Abstract
Background
Sleeve gastrectomy (SG) is the most common bariatric procedure performed worldwide. It accounts for more than 50% of primary bariatric surgeries performed each year. Recent long-term data has shown an alarming trend of weight recidivism. Some authors have proposed the concurrent use of a non-adjustable gastric band to decrease long-term sleeve failure.
Objective
To compare the outcomes (weight loss) and safety (rate of complication and presence of upper GI symptoms) between SG and BSG.
Methods
A systematic search with no language or time restrictions was performed to identify relevant observational studies and randomized controlled trials (RCT) evaluating people with morbid obesity undergoing SG or SGB for weight loss. An inverse-of-the-variance meta-analysis was performed by random effects model. Heterogeneity was assessed using Cochrane X2 and I2 analysis.
Results
A total of 7 observational studies and 3 RCT were included in the final analysis. There were 911 participants pooled from observational studies and 194 from RCT. BSG showed a significant higher excess of weight loss (% EWL). The difference among groups was clinically relevant after the third year where the weighted mean difference (SMD) was 16.8 (CI 95% 12.45, 21.15, p < 0.0001), while at 5 years, a SMD of 25.59 (16.31, 34.87, p < 0.0001) was noticed. No differences related to overall complications were noticed. Upper GI symptoms were up to three times more frequent in the BSG group (OR 3.26. CI 95% 1.96, 5.42, p < 0.0001).
Conclusions
According to the results, BSG is superior to SG in weight loss at 5 years but is associated with a higher incidence of upper GI symptoms. However, these conclusions are based mainly on data obtained from observational studies. Further RCT are needed to evaluate the effect and safety of BSG.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sleeve gastrectomy (SG) is the most common bariatric procedure performed worldwide. It accounts for 53.6% of all primary bariatric surgeries completed and has continues to gain popularity [1]. Its widespread adoptions is related to an overall lower risk of surgical and medical complications, a shorter operative time, and its technical simplicity over the Roux-en-Y gastric bypass (RGYB) [2]. However, as mid- and long-term data become available, some have called into question the overall efficacy and durability of weight loss and comorbidity resolution associated with this procedure. In fact, compared to RYGB, the SG has been associated with lower rates of diabetes mellitus (DM) and hyperlipidemia remission, higher rates of weight regain, and a higher proportion of patients who fail to achieve adequate weight loss. The latter is especially pronounced in patients with body mass index (BMI) great than 40 kg/m2 [3,4,5,6,7,8].
There are many technical and patient-related factors that can lead to inadequate weight loss and weight regain after bariatric surgery. In the RYGB, the most commonly cited reason for failure is a progressive dilation of the gastric pouch or the anastomosis [9, 10]. Similarly, leaving a remnant fundus during sleeve gastrectomy has been attributed to long-term failure after sleeve gastrectomy. In order to tackle this issue, Fobi et al. described the banded gastric bypass (BGBP), which used a silastic ring tube around the proximal portion of the gastric pouch to prevent stoma and pouch dilation. The concomitant use of the band during RYGB led to a higher overall weight loss and lower rates of weight regain with minimal side effects [11]. Following suit, few authors began advocating for the placement a non-adjustable ring or band around the proximal portion of the stomach during SG to prevent gastric pouch dilation. Similar to the BGBP, banded SG (BSG) has reported with higher weight loss and overall lower rates of weight regain [12,13,14]. We aimed to perform a systematic review and meta-analysis of patients undergoing either the standard SG or the BSG with regard to weight loss, complication rate, and frequency of upper GI symptoms.
Methods
Search Strategy
Information Sources
A systematic search was performed on October 2020 and updated on April 2021 to identify relevant studies published in English, French, Spanish, Italian, or German using the following databases: PubMed, Web of Science, EBSCO host, Scopus, EMBASE, and the Cochrane Central Library. There was no time limitation for this search. Relevant publications were manually searched for additional information. In relevant studies with missing information, the corresponding authors were reached by available e-mail.
Search Strategy
For the search strategy, the following terms were included in the search for articles: “banded sleeve gastrectomy” or “banded sleeve” or “banded gastrectomy” or “banded-sleeve gastrectomy” or “non-banded sleeve gastrectomy” or “non-banded sleeve” or “non-banded gastrectomy” or “non banded sleeve gastrectomy” or “non banded sleeve” or “non banded gastrectomy” or “nonadjustable banded procedures” or “nonadjustable banded sleeve” or “nonadjustable banded sleeve gastrectomy” or “nonadjustable banded bariatric procedure*” or “nonadjustable banded bariatric surg*” or “non-adjustable banded sleeve” or “non-adjustable banded bariatric procedure*” or “non-adjustable banded bariatric surg* procedure*.”
Protocol and Registration
The protocol was registered in the prospective international register of systematic reviews before article selection process (PROSPERO: CRD42020212175).
Study Selection
All identified abstracts were assessed by 2 independent evaluators (G.P-B. and G.R-V.). Disagreement between evaluators was mitigated by the participation of a third independent investigator (R.M). The study protocol was agreed on by all authors and reviewed. We followed a PICO (population, intervention, comparison, and outcomes) format for intervention literature reviews and included the following:
-
1.
Population: adult participants of any gender undergoing SG or BSG due to morbid obesity,
-
2.
Intervention: banded sleeve gastrectomy (BSG) which consists in a vertical gastric reservoir and placement of a non-adjustable banding device at 4 to 6 cm distal to the gastroesophageal junction,
-
3.
Comparison: non-banded sleeve gastrectomy
-
4.
Outcomes: weight loss up-to 5 years after surgery, described as follows [15]:
-
Percent total weight loss (%TWL) = [(Initial weight) − (Postoperative weight at time of measurement) / (Initial weight)] × 100
-
Percent excess of weight loss (%EWL) = [(Initial weight) − (Postoperative weight at the time of measurement) / (Initial Weight) − (Ideal Weight − defined as weight corresponding to a BMI of 25 kg/m2)] × 100
Secondary outcomes were surgical complications, or the development of upper GI symptoms related to any of the procedures.
Criteria for inclusion in this systematic review were as follows: (1) observational studies (retrospective or prospective cohorts, case–control studies, and case series) or randomized controlled trials including patients with morbid obesity who underwent BSG and were compared with controls who underwent SG for weight loss. Studies performing any bariatric surgery other than BSG and SG were manually removed from the final analysis, as well as in those where adjustable banding devices were used. Studies meeting the inclusion criteria were retrieved and their text was reviewed in full. In cases of relevant studies with missing information, corresponding authors were contacted to acquire additional data.
Data Extraction
Information extracted from eligible studies included basic study data (last name of the first author, year of publication, country, design, sample size), demographic data (gender — % male patients, age, baseline BMI), surgical parameters (operative time, type of band used, length of the band, distance of the band placement from the gastroesophageal junction, size of gastric calibration tube, distance of gastric transection from the pylorus), weight loss parameters (EWL and/or TWL), complications (leak, major bleeding, death, deep wound infection (abdominal abscess, reoperation, stricture or stenosis, band erosion, and/or migration), and presence of upper gastrointestinal symptoms (GERD, dysphagia, food intolerance, or persistent vomiting). Two of the corresponding authors from the selected studies were contacted to obtain further information.
Risk of Bias Assessment
Two reviewers (G.P-B. and G.R-V.) independently evaluated the quality of the selected articles. The quality assessment of the observational studies was carried out using the Newcastle–Ottawa Scale for cohort studies. Each article was evaluated on three main features: selection of the study groups, ascertainment of the exposure, and comparability of the groups. A star was given for each signaling question within each dimension. With a total of nine possible stars, studies with seven or more stars were considered high-quality for the purposes of our review [16]. For randomized controlled trials, the quality of the selected studies was evaluated according to the Cochrane Collaboration tool for randomized controlled trials [17]. This tool consists of six questions that address sequence generation, allocation concealment, blinding, incomplete data, reporting bias, and other biases. Answers regarding bias were categorized as low risk, high risk, and unclear risk. Results from these questions were graphed and assessed using Review Manager (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration).
Statistical Analysis
Data synthesis and statistical analysis were performed using RevMan 5.4 software (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration). Weighted mean differences (SMD) and 95% confidence intervals (95% CI) were calculated for continuous data, whereas the Mantel–Haenszel test was used to estimate the odds ratio (OR) with 95% CI for matched categorical (dichotomous) data. Statistical significance was set at p < 0.05 or < 5%. The quantitative synthesis was performed using random effects model. In case of two or less studies for a given outcome, fixed effect was preferred for pooling the effect estimate. Between-study heterogeneity/variability was assessed using the Τau2, Χ2 (Cochrane Q), and I2 statistics. Publication bias was visually assessed by funnel plots [18, 19] quantified by the Egger method (weighted linear regression of the treatment effect on its standard error) [20] when more than 10 studies were available for a given outcome.
Sensitivity analysis regarding weight loss as the main outcome using only the information among randomized controlled trials (RCTs) as the preferred source for quality recommendations was not able due to lack of data.
Results
Study Selection
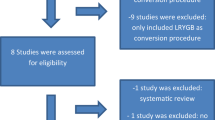
A total of 1217 studies (G.P-B. and G.R-V.) were identified from the previously described search strategy. After remove of duplicates, 992 studies underwent title and abstract assessment, from which only 58 were eligible for full text analysis. After review of the full-text, a total of 48 were excluded, from which 23 were conference abstracts with incomplete data, 11 were case reports or multimedia articles, 4 were excluded due to duplication of data with other studies, 3 did not provide adequate data about the outcomes of interest, 2 were in-animal studies, 2 were commentaries or letters, 2 were review articles, and 1 was related to an adjustable banding device, leaving only 10 articles for the final synthesis. Figure 1 shows the PRISMA flow chart of the included articles. Among these, three manuscripts were randomized controlled trials, six nested case–control studies, and 1 was a cohort study. Summary of the included studies is shown in Table 1 [6, 13, 21,22,23,24,25,26,27,28].
Risk of Bias Assessment
The three included randomized controlled trials showed low risk of bias in random sequence generation. However, allocation and blinding of the intervention are impossible due to the nature of the intervention itself, which is a specific type of surgical procedure. Thus, the three studies were rated as high risk of bias in these two items. On the other hand, these studies were considered low risk of bias in the incomplete outcome, selective reporting, and other biases categories. Supplemental Figs. 1 and 2 show the individual assessment for each included RCT. Regarding the observational studies, initial concordance of the NOS scores among reviewers was 87.5%. There was a single article with a discrepancy among reviewers. This article was re-reviewed, and a score mutually agreed upon. Ultimately, there were two papers that had a high risk of bias. The remaining five manuscripts had a low risk as shown in Table 2.
Characteristics of the Population
A total of 1105 patients were included, 436 underwent BSG and 669 SG. However, sample size may be an over-estimations due to shared patients among the studies from Bhandari [25, 29], Fink [23, 26], Gentileschi [28], and Tognoni [22]. One hundred ninety-eight patients (45.4%) undergoing BSG were men, compared to 277 (41.4%) undergoing SG (p = 0.11). Patients who underwent BSG were slightly younger (SMD — 0.36 years; CI 95% − 0.69, − 0.04, p = 0.03) but, this was not considered to be clinically relevant. Baseline BMI was similar among groups (SMD 0.35 CI 95% − 0.02. 0.72; p = 0.06). The follow-up period ranged between 12 and 60 months.
The SG resection technique was similar in most of the series. Dissection of the greater curvature was commonly performed 4–6 cm from the pylorus, except in one series where it was described 2 to 3 cm from the pylorus [21]. All authors used a gastric calibration tube (GCT) between 35 and 38 Fr except for Lemmens et al. who used a 40 Fr GCT [24]. The non-adjustable bands were placed between 4 and 6 cm from the gastroesophageal junction and the size ranged between 6.5 and 7.5 cm. Bhandari and Gentileschi [6, 22, 25, 28] used the GaBP Ring Autolock® non-adjustable band. Fink, Lemmens, and Karcz [13, 23, 26, 27] all used the Minimizer® Gastric ring device. Soliman et al. described the placement of a 6.5–7-cm band of double-layer Gortex ® mesh [21]. Operative time was similar among groups for the observational studies (SMD 2.72 min, CI 95% − 3.73, 9.17; p 0.41) (Fig. 2), as well as in the study of Tognoni [22] where it was 84.6 ± 30.13 min for the BSG group and 74.6 ± 14.48 min for the SG group (p = 0.14).
Outcome Synthesis
Observational Studies
Main Outcome — Weight Loss
Time intervals for follow-up and weight loss parameters differed between the studies included in this systematic review. The percentage of excess of weight loss (% EWL) at 12, 36, and 60 months were used for the final analysis. Data at 12 and 36 months was available in 5 studies, and at 60 months in 3 of the studies [13, 21, 23,24,25,26, 29]. BSG showed a significant higher %EWL at each of the analyzed time points as shown in Fig. 3. The difference among groups was clinically relevant after the third year where SMD was 17.6 (CI 95% 12.95, 22.23, p < 0.005), while at 5 years, SMD was 25.59 (16.31, 34.87, p < 0.0001). Heterogeneity for weight loss among observational studies at 1, 3, and 5 years showed a Q < 0.05 in all cases and the proportion was measured as 78%, 73%, and 61% showing that heterogeneity was due to true effect rather than random error. Due to the small number of studies in this analysis, we could not assess publication bias.
Secondary Outcomes
-
1.
Early complications
Data of early complications were available in all the included series. No significant differences were noticed regarding leaks, postoperative bleeding, infectious complications, or death. Just one leak was registered in a patient who underwent SG [6], while there were 2 mortalities not related to the procedures recorded, 1 in each group [6, 13]. Postoperative bleeding comparison is shown in Fig. 4. A Q statistic of 0.99 and a I2 of 0% demonstrates low heterogeneity for this outcome.
-
2.
Reoperation
The overall (early and late) reoperations frequency was compared between procedures. A total of 25 (7.37%) reinterventions were reported in the BSG group and 39 (6.8%) in the SG group.
For the BSG, some of these were due to bleeding at the staple line, band slippage, functional stenosis or regurgitation related to the band, non-responsive GERD, and insufficient weight loss, whereas for the SG, interventions were due to bleeding, intraabdominal abscess, postincisional hernia, insufficient weight loss and/or significant weight regain, and intractable GERD requiring conversion to RYGB. The analysis showed a non-significant difference in reoperations between the groups (OR 0.82, CI 95% 0.47, 1.43, p = 0.48) (Fig. 5). Despite an I2 of 34% heterogeneity, Q statistics show that heterogeneity comes from random error rather than true sample (p = 0.018).
-
3.
Band-related complications
Band-related complications consisted of one band slippage [26] and 6 patients diagnosed with functional stenosis [13, 24]. All these patients underwent revisional surgery, where the bands were removed, or the band-length was increased up to 7.5 cm. Neither erosions nor perforation was described.
-
4.
Upper GI symptoms
-
(a)
Dysphagia, food intolerance, vomiting, and regurgitation
All of the studies included data related to upper gastrointestinal (GI) symptoms. The presence of regurgitation, vomiting, food intolerance, and mild to severe dysphagia were used for the final analysis. Overall, upper GI symptoms were three times more frequent in the BSG group (66 patients) as compared to the SG group (23 patients) (OR 3.92 CI 95% 1.55, 9.88, p = 0.004) (Fig. 6). Also, upper GI symptoms showed a 55% heterogeneity by I2 but a Q statistic of p = 0.05, which demonstrates that no true heterogeneity was found.
-
(b)
Gastroesophageal reflux (GERD)
Data related to GERD was available in 5 studies which were included in the analysis [13, 21, 23, 25, 26]. The prevalence of de novo GERD or worsening pre-existing reflux after surgery was similar between BSG group (15.7%) and the SG group (11.95%), OR 1.05(CI 95% 0.58, 1.91, p = 0.87) (Fig. 7). No true heterogeneity was found in this outcome (I2 = 0%; Q = 0.087).
Randomized Controlled Trials
Main Outcome — Weight Loss
At 3 years, Fink et al. [27] reported an EWL of 73.9% for the BSG group, whereas it was 62.3% for the SG group. Gentileschi [28] reported data as percent of excess of BMI loss (%EBMIL) at 3 years for each group of 103.4% and 86.29%, respectively. Due to the lack of standard media deviations values and the use of different weight loss parameters, it was not possible to compare weight loss among each group.
Secondary Outcomes
-
1.
Early complications
There were no significant differences in frequency of staple line leaks, postoperative bleeding, infections, or deaths. Postoperative bleeding was recorded in 3 patients, 2 in the SG group [22, 27], and 1 in the BSG group [22] (OR 0.59, CI 95% 0.08, 4.61; p = 0.62).
-
2.
Reoperation and band-related complications
In terms of reoperations (early and late), 3 patients underwent revisional surgery in each group. In the BSG group, 2 patients underwent conversion to RYGB due to GERD and in 1 patient, the band was removed due to slippage [27]. In the SG group, 2 patients were converted to RYGB due to GERD and one patient was reoperated to repair an incisional hernia [27, 28] (OR 1, CI 95% 0.22, 4.55; p = 1).
-
3.
Upper GI symptoms
-
(a)
Dysphagia, food intolerance, vomiting, and regurgitation
No significant differences were noticed regarding upper GI symptoms (regurgitation, vomiting, food intolerance, and mild to severe dysphagia) as shown in Fig. 8. Regurgitation was the most frequent symptom in the BSG group; however, no patient required revision for this symptom [27] (OR 2.15, CI 95% 0.62, 7.46; p = 0.23).
-
(b)
Gastroesophageal reflux (GERD)
Data regarding GERD was just available in one RCT [27] and no significant difference was recorded. There were 3 (6.7%) cases in the BSG group and 4 (8.7%) in the SG group (OR 0.75, CI 95% 0.16, 3.56; p = 0.72).
Discussion
The laparoscopic SG has become an established bariatric procedure in the treatment of morbid obesity and its related complications. Despite initially having promising results, long-term data has shown that 27.8% of patients will have inadequate weight loss, defined as an EWL < 50%, or significant weight regain in the long-term. The term “sleeve failure” has been proposed to describe this group of patients whose weight or comorbidities do not respond to SG or those who relapse after having adequate weight loss. Ultimately, up to 13% of the patients will eventually require either revision or conversion to another bariatric procedure as a result of sleeve failure [30]. In our meta-analysis, we found that patients who undergo banded sleeve gastrectomy have better long-term weight loss compared to those who undergo sleeve gastrectomy alone.
Many authors have described mechanisms that predispose a patient to inadequate weight loss after SG. These mechanisms can be considered either surgeon dependent, patient dependent, or simply attributed to the chronic and relapsing nature of obesity as a disease. One of the commonly attributable mechanisms for sleeve failure is a dilation of the sleeve, which can reduce the restrictive effects of surgery. Some authors suggest that revisional surgery with reduction of the gastric volume by performing a re-sleeve gastrectomy (RSG) is a viable option [9, 10, 31]. However, the long-term results have not been encouraging with only 28% of the patients having a persistent weight loss [32]. Similar to sleeve failure, mechanisms for failure after a RYGB have been proposed and have some degree of overlap, which center around dilatation of the gastric pouch and the gastrojejunal anastomosis. The long-term care of these treatment resistant patients is challenging and has led authors to propose treatments or interventions to prevent such failures after bariatric surgery.
Fobi et al. initially described the Banded-RGYB (BRGYB) in the 1980s where a non-adjustable ring was placed near the gastro-esophageal junction. This ultimately led to a higher weight loss in the short- and medium-term periods as well as lower risk for weight regain with an acceptable rate of dysphagia and local complications [11, 33, 34]. Following the same concept, some authors have proposed the use of a non-adjustable ring or band at the time of SG to decrease weight recidivism. Results in early studies are very encouraging but are limited to single institution experiences with an overall limited number of patients [12,13,14, 22, 23, 26, 27, 35].
The primary objective of this meta-analysis was to compare the weight loss among BSG and SG. Based on the %EWL at 1, 3, and 5 years with observational studies, BSG showed significantly greater weight loss compared to SG. However, at 1 year, the mean %EWL difference between groups was only 6.8. After 3 years, the standard mean difference (SDM) of %EWL between groups was 17.6 (CI 95% 12.95, 22.23), and at 5 years, this was 25.59 (CI 95% 16.31, 34.87), which are clinically and statistically significant differences. Although there were only 3 studies included for the analysis at 5 years, a total of 92 and 140 patients were included in the BSG and SG groups, respectively, and the sample size was ample enough to assess a hypothesized difference of at least 10%. SMD differences of %EWL between procedures at 3 and 5 years were greater than 10% in each of the included studies, which ranged between 10.5 and 24.3% [24, 26] at 3 years, and between 14.95 and 30% at 5 years [25, 26].
Interestingly, two of the observational studies provided data in patients with super obesity (BMI > 50 kg/m2) (Bhandari, and Lemmens) [24, 29]. Limiting analysis to the data included in these studies, we found that at 3 years, the pooled mean %EWL for the BSG ranged between 80.4 and 85.11%, whereas this was 62.3% for the SG in both studies. The pooled SMD difference of %EWL at 3 years for these 2 studies was 22.58 (CI 95% 19.1; 26.11, p < 0.00001) in favor of the BSG (BSG n = 25, SG n = 189) [6, 24]. However, more data is required to demonstrate the superiority of the BSG over SG for the management of patients with super obesity.
BSG has been compared with other procedures such as the One-anastomosis Gastric Bypass (OAGB/MGB) and the reported weight loss has been slightly higher for the BSG than for OAGB/MGB, with an %EWL of 84% vs %EWL of 79% at 6 years [35]. Another possible advantage for the BSG is that none of the patients who underwent BSG showed nutritional deficiencies, whereas 20% of patients of the OAGB/MGB group developed hypoproteinemia or other nutrient deficiencies. Nonetheless, the OAGB/MGB is remained superior in terms of comorbidities resolution, as more than 85% of patients with DM and hypertension showed complete remission, whereas these rates were 75% and 64% for the patients who underwent BSG [35].
Regarding weight regain, a total of 29 patients of the SG group underwent a revision, whereas only 2 patients of the BSG underwent revision due to insufficient weight loss. However, the analysis of weight recidivism was difficult to assess with the available data. In fact, only 4 studies included information on follow-up beyond 3 years. Thus, the real number of patients that had significant weight regain, insufficient weight loss, or those who required revision was not available.
An overall low rate of band-related complications was demonstrated in the present meta-analysis. Functional stenosis was the most commonly reported complication, and it was seen in 6 (1.4%) patients. There were 2 band-slippage events (0.45%) and no band erosions or perforations reported. These results are consistent with meta-analyses that have reported complications in banded-RYGB. These studies also show a very low frequency of band-related complications and in fact, none reported band-erosion. Nonetheless, band slippage and migration ranged between 1.5 and 13.7% and gastric outlet stenosis was seen in 2.8% [36,37,38].
The presence of upper GI symptoms such as vomiting, regurgitation, food intolerance, or dysphagia was up to three times more common after BSG compared to SG when considering only observational studies. Interestingly, there was no difference in the presence of these symptoms when randomized controlled trials were pooled into the analysis. When food intolerance and dysphagia were analyzed, we found that most patients reported only mild symptoms, which were mostly related to intake of solid foods. It is important to highlight that these events were much more frequent in early series where a band < 7 cm was placed. Subsequently, Fink et al. modified the size of the band up to 7.5 cm after their initial experience [23] with some improvement in the frequency of these symptoms. Similarly, Lemmens et al. reported a few cases of gastric outlet stenosis with his use of a 6.5-cm band, which, when he adjusted to using a 7.5-cm band, showed some improvement of symptoms [24]. When the prevalence of upper GI symptoms between the series who used a 6.5 cm and > 7 cm was analyzed, we found that 37.2% of the patients had a least one upper GI symptom (mild to moderate dysphagia, regurgitation, vomiting, or food intolerance) when a band of 6.5 cm was placed, whereas these were present only in 11.4% in patients where a band > 7 cm was placed (p < 0.0001). Still, this proportion was significantly higher compared to SG, where the pooled frequency of upper GI symptoms was only 6% (p = 0.016). However, the fact that 2 of the authors used different sizes of the band because they noticed a higher presence of symptoms in patients with a 6.5-cm band-size, as previously mentioned could limit the comparison.
To the best to our knowledge, this is the first meta-analysis that compares BSG and SG in terms of weight loss and upper GI symptoms. Some of the limitations of the present meta-analysis are that only 2 of the included series (3 studies) are randomized control trials (RCT), which highlights the urgent need for more RCT in this important topic. Related to this, most of the conclusions of the present study are based on the findings of observational studies which confer selection bias and are major limitation.
Secondly, there was a lack of standardization of weight-loss parameters, and most of the studies used the %EWL to report the history of weight loss; however, in some others, the %EBMIL (excess of BMI loss) and %TWL were employed. This made it impossible to perform a sensitivity analysis using data of the RCT regarding weight loss.
Another important issue to consider is the lack of band size standardization. As discussed previously, a smaller size could be related to a greater risk of upper GI symptoms, which can alter the weight loss effect do to the presence of the symptoms.
Also, despite the low incidence of band-related complications, the follow-up in these studies was limited to 5 years and surgeon late onset band-complications such as erosion, migration, and/or perforation could take longer to be noticed.
Finally, and related to the latter, another major limitation was the small sample size with complete follow-up at 3 and 5 years, and this may limit the conclusions that can be drawn regarding the safety and effect of the BSG at medium (2–5 years) and long term (> 5 years).
References
Angrisani L, Santonicola A, Iovino P, Vitiello A, Higa K, Himpens J, et al. IFSO worldwide survey 2016: primary, endoluminal, and revisional procedures. Obes Surg. 2018;28(12):3783–94.
Li J-F, Lai D-D, Lin Z-H, Jiang T-Y, Zhang A-M, Dai J-F. Comparison of the long-term results of Roux-en-Y gastric bypass and sleeve gastrectomy for morbid obesity. Surg Laparosc Endosc Percutan Tech. 2014;24(1):1–11.
Wang Y, Song Y, Chen J, Zhao R, Xia L, Cui Y, et al. Roux-en-Y gastric bypass versus sleeve gastrectomy for super super obese and super obese: systematic review and meta-analysis of weight results, comorbidity resolution. Obes Surg. 2019;29(6):1954–64.
Vitiello A, Berardi G, Velotti N, De Palma GD, Musella M. Should sleeve gastrectomy be considered only as a first step in super obese patients? 5-year results from a single center. Surg Laparosc Endosc Percutan Tech. 2020;31(2):203–207.
Boza C, Daroch D, Barros D, León F, Funke R, Crovari F. Long-term outcomes of laparoscopic sleeve gastrectomy as a primary bariatric procedure. Surg Obes Relat Dis. 2014;10(6):1129–33.
Bhandari M, Ponce de Leon-Ballesteros G, Kosta S, Bhandari M, Humes T, Mathur W, et al. Surgery in patients with super obesity: medium-term follow-up outcomes at a high-volume center. Obesity. 2019;27(10):1591–7.
Himpens J, Dobbeleir J, Peeters G. Long-term results of laparoscopic sleeve gastrectomy for obesity. Ann Surg. 2010;252(2):319–24.
Bohdjalian A, Langer FB, Shakeri-Leidenmühler S, Gfrerer L, Ludvik B, Zacherl J, et al. Sleeve gastrectomy as sole and definitive bariatric procedure: 5-year results for weight loss and ghrelin. Obes Surg. 2010;20(5):535–40.
Baltasar A, Serra C, Pérez N, Bou R, Bengochea M. Re-sleeve gastrectomy. Obes Surg. 2006;16(11):1535–8.
Gagner M, Rogula T. Laparoscopic reoperative sleeve gastrectomy for poor weight loss after biliopancreatic diversion with duodenal switch. Obes Surg. 2003;13(4):649–54.
Fobi MAL, Lee H. The surgical technique of the fobi-pouch operation for obesity (the transected silastic vertical gastric bypass). Obes Surg. 1998;8(3):283–8.
Frezza EE, Herbert H, Wachtel MS. Combined laparoscopic gastric banding and stomach reduction (GBSR): initial experience after 1 year. Obes Surg. 2008;18(6):690–4.
Karcz WK, Karcz-Socha I, Marjanovic G, Kuesters S, Goos M, Hopt UT, et al. To band or not to band—early results of banded sleeve gastrectomy. Obes Surg. 2014;24(4):660–5.
Chamany T, Makam R, Kanth R. Early experience of laparoscopic banded sleeve gastrectomy using gabp ring. Abstracts from the 19th World Congress of the International Federation for the Surgery of Obesity & Metabolic Disorders (IFSO), Montreal, Canada 26–30 August 2014. Obes Surg. 2014;24(8):1136–378.
Brethauer SA, Kim J, el Chaar M, Papasavas P, Eisenberg D, Rogers A, et al. Standardized outcomes reporting in metabolic and bariatric surgery. Obes Surg. 2015;25(4):587–606.
Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Univ Ottawa. 2014
Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane handbook for systematic reviews of interventions version 6.2 (updated February 2021) [Internet]. Cochrane; 2021. Available from: www.training.cochrane.org/handbook
Stuck AE, Rubenstein LZ, Wieland D. Bias in meta-analysis detected by a simple, graphical test. Asymmetry detected in funnel plot was probably due to true heterogeneity. BMJ. 1998;316(7129):469.
Biljana M, Jelena M, Branislav J, Milorad R. Bias in meta-analysis and funnel plot asymmetry. Stud Health Technol Inform. 1999;68:323–8.
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Soliman AMS, Lasheen M. Effect of banded laparoscopic sleeve gastrectomy on weight loss maintenance: comparative study between banded and non-banded sleeve on weight loss. Bariatr Surg Pract Patient Care. 2015;10(3):99–104.
Tognoni V, Benavoli D, Bianciardi E, Perrone F, Ippoliti S, Gaspari A, et al. Laparoscopic sleeve gastrectomy versus laparoscopic banded sleeve gastrectomy: first prospective pilot randomized study. Gastroenterol Res Pract. 2016;2016:1–5.
Fink JM, Hoffmann N, Kuesters S, Seifert G, Laessle C, Glatz T, et al. Banding the sleeve improves weight loss in midterm follow-up. Obes Surg. 2017;27(4):1098–103.
Lemmens L, Van Den Bossche J, Zaveri H, Surve A. Banded sleeve gastrectomy: better long-term results? A long-term cohort study until 5 years follow-up in obese and superobese patients. Obes Surg. 2018;28(9):2687–95.
Bhandari M, Mathur W, Kosta S, Mishra AK, Cummings DE. Banded versus nonbanded laparoscopic sleeve gastrectomy: 5-year outcomes. Surg Obes Relat Dis. 2019;15(9):1431–8.
Fink JM, von Pigenot A, Seifert G, Laessle C, Fichtner-Feigl S, Marjanovic G. Banded versus nonbanded sleeve gastrectomy: 5-year results of a matched-pair analysis. Surg Obes Relat Dis. 2019;15(8):1233–8.
Fink JM, Hetzenecker A, Seifert G, Runkel M, Laessle C, Fichtner-Feigl S, et al. Banded versus nonbanded sleeve gastrectomy. Ann Surg. 2020;272(5):690–5.
Gentileschi P, Bianciardi E, Siragusa L, Tognoni V, Benavoli D, D’Ugo S. Banded sleeve gastrectomy improves weight loss compared to nonbanded sleeve: midterm results from a prospective randomized study. J Obes. 2020;1(2020):1–7.
Bhandari M, Ponce de Leon-Ballesteros G, Kosta S, Bhandari M, Humes T, Mathur W, et al. Surgery in patients with super obesity: medium-term follow-up outcomes at a high-volume center. Obesity. 2019;27(10).
Clapp B, Wynn M, Martyn C, Foster C, O’Dell M, Tyroch A. Long term (7 or more years) outcomes of the sleeve gastrectomy: a meta-analysis. Surg Obes Relat Dis. 2018;14(6):741–7.
Nedelcu M, Noel P, Iannelli A, Gagner M. Revised sleeve gastrectomy (re-sleeve). Surg Obes Relat Dis. 2015;11(6):1282–8.
Noel P, Nedelcu A, Eddbali I, Gagner M, Danan M, Nedelcu M. Five-year results after resleeve gastrectomy. Surg Obes Relat Dis. 2020;16(9):1186–91.
Fobi MA, Lee H. SILASTIC ring vertical banded gastric bypass for the treatment of obesity: two years of follow-up in 84 patients [corrected]. J Natl Med Assoc. 1994;86(2):125–8.
Fobi MAL. Placement of the GaBP ring system in the banded gastric bypass operation. Obes Surg. 2005;15(8):1196–201.
Salvi P, Kosta S, Fobi M, Bhandari M, Reddy M, Gusani R, et al. Banded sleeve gastrectomy and one anastomosis gastric bypass/mini-gastric bypass for treatment of obesity: a retrospective cohort comparative study with 6 years follow-up. Obes Surg. 2020;30(4):1303–9.
Shoar S, Khorgami Z, Brethauer SA, Aminian A. Banded versus nonbanded Roux-en-Y gastric bypass: a systematic review and meta-analysis of randomized controlled trials. Surg Obes Relat Dis. 2019;15(5):688–95.
Magouliotis DE, Tasiopoulou VS, Svokos KA, Svokos AA, Sioka E, Tzovaras G, et al. Banded vs. non-banded Roux-en-Y gastric bypass for morbid obesity: a systematic review and meta-analysis. Clin Obes. 2018;8(6):424–33.
Buchwald H, Buchwald JN, McGlennon TW. Systematic review and meta-analysis of medium-term outcomes after banded Roux-en-Y gastric bypass. Obes Surg. 2014;24(9):1536–51.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conclusions
According to the results, BSG is a safe and effective procedure with acceptable rates of perioperative and band-related complications. Mid- (3 years) and long-term (5 years) weight loss may be superior in BSG compared to SG but may come at the expense of a higher incidence of upper GI symptoms such as food intolerance, vomiting, and regurgitation. A 6.5-cm band is associated to a higher incidence of upper GI symptoms, in comparison to a band greater than 7 cm.
However, these findings are mainly based on data obtained from observational studies. Further RCT are needed to validate these results, as well as studies with longer follow-up to assess safety and efficacy of BSG at long term.
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent Statements
Non-applicable.
Conflict of Interest
Guillermo Ponce de Leon-Ballesteros has nothing to disclose. Gustavo Romero-Velez has nothing to disclose. Raigam Jafet Martinez-Portilla has nothing to disclose. Xavier Pereira has nothing to disclose. Ivonne Roy-Garcia has nothing to disclose. Mathias AL Fobi has ownership of share in Bariatec Corporation. Miguel F Herrera has nothing to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
- Banded sleeve gastrectomy (BSG) has been proposed as an alternative to SG as it can limit gastric pouch dilation.
- Seven case-control studies and three randomized clinical trials have compared the outcomes between SG and BSG.
- BSG has significant higher %EWL compared to SG.
- BSG showed a higher frequency of upper gastrointestinal symptoms on follow-up on observational studies. According to the RCT, there was no significant difference among groups.
Article Category: Systematic review. No previous presentation in a meeting or to a society.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ponce de Leon-Ballesteros, G., Romero-Velez, G., Martinez-Portilla, R.J. et al. Comparison of Outcomes Between Banded and Non-banded Sleeve Gastrectomy: a Systematic Review and Meta-analysis. OBES SURG 32, 1–12 (2022). https://doi.org/10.1007/s11695-022-06043-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-022-06043-7