetics of all samples can be very well fitted by the pseudo-first-order rate model. The k value of BPA self-degradation was 0.0000673 /min, which could be neglected. In addition, the calculated k values for fabricated Bi2O3/BiFeO3 composite, Bi2O3, BiFeO3, and P25 were 0.00871, 0.00238, 0.00389, and 0.001 /min, respectively. Namely, the reaction rate constant over fabricated Bi2O3/BiFeO3 composite is 2.23, 3.65, and 8.71 times higher than that of BiFeO3, Bi2O3, and P25, respectively, proving that Bi2O3/BiFeO3 composite was a promising photocatalyst.
Recyclability
To investigate the recyclability of Bi2O3/BiFeO3 photocatalyst, the recycled experiments were carried out. After each photocatalytic reaction, the Bi2O3/BiFeO3 photocatalyst was centrifugated and washed by anhydrous alcohol for reuse, and then, the next cycle was started with the photocatalyst dosage of 0.5 g/L, initial BPA concentration of 10 mg/L, solution pH of 6.3, and irradiation time of 300 min. From Fig. 10, the removal rate decreased from 96.9 to 71.4% after three cycles, but it is clearly shown that the photocatalytic activity of Bi2O3/BiFeO3 was still higher than Bi2O3 or BiFeO3, suggesting that Bi2O3/BiFeO3 was a stable and efficient photocatalyst.
Photocatalytic mechanism
On the basis of experimental results, a possible photocatalytic mechanism was proposed for the photodegradation of BPA over Bi2O3/BiFeO3 composite (Fig. 11).
When Bi2O3/BiFeO3 photocatalyst was subjected to the visible-light irradiation, both Bi2O3 and BiFeO3 were excited with photogenerated electrons and holes produced. The photogenerated electrons in the CB of Bi2O3 would transfer to CB of BiFeO3, and the holes in the VB of Bi2O3 would transfer to VB of BiFeO3. This could help to reduce the recombination of electron–hole pairs, and thus, the photocatalytic activity of Bi2O3/BiFeO3 composite could be enhanced. It was known that the standard redox potentials of O2/·O2− and ·OH/H2O were + 0.13 and + 2.68 eV at pH 7, respectively (Chen et al. 2015). In this system, the CB edge of Bi2O3 was more negative than + 0.13 eV, indicating that ·O2− radicals could be produced. In addition, the VB edge of Bi2O3 was more positive than + 2.68 eV, indicating that the photogenerated holes in the VB of Bi2O3 had enough ability to oxidize H2O to give ·OH radicals. According to the above results, ·OH and ·O2− radicals were produced in the process and then participated in the photocatalytic reaction. Besides, organic pollutants would be oxidized via the excess of holes in the valence band of Bi2O3 and BiFeO3. In a word, ·OH radicals, ·O2− radicals, and holes simultaneously participated in the degradation mechanism.
The possible reactions in the photodegradation process of BPA were as follows:
Conclusions
In this paper, Bi2O3/BiFeO3 composite photocatalyst was successfully fabricated by a one-step sol–gel method using Bi(NO3)3·5H2O as bismuth source and Fe(NO3)3·9H2O as iron source. Effect of various reaction parameters on the BPA degradation under visible light was investigated in detail and concluded that the catalyst dosage of 0.5 g/L, initial BPA concentration of 10 mg/L, solution pH 6.3, and 63.9% of Bi2O3 in the composite was favorable to achieve maximum efficiency. The reaction rate constant over optimized Bi2O3/BiFeO3 composite is 2.23, 3.65, and 8.71 times higher than that of BiFeO3, Bi2O3, and P25, respectively. Bi2O3/BiFeO3 showed high photocatalytic activity after three cycles, suggesting that it was a stable photocatalyst. According to the theoretical calculation and the experimental results, ·OH radicals, ·O2− radicals, and valence band holes played significant roles in the photocatalytic reaction.
References
Chang X, Huang J, Cheng C, Sha W, Li X, Ji G, Deng S, Yu G (2010) Photocatalytic decomposition of 4-t-octylphenol over NaBiO3 driven by visible light: catalytic kinetics and corrosion products characterization. J Hazard Mater 173:765–772. https://doi.org/10.1016/j.jhazmat.2009.08.148
Chen Y, Tian G, Shi Y, Xiao Y, Fu H (2015) Hierarchical MoS2/Bi2MoO6 composites with synergistic effect for enhanced visible photocatalytic activity. Appl Catal B Environ 164:40–47. https://doi.org/10.1016/j.apcatb.2014.08.036
Chen WY, Shen YP, Chen SC (2016) Assessing bisphenol A (BPA) exposure risk from long-term dietary intakes in Taiwan. Sci Total Environ 543:140–146. https://doi.org/10.1016/j.scitotenv.2015.11.029
Cheng L, Kang Y (2014) Selective preparation of Bi2O3 visible light-driven photocatalyst by dispersant and calcination. J Alloys Compd 585:85–93. https://doi.org/10.1016/j.jallcom.2013.08.010
Falconer IR, Chapman HF, Moore MR, Ranmuthugala G (2006) Endocrine-disrupting compounds: a review of their challenge to sustainable and safe water supply and water reuse. Environ Toxicol 21:181–191. https://doi.org/10.1002/tox.20172
Fan Y, Chen C, Tang Z, Ni Y, Lu C (2015) Synthesis and characterization of g-C3N4/BiFeO3 composites with an enhanced visible light photocatalytic activity. Mater Sci Semicond Process 40:439–445. https://doi.org/10.1016/j.mssp.2015.06.054
Feng Y, Yan X, Liu C, Hong Y, Zhu L, Zhou M, Shi W (2015) Hydrothermal synthesis of CdS/Bi2MoO6 heterojunction photocatalysts with excellent visible-light-driven photocatalytic performance. Appl Surf Sci 353:87–94. https://doi.org/10.1016/j.apsusc.2015.06.061
Gao X, Wang Z, Fu F, Li X, Li W (2015) 2D double-layer-tube-shaped structure Bi2S3/ZnS heterojunction with enhanced photocatalytic activities. Physica B 474:81–89. https://doi.org/10.1016/j.physb.2015.06.002
Gao X, Dai Y, Fu F, Hua X (2016) 2D laminated cylinder-like BiFeO3 composites: hydrothermal preparation, formation mechanism, and photocatalytic properties. Solid State Sci 62:6–12. https://doi.org/10.1016/j.solidstatesciences.2016.10.014
Humayun M, Zada A, Li Z, Xie M, Zhang X, Qu Y, Raziq F, Jing L (2016) Enhanced visible-light activities of porous BiFeO3 by coupling with nanocrystalline TiO2 and mechanism. Appl Catal B: Environ 180:219–226. https://doi.org/10.1016/j.apcatb.2015.06.035
Jandegian CM, Deem S, Bhandari R, Holliday CM, Nicks D, Rosenfeld CS, Selcer KW, Tillitt DE, Vom Saal F, Velez-Rivera V, Yang Y, Holliday DK (2015) Developmental exposure to bisphenol A (BPA) alters sexual differentiation in painted turtles (Chrysemys picta). Gen Comp Endocrinol 216:77–85. https://doi.org/10.1016/j.ygcen.2015.04.003
Keykavoos R, Mankidy R, Ma H, Jones P, Soltan J (2013) Mineralization of bisphenol A by catalytic ozonation over alumina. Sep Purif Technol 107:310–317. https://doi.org/10.1016/j.seppur.2013.01.050
Kim JK, Kim SS, Kim WJ (2005) Sol-gel synthesis and properties of multiferroic BiFeO3. Mater Lett 59:4006–4009. https://doi.org/10.1016/j.matlet.2005.07.050
Konstantinou IK, Albanis TA (2004) TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: kinetic and mechanistic investigations. Appl Catal B Environ 49:1–14. https://doi.org/10.1016/j.apcatb.2003.11.010
Kumar SG, Rao KSRK (2015) Tungsten-based nanomaterials (WO3 & Bi2WO6): modifications related to charge carrier transfer mechanisms and photocatalytic applications. Appl Surf Sci 355:939–958. https://doi.org/10.1016/j.apsusc.2015.07.003
Kumar SG, Rao KSRK (2017) Comparison of modification strategies towards enhanced charge carrier separation and photocatalytic degradation activity of metal oxide semiconductors (TiO2, WO3 and ZnO). Appl Surf Sci 391:124–148. https://doi.org/10.1016/j.apsusc.2016.07.081
Lam SM, Sin JC, Mohamed AR (2017) A newly emerging visible light-responsive BiFeO3 perovskite for photocatalytic applications: a mini review. Mater Res Bull 90:15–30. https://doi.org/10.1016/j.materresbull.2016.12.052
Li D, Zhou Z, Qing D, He Y, Wu T, Miao M, Wang J, Weng X, Ferber JR, Herrinton LJ, Zhu Q, Gao E, Checkoway H, Yuan W (2010) Occupational exposure to bisphenol-A (BPA) and the risk of self-reported male sexual dysfunction. Hum Reprod 25:519–527. https://doi.org/10.1093/humrep/dep381
Li J, Zhao W, Guo Y, Wei Z, Han M, He H, Yang S, Sun C (2015) Facile synthesis and high activity of novel BiVO4/FeVO4 heterojunction photocatalyst for degradation of metronidazole. Appl Surf Sci 351:270–279. https://doi.org/10.1016/j.apsusc.2015.05.134
Lin X, Lv P, Quan Q, Li H, Zhai H, Liu C (2012) Bismuth titanate microspheres: directed synthesis and their visible light photocatalytic activity. Appl Surf Sci 258:7146–7153. https://doi.org/10.1016/j.apsusc.2012.04.019
Liu Y, Ding Z, Lv H, Guang J, Li S, Jiang J (2015) Hydrothermal synthesis of hierarchical flower-like Bi2WO6 microspheres with enhanced visible-light photoactivity. Mater Lett 157:158–162. https://doi.org/10.1016/j.matlet.2015.05.024
Majid F, Mirza ST, Riaz S, Naseem S (2015) Sol-Gel synthesis of BiFeO3 nanoparticles. Mater Today Proc 2:5293–5297. https://doi.org/10.1016/j.matpr.2015.11.038
Manfo FP, Jubendradass R, Nantia EA, Moundipa PF, Mathur PP (2014) Adverse effects of bisphenol A on male reproductive function. Rev Environ Contam Toxicol 228:57–82. https://doi.org/10.1007/978-3-319-01619-1_3
Paulose T, Speroni L, Sonnenschein C, Soto AM (2015) Estrogens in the wrong place at the wrong time: fetal BPA exposure and mammary cancer. Reprod Toxicol 54:58–65. https://doi.org/10.1016/j.reprotox.2014.09.012
Puangpetch T, Sommakettarin P, Chavadej S, Sreethawong T (2010) Hydrogen production from water splitting over Eosin Y-sensitized mesoporous-assembled perovskite titanate nanocrystal photocatalysts under visible light irradiation. Int J Hydrogen Energy 35:12428–12442. https://doi.org/10.1016/j.ijhydene.2010.08.138
Son LT, Takaomi K (2011) Hollow-fiber membrane absorbents embedded molecularly imprinted polymeric spheres for bisphenol A target. J Membr Sci 384:117–125. https://doi.org/10.1016/j.memsci.2011.09.013
Sood S, Umar A, Mehta SK, Kansal SK (2015) α-Bi2O3 nanorods: an efficient sunlight active photocatalyst for degradation of Rhodamine B and 2,4,6-trichlorophenol. Ceram Int 41:3355–3364. https://doi.org/10.1016/j.ceramint.2014.10.038
Sun B, Zhou G, Gao T, Zhang H, Yu H (2016) NiO nanosheet/TiO2 nanorod-constructed p–n heterostructures for improved photocatalytic activity. Appl Surf Sci 364:322–331. https://doi.org/10.1016/j.apsusc.2015.12.158
Takamiya M, Magan N, Warner PJ (2008) Impact assessment of bisphenol A on lignin-modifying enzymes by basidiomycete Trametes versicolor. J Hazard Mater 154:33–37. https://doi.org/10.1016/j.jhazmat.2007.09.098
Tong T, Zhang H, Chen J, Jin D, Cheng J (2016) The photocatalysis of BiFeO3 disks under visible light irradiation. Catal Comm 87:23–26. https://doi.org/10.1016/j.catcom.2016.08.030
Vom Saal FS, Welshons WV (2014) Evidence that bisphenol A (BPA) can be accurately measured without contamination in human serum and urine, and that BPA causes numerous hazards from multiple routes of exposure. Mol Cell Endocrinol 398:101–113. https://doi.org/10.1016/j.mce.2014.09.028
Xiao X, Hu R, Liu C, Xing C, Qian C, Zuo X, Nan J, Wang L (2013) Facile large-scale synthesis of β-Bi2O3 nanospheres as a highly efficient photocatalyst for the degradation of acetaminophen under visible light irradiation. Appl Catal B: Environ 140–141:433–443. https://doi.org/10.1016/j.apcatb.2013.04.037
Yan Y, Zhou Z, Cheng Y, Qiu L, Gao C, Zhou J (2014a) Template-free fabrication of α- and β-Bi2O3 hollow spheres and their visible light photocatalytic activity for water purification. J Alloys Compd 605:102–108. https://doi.org/10.1016/j.jallcom.2014.03.111
Yan Y, Zhou Z, Zhao X, Zhou J (2014b) A controlled anion exchange strategy to synthesize core-shell beta-bismuth oxide/bismuth sulfide hollow heterostructures with enhanced visible-light photocatalytic activity. J Colloid Interface Sci 435:91–98. https://doi.org/10.1016/j.jcis.2014.08.027
Yap PS, Lim TT, Lim M, Srinivasan M (2010) Synthesis and characterization of nitrogen-doped TiO2/AC composite for the adsorption–photocatalytic degradation of aqueous bisphenol-A using solar light. Catal Today 151:8–13. https://doi.org/10.1016/j.cattod.2010.01.012
Acknowledgements
This work was jointly supported by the National Natural Science Foundation of China (Nos. 21163023 and 21261026) and Key Program of Yunnan Province Foundation (No. 2013FA005).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shi, Y., Xiao, M., Luo, L. et al. Bi2O3/BiFeO3 heterostructure: preparation, characterization, and photocatalytic activity. Chem. Pap. 72, 1327–1337 (2018). https://doi.org/10.1007/s11696-018-0384-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-018-0384-z