Abstract
Background
Laparoscopic adjustable gastric banding (LAGB) is associated with high long-term failure rates, often requiring conversion to an alternative bariatric procedure. The most efficacious procedure after failed LAGB is subject to debate. Our objective was to compare 12-month weight loss following laparoscopic sleeve gastrectomy (LSG) vs. laparoscopic Roux-en-Y gastric bypass (LRYGB) performed for insufficient weight loss or weight regain after LAGB.
Methods
A systematic search was conducted in PubMed and Medline for English language studies comparing weight loss after a conversion surgery for failed gastric banding. We examined studies with patients who had at least 1-year follow-up and included conversions to both LSG and LRYGB. A fixed effects model was created, and variance measures were calculated to measure heterogeneity. Both were analyzed for significance. All statistical analyses were conducted with the “meta” package in R 3.3.2.
Results
The initial search produced 17 studies. Six studies, consisting of 205 LSG and 232 LRYGB patients, met our inclusion criteria and were included in the review. Heterogeneity among studies was high (Q = 23.1; p < 0.001). There was no statistically significant difference in weight loss after 12 months between the groups (p = 0.14).
Conclusions
It remains unknown which conversion procedure is more appropriate to perform after a failed gastric band in order to achieve the highest weight loss potential. In our meta-analysis, there was no difference in weight loss after 12 months in patients who were converted to LSG or LRYGB. Further studies and longer follow-up comparisons are required before firm conclusions can be drawn.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Long-term weight loss with 47% excess weight loss (%EWL) maintained up to 15 years after laparoscopic adjustable gastric band (LAGB) has been reported [1]. However, high long-term weight loss failure and complication rates are also well known [2]. Candidates for a conversion procedure after LAGB include those who have had at least five adjustments without reaching target weight loss in 2 years, pouch enlargement, band slippage, or erosion [3]. As such, 10–60% patients require removal with or without conversion to an alternative bariatric procedure [4, 5]. A long-term retrospective study with follow-up of nearly 14 years showed that the LAGB was removed in 59.4% of the patients and comorbidities were not improved [6]. As such, the current trend for failed weight loss after LAGB is removal and conversion to another procedure. The most efficacious procedure after failed LAGB is subject to debate.
Laparoscopic Roux-en-Y gastric bypass (LRYGB) has traditionally been considered the revisional procedure of choice, as it has had a long history of feasibility and success [7]. The addition of a malabsorptive component may be an important mechanism for weight loss in patients who failed a purely restrictive procedure. LRYGB is associated with significant physiologic and metabolic changes related to gastrointestinal hormones, although the mechanisms underlying its efficacy are far from being completely elucidated [8]. Potential disadvantages of LRYGB include the risks of dumping syndrome, malabsorption, marginal ulceration, and internal hernia [4, 9].
In the recent years, laparoscopic sleeve gastrectomy (LSG) has gained favor as a primary bariatric procedure as it has been shown to provide durable weight loss as well as amelioration of comorbidities [4]. There is evidence that LSG offers not only restrictive properties but also improves glucose metabolism with neurohormonal changes [10, 11]. As a primary procedure, LSG has shown an almost equivalent weight loss potential to LRYGB and possibly a better safety profile [12]. Due to its success, LSG has been increasingly utilized as a conversion procedure following failed gastric band [13].
Literature comparing weight loss efficacy between LRYGB and LSG is scant, especially in long-term follow-up. Therefore, we conducted a meta-analysis of the published literature to assess and compare 12-month weight loss following LSG vs. LRYGB performed for inadequate weight loss after LAGB.
Methods
Search Strategy and Article Selection
A systematic search was conducted in PubMed and Medline (February 2016 to June 2016) for studies using the following terms in every possible combination: “failed weight loss or complications of laparoscopic gastric band,” “revision or conversion surgery after failed gastric band,” and “laparoscopic sleeve gastrectomy versus laparoscopic roux-en-y gastric bypass for failed gastric band.” Inclusion criteria were studies (1) written in the English language, (2) with at least 1-year follow-up and including both conversion LSG and LRYGB study groups, and (3) with efficacy of weight loss defined in percent excess weight loss (%EWL). One- or two-stage conversion procedures were included. Studies that included conversion procedure other than LSG or LRYGB, i.e., biliopancreatic diversion with duodenal switch (BPDDS), were excluded.
Data Extraction
For each eligible study, the following data were extracted: demographics, procedure, and weight loss (%EWL at 12 months).
Statistical Analysis
All statistical analyses were conducted with the “meta” package in R 3.3.2 [14]. Heterogeneity was examined by calculating T2 (an estimate of τ2), Cochran’s Q, and I2. Cochran’s Q was tested for significance. Given the small number of studies included, a fixed effects model was created and tested for significance.
Results
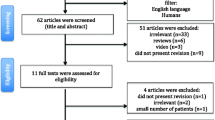
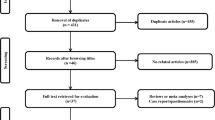
The flow diagram of the literature search is shown in Fig. 1. The initial search produced 23 studies. Six studies were excluded because they only included LSG as a conversion procedure. Nine studies were excluded as they only included LRYGB as a conversion procedure. The remaining eight were assessed for eligibility. One study was removed as it was a systematic review. Another article was excluded because it did not report a 12-month follow-up. Six retrospective studies, consisting of 205 LSG and 232 LRYGB patients, met our inclusion criteria and were included in the review. See Table 1 for demographic details.
Percent excess weight loss (%EWL) at 12 months was utilized from each included study for the meta-analysis (Fig. 2). Only two studies [9, 15] reported %EWL beyond 12 months, and thus, there was not enough data to analyze. Conversion procedures were included regardless of whether they were performed in one or two stages. Where available, the stated reasons for the respective conversion procedure are presented in Table 2. Only one study [15] demonstrated LRYGB to be more efficacious in %EWL with statistical significance while another [3] showed a trend toward a better %EWL but without statistical significance. The four remaining reports [4, 9, 16, 17] revealed no statistical difference between the two.
Statistical measures of heterogeneity among studies reflected those varying results. Cochran’s Q (23.1) was significant (p < 0.001), indicating that the effects across studies vary. With an I2 of 87% (95% CI: 68.8%–94.6%), it appears that most of the differences among studies reflect real, not random, differences in effects. Likewise, our estimate of τ2 (T2) is 234.0, indicating that the within-study variation does not account for the differences we see between studies.
Analysis of the fixed effects model (Fig. 3) revealed no statistically significant difference in weight loss after 12 months between groups (p = 0.14). All statistical analyses were conducted with the meta package in R 3.3.2 [14].
Discussion
Because of insufficient weight loss and/or complications, a significant number of patients require gastric band removal or conversion to another procedure. It is controversial which conversion bariatric procedure should be performed in non-responders to gastric banding. Proponents of LRYGB argue that these patients failed a restrictive procedure, and therefore, the conversion procedure should include a malabsorptive component [15]. On the other hand, LSG has rapidly gained acceptance as primary as well as secondary bariatric procedure for several reasons. LSG does not alter intestinal continuity and conveys a lower risk of vitamin and mineral deficiencies [18]. In addition, compared to LRYGB, there is an elimination of dumping syndrome, malabsorption, marginal ulceration, and internal hernia [15]. A potential disadvantage of LSG is the reported higher risk of gastric-esophageal junction leak derived from stapling in scarred tissue and the compromise of vascular supply during left crus dissection [15]. However, additional literature reports similar leak rates between LSG and LRYGB [4].
In contrast to our study, the systematic review of revisional surgery after failed gastric band conducted by Elnahas et al. [5] concluded that LRYGB or BPDDS were superior to LSG in weight loss efficacy. However, a meta-analysis was not conducted in their study due to significant variation in study design and outcome measurement. As a unique strength of our study, a single measurement methodology of weight loss (%EWL), common to the six included studies, was used for analysis. The meta-analysis revealed that neither conversion LSG or LRYGB has an advantage over the other in terms of weight loss, and that both procedures result in successful weight loss after 1 year.
Our investigation highlights the complexity of weight loss following metabolic and bariatric procedures and the fact that its physiology is beyond restriction vs. malabsorption. Recent research has revealed numerous hormonal changes affected by LSG, elevating this procedure beyond its mere restrictive property [8, 10, 19]. A study of LSG and LRYGB demonstrated that both procedures affect glucose and insulin sensitivity as well as glucagon and GLP-1 levels [20]. These similarities suggest the viability of either procedure to address insufficient weight loss after gastric band.
Several limitations of the current meta-analysis exist. Long-term follow-up data were lacking in the studies examined; therefore, only a 12-month %EWL was used as the outcome. In the majority of the studies, the percent of patients who returned for follow-up decreased over time; this may be due to the fact that patients are coming from other states or countries to visit major bariatric centers where their surgery was performed [15].
In addition, patient differences complicate the meta-analysis. For example, in one study [16], the mean BMI for patients undergoing conversion LSG was significantly lower than those who underwent LRYGB. In contrast, in Gonzalez-Heredia et al.’s study [3], LSG was more likely to be performed for patients with BMI > 60. There appears to be a trend that patients with GERD or diabetes underwent LRYGB; however, this difference was not quantified or consistent in all the studies. Table 2 highlights the various reasons why a certain conversion procedure was selected. Patient selection to each conversion procedure was not controlled and may have contributed to the heterogeneity we found in the studies. Alternatively, it may be that the heterogeneity merely reflects that LSG may be better for patients with certain characteristics, whereas LRYGB may be more beneficial for others. Future research should try to determine whether this is the case, and if so, which characteristics are associated with better outcomes for LSG, and which with LRYGB.
In another study [9], a higher number of patients underwent conversion to LSG as a staged procedure compared to LRYGB. While weight loss failure may be the most common reason, this was not the sole reason for conversion in the studies included in our meta-analysis. This finding reflects the other indications for band removal, i.e., band erosion, slippage, etc. Our study was not able to isolate these patients for exclusion. Nevertheless, weight loss occurred following conversion, as would be expected, and was tracked and reported in these studies.
Lastly, all study designs were retrospective, single cohort, and without control groups. It remains unknown which conversion procedure is more appropriate to perform after a failed gastric band in order to achieve the highest weight loss potential in the long term. In our meta-analysis, there was true heterogeneity in outcomes among the studies and no difference in weight loss after 12 months in patients who were converted to LSG or LRYGB. The overall condition and comorbidities of the patient are crucial factors in selection of the most successful procedure. Diabetes and symptomatic reflux disease should likely sway the clinician’s recommendation toward LRYGB, while having significant intraabdominal adhesions or Crohn’s disease, etc., might influence the surgeon to perform a LSG. It is possible that patient characteristics drove the differences in effects that we saw among studies.
The data presented in our study demonstrated that there was no difference in weight loss outcomes in the short term (12 months) between conversion LSG and LRYGB performed to address inadequate weight loss following LAGB. Further longer follow-up studies should focus solely on weight loss failure after gastric band placement, excluding other indications for band removal. An analysis of a larger database, such as the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MSAQIP), or a randomized prospective trial through a multi-institutional effort will be necessary to elucidate the ideal procedure to perform after failed LAGB.
References
Himpens J, Cadière G, Bazi M, et al. Long-term outcomes of laparoscopic adjustable gastric banding. Arch Surg. 2011;146(7):802–7. https://doi.org/10.1001/archsurg.2011.45.
O’Brien PE, MacDonald L, Anderson M, et al. Long-term outcomes after bariatric surgery: fifteen-year follow-up of adjustable gastric banding and a systematic review of the bariatric surgical literature. Ann Surg. 2013;257(1):87. http://www.ncbi.nlm.nih.gov/pubmed/23235396–94.
Gonzalez-Heredia R, Masrur M, Patton K, et al. Revisions after failed gastric band: sleeve gastrectomy and roux-en-Y gastric bypass. Surg Endosc. 2015;29(9):2533–7. https://doi.org/10.1007/s00464-014-3995-7. http://www.ncbi.nlm.nih.gov/pubmed/25427419
Yeung L, Durkan B, Barrett A, et al. Single-stage revision from gastric band to gastric bypass or sleeve gastrectomy: 6- and 12-month outcomes. Surg Endosc. 2016;30(6):2244–50. https://doi.org/10.1007/s00464-015-4498-x. http://www.ncbi.nlm.nih.gov/pubmed/26335074
Elnahas A, Graybiel K, Farrokhyar F, et al. Revisional surgery after failed laparoscopic adjustable gastric banding: a systematic review. Surg Endosc. 2013;27(3):740–5. https://doi.org/10.1007/s00464-012-2510-2. http://www.ncbi.nlm.nih.gov/pubmed/22936440
Froylich D, Abramovich-Segal T, Pascal G, et al. Long-term (over 10 years) retrospective follow-up of laparoscopic adjustable gastric banding. Obes Surg. 2018;28(4):976–80. https://doi.org/10.1007/s11695-017-2952-7. https://search.proquest.com/docview/1966294282
Aarts E, Koehestanie P, Dogan K, et al. Revisional surgery after failed gastric banding: results of one-stage conversion to RYGB in 195 patients. Surg Obes Relat Dis. 2014;10(6):1077–83. https://doi.org/10.1016/j.soard.2014.07.006. http://www.ncbi.nlm.nih.gov/pubmed/25443075
Holst JJ, Madsbad S, Bojsen-Møller KN, et al. Mechanisms in bariatric surgery: gut hormones, diabetes resolution, and weight loss. Surg Obes Relat Dis. 2018;14(5):708–14. https://doi.org/10.1016/j.soard.2018.03.003. https://www.sciencedirect.com/science/article/pii/S1550728918301242
Carr WRJ, Jennings NA, Boyle M, et al. A retrospective comparison of early results of conversion of failed gastric banding to sleeve gastrectomy or gastric bypass. Surg Obes Relat Dis. 2015;11(2):379–84. https://doi.org/10.1016/j.soard.2014.07.021. http://www.ncbi.nlm.nih.gov/pubmed/25443072.
Dimitriadis E, Daskalakis M, Kampa M, et al. Alterations in gut hormones after laparoscopic sleeve gastrectomy: a prospective clinical and laboratory investigational study. Ann Surg. 2013;257(4):647–54. https://doi.org/10.1097/SLA.0b013e31826e1846. https://www.ncbi.nlm.nih.gov/pubmed/23108120
Anderson B, Switzer N, Almamar A, et al. The impact of laparoscopic sleeve gastrectomy on plasma ghrelin levels: a systematic review. Obes Surg. 2013;23(9):1476–80. https://doi.org/10.1007/s11695-013-0999-7. http://www.ncbi.nlm.nih.gov/pubmed/23794092
Acholonu E, McBean E, Court I, et al. Safety and short-term outcomes of laparoscopic sleeve gastrectomy as a revisional approach for failed laparoscopic adjustable gastric banding in the treatment of morbid obesity. Obes Surg. 2009;19(12):1612–6. https://doi.org/10.1007/s11695-009-9941-4. http://www.ncbi.nlm.nih.gov/pubmed/19711138
Goitein D, Feigin A, Segal-Lieberman G, et al. Laparoscopic sleeve gastrectomy as a revisional option after gastric band failure. Surg Endosc. 2011;25(8):2626–30. https://doi.org/10.1007/s00464-011-1615-3. http://www.ncbi.nlm.nih.gov/pubmed/21416182
Schwarzer G, Carpenter JR, Rücker G. Meta-analysis with R. Cham: Springer Verlag; 2015. https://doi.org/10.1007/978-3-319-21416-0. http://lib.myilibrary.com?ID=840322
Marin-Perez P, Betancourt A, Lamota M, et al. Outcomes after laparoscopic conversion of failed adjustable gastric banding to sleeve gastrectomy or roux-en-Y gastric bypass. Br J Surg. 2014;101(3):254–60. https://doi.org/10.1002/bjs.9344. http://onlinelibrary.wiley.com/doi/10.1002/bjs.9344/abstract
Khoursheed M, Al-Bader I, Mouzannar A, et al. Sleeve gastrectomy or gastric bypass as revisional bariatric procedures: retrospective evaluation of outcomes. Surg Endosc. 2013;27(11):4277–83. https://doi.org/10.1007/s00464-013-3038-9. http://www.ncbi.nlm.nih.gov/pubmed/23756590
Liu K, Diana M, Vix M, et al. Revisional surgery after failed adjustable gastric banding: institutional experience with 90 consecutive cases. Surg Endosc. 2013;27(11):4044–8. https://doi.org/10.1007/s00464-013-3056-7. http://www.ncbi.nlm.nih.gov/pubmed/23836121
Al Sharqawi N, Al Sabah S, Al Mulla A, et al. Conversional surgery: single-step conversion of laparoscopic adjustable gastric band to laparoscopic sleeve gastrectomy. Obes Surg. 2014;24(10):1808–11. https://doi.org/10.1007/s11695-014-1358-z. http://www.ncbi.nlm.nih.gov/pubmed/25005810
Palikhe G, Gupta R, Behera B, et al. Efficacy of laparoscopic sleeve gastrectomy and intensive medical management in obese patients with type 2 diabetes mellitus. Obes Surg. 2014;24(4):529–35. https://doi.org/10.1007/s11695-013-1114-9. http://www.ncbi.nlm.nih.gov/pubmed/24272885
Nannipieri M, Baldi S, Mari A, et al. Roux-en-Y gastric bypass and sleeve gastrectomy: mechanisms of diabetes remission and role of gut hormones. J Clin Endocrinol Metabol. 2013;98(11):4391–9. https://doi.org/10.1210/jc.2013-2538.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Informed consent was not a necessary component of this study.
This article does not contain any studies with human participants or animals performed by any of the author.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhou, R., Poirier, J., Torquati, A. et al. Short-Term Outcomes of Conversion of Failed Gastric Banding to Laparoscopic Sleeve Gastrectomy or Roux-En-Y Gastric Bypass: a Meta-Analysis. OBES SURG 29, 420–425 (2019). https://doi.org/10.1007/s11695-018-3538-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-018-3538-8