Abstract
Purpose
Bariatric surgery is a treatment option for those affected by severe obesity. This study investigated changes in gut microbiota and serum biomarkers after laparoscopic sleeve gastrectomy (LSG).
Materials and Methods
A total of 126 patients with morbid obesity who underwent LSG were enrolled in this study. Routine biochemical tests, hormonal (insulin and glucagon), and cytokine levels (IL-6, IL-1β, TNF-α, IL-10, and TGF–β 1) were measured, in addition, real-time PCR (quantitative PCR, qPCR) quantitated gut microbiota. All the parameters were measured pre-operatively, 3, and 12 months post-surgery (F0, F3, and F12, respectively).
Results
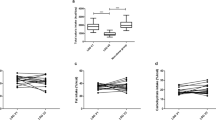
At F3, the level of FBS, HbA1c, HOMA-IR, triglyceride, cholesterol, LDL, BUN, creatinine, urea, SGOT, SGPT, IL-1β, IL-6, IFNγ, insulin, glucagon, the abundance of Prevotella and Bacteroides fragilis group, as well as the concentration of Firmicutes spp. showed significant decrease (P < 0.01), and HDL level, Akkermansia muciniphila and Roseburia spp. abundance, and Bacteroidetes and Bifidobacterium spp. concentration showed significant increase (P < 0.0001). The observed pattern continued or remained stable at F12 for all of these variables. IL-10 and TGF-β1 remained unchanged until F3 and showed a significant drop at F12. At F3, Clostridium cluster IV increased significantly and remained at that level afterward. Moreover, concentration of Phylum Actinobacteria showed an initial drop at F3 and a later increase at F12 (P < 0.0001).
Conclusion
LSG is associated with a significant improvement in serum biomarkers, as well as significant changes in fecal microbiota. Future systems biology analyses would shed more light on the underlying interactions of these parameters, and could help in developing novel diagnostic and therapeutic strategies for obesity management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is a complex health problem resulting from a combination of environmental and genetic factors [1, 2]. Obesity is associated with a number of medical complications such as diabetes mellitus, cardiovascular diseases, hypertension, and cancer [1]. According to the recent estimates, overweight and obesity are among the five major risk factors for human health, and at least 2.8 million adults die each year due to obesity epidemic [3]. Although obesity is largely preventable through physical activity, diet change, behavior therapy, and pharmacotherapy, the bariatric surgery continues to be an option for the management of morbid obesity when the abovementioned interventions fail to solve the problem [4].
Laparoscopic sleeve gastrectomy (LSG) is an innovative surgical approach for the management of morbid obesity [5]. It is been reported that LSG can reduce excess body weight and caloric intake up to 50–70% [6]. Other mechanisms, including changes in gastrointestinal hormone, alteration in energy expenditure, and regulation of the gut microbiota (GM), are thought to contribute to the post-surgery benefits [6].
The human intestinal tract is the natural habitat of a diverse and complex microbial community that plays a key role in maintaining host body homeostasis [2, 6]. Animal and human studies revealed that the GM is a pivotal factor in energy extraction from food and host metabolic pathways, which are interrelated and are responsible for the development of metabolic disorders such as obesity [7, 8].
Although a number of studies have sought to characterize alterations in GM [9, 10] and serum biomarkers [5, 6, 10] after weight loss surgery, there are several factors that can affect these parameters that are not fully addressed yet, including genetics, diet, age, child birth method (for women), certain medications, especially antibiotics and probiotics [11]. Many of these factors are inter-related, and should be considered in a holistic manner. Also, there are few studies that have followed these variations for a long period of time, with no studies being conducted in the Iranian population. So, additional research is needed to determine variations in the GM and serum biomarkers, while adjusting for the role of other influencing factors.
Therefore, the aim of this study was to analyze the specific changes in GM composition and serum biomarkers that may be caused by LSG in patients with morbid obesity over a 1-year follow-up.
Materials and Methods
Participants
Morbidly obese patients (N = 126), who underwent LSG at The Department of Surgery in Shariati Educational Hospital (Tehran, Iran) from September 2018 to January 2020 were included in this longitudinal study. Each subject was provided with a standardized questionnaire regarding sociodemographic, anthropometric, lifestyle, and underlying medical history. Also, all patients were prescribed at least a 2-week pre-operative, strict calorie controlled diet.
The inclusion criteria were [1] meeting the National Institute of Health and Care Excellence guidelines for weight loss surgery [12]; [2]: age 30 to 50 years; [3] BMI > 40 kg/m2 or > 35 kg/m2 with obesity-related complications; [4] without dairy allergy; [5] no infectious disease; [6] no use of medication or dietary supplements affecting the findings during the follow-up or 6 months prior to the study. Moreover, the exclusion criteria were [1] recent illnesses; [2] genetic or psychotic disorders; and [3] drug abuse.
The Research Ethics Committee of the Pasteur Institute of Iran, Tehran, approved this study (Approval ID: IR.PII. REC.1397.029).
Stool Samples and DNA Extraction
Stool samples were collected in a sterile container and stored at − 80 °C until DNA extraction. DNA was extracted from stool samples using the DNA purification Kit (QIAGEN, GmbH, Hilden, Germany) following the manufacturer’s instructions. Purity of the extracted DNA was measured using a Nanodrop spectrophotometer (BioTek Instruments, Inc., Winooski, VT, USA) and stored at − 20 °C until processed.
Characterization of the GM by qPCR
The abundance of the bacterial communities in stool samples was evaluated using absolute qPCR (StepOne™ Real-Time PCR System, Applied Biosystems, USA) with 16S rRNA gene-based specific primers for phylum and species (Metabion, Germany, Table 1). Amplification reactions were carried out in a total volume of 20 μl containing 10 μL 2× QPCR Green Master Mix HRox (Biotechrabbit GmbH, Hennigsdorf, Germany), 1 μL of each primer (5 μM) and 2 μL of target DNA. The qPCR temperature cycling conditions were as follows: one cycle of initial denaturation at 95 °C for 3 min, 40 cycles of 95 °C for 15 s, and annealing at specific temperature for 30 s (Table 1). Standard curves were made using tenfold serial dilutions of Phylum Proteobacteria genomic DNA and then, the number of copies of amplified 16S rRNA for each species determined using the following equation:
Number of copies = (DNA concentration (ng/μl) × [6.022 × 1023]) / (length of template (bp) × [1 × 109] × 650).
Blood Biochemical and Cytokine Parameters
Biochemistry and cytokine parameters were detected from all patients at each time point after an overnight fast. Routine biochemical parameters including cholesterol (Chol), high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglycerides (Tg), fasting blood sugar (FBS), aspartate aminotransferase (SGOT), alanine aminotransferase (SGPT), blood urea nitrogen (BUN), creatinine (CRE), urea, and alkaline phosphatase (ALP) were determined using a standard autoanalyzer method (COBAS MIRA® Plus). Glycated hemoglobin (HbA1c) was measured on whole blood samples with an immunoturbidimetric assay (Hitachi 917). To quantify insulin resistance and beta cell function, the homeostasis model assessment insulin resistance (HOMA-IR) index was calculated using the formula: fasting insulin (μU/mL) × fasting glucose (mmol/L) / 22.5.
The serum concentrations of insulin, glucagon, inflammatory cytokines (IL-6, IL-1β, and TNF-α), and anti-inflammatory cytokines (IL-10 and TGF–β 1) were determined using commercially quantitative enzyme-linked immunosorbent assay (ELISA) kits (Abcam, Cambridge, UK).
Each measure was determined in duplicate and the median of the two measurements was reported.
Statistical Analysis
Continuous variables were summarized using median (min–max) or mean ± standard deviation. Categorical variables were described as number (n) and percentage (%). Changes in biochemical factors, cytokines, and gut microbiota were evaluated over the 1-year follow-up using Repeated Measure ANOVA test. Given the trend was statistically significant for all of these variables (except BUN, creatinine, and urea), the trend for each variable over follow-up time was visually inspected. We also performed paired t test to assess changes before and 3 months after surgery as well as 3 and 12 post-surgery. The level of significance was defined as P < 0.05. data was analyzed in Stata software (v. 14).
Results
Characteristics of Participants
A total of 126 patients with morbid obesity (96 females and 30 males) who were candidate for LSG were finally included in this study. The mean age was 37.3 ± 6.3. Most of the participants (93.7%) were urban residents, married (68.3%), and had educational training beyond 12 years (64.29%). Frequency of type 2 diabetes and hypothyroidism were 12.7% and 20.6%, respectively. Frequency of major gastrointestinal disorders was below 10% (9.5% for stomach ulcer caused by H. pylori, 9% for fatty liver, and 8.7% for gallstone history). Considering participants’ pre-operative diet and life-style, majority of them (70%) reported fast food consumption, 40% reported caffeine consumption, 32.5% reported current tobacco use, and 19.1% reported current alcohol consumption (Table 2).
Clinical and Biomarker Analysis
Preoperative weight and BMI were 121.4 ± 23.9 kg and 43.27 ± 5.4 kg/m2, respectively.
At F3, weight and BMI were 94.96 ± 19.68 kg and 33.88 ± 5.12 kg/m 2, respectively. Also, the level of all biochemical indices measured in this study (except ALP) was significantly improved (P < 0.01), with FBS, HbA1c, HOMA-IR, triglyceride, cholesterol, LDL, BUN, creatinine, urea, SGOT, and SGPT showing significant decrease and HDL showing significant increase. These improvements continued over the 12-month follow-up period, and the changing patterns were great enough to be statistically significant for all mentioned variables, except for LDL, BUN, urea, and ALP (Table 3).
At F12, weight and BMI were 82.51 ± 15.77 kg and 29.54 ± 4.42 kg/m2, respectively. Three cytokines including IL-1β, IL-6, and IFNγ showed significant decrease after LSG surgery over the 12-month follow-up period.
IL-10 remained unchanged 3 months after surgery, but in month 12, it showed a dramatic drop which was also statistically significant (P < 0.0001). TGF-β 1 showed more unstability, which was evident by 380.6 units increase in month 3 after surgery compared to the pre-operation value, and a dramatic decrease in month 12 after surgery (2073.8 units increase in month 12 vs. month 3 after surgery, P < 0.0001, Table 3).
Both insulin and glucagon showed gradual significant decrease over months 3 and 12 after surgery (P < 0.0001, Table 3).
Fecal Microbiota Analysis
Changes in fecal microbiota before, 3, and 12 months after LSG are shown in Table 4. The abundance of Akkermansia muciniphila and Roseburia spp. showed significant increase over months 3 and 12 after surgery. The same pattern of change was also observed in the concentration of Bacteroidetes and Bifidobacterium spp. (P < 0.0001). On the other hand, the abundance of Prevotella and Bacteroides fragilis group as well as the concentration of Firmicutes spp. showed significant decrease in months 3 and 12 after surgery (P < 0.0001). This was also observed for the Firmicutes/Bifidobacterium ratio (P < 0.0001). The abundance of Clostridium cluster IV significantly increased in month 3 and remained unchanged in month 12. Also, the concentration of phylum Actinobacteria showed a significant decrease in month 3 (compared to pre-operation time), and a subsequent significant increase in month 12 post-surgery when compared to month 3 after surgery (P < 0.0001).
Discussion
Recent evidence suggests that GM and serum biomarkers play vital role not only in the maintenance of host health, but also in the development of several metabolic disorders, including obesity-related diseases [7, 8]. Weight loss surgery modifies the GM and serum biomarkers [6, 9, 10]; however, major alterations in these parameters have not been surveyed in detail.
The results from the sociodemographic and clinical questionnaire before LSG revealed hypothyroidism, fatty liver, and diabetes were the most common comorbidities associated with obesity. Present findings are consistent with previous studies [18, 19].
Our results in agreement with previous findings [6, 10] revealed a significant weight loss and metabolic improvement after LSG. In the same context, glucose, insulin, glucagon, HbA1c, and HOMA-IR were significantly decreased after surgery. These changes could be explained by the weight loss and the improvement of insulin sensitivity. Furthermore, lipids were also significantly affected by the LSG. In this regard, Tg, Chol, and LDL were significantly lower postoperatively, while HDL was higher, indicating regulation of lipid profile, probably connected with the LSG. In particular, our data showed that ALP transitory increased at F3 and decreased at F12, after LSG. Similar results were reported previously in two studies [20, 21]; this is a topic for further investigation and analysis. In addition, our findings suggest that the decreased levels of AST and ALT after LSG improve the liver function of patients with morbid obesity. However, there were no significant differences with respect to biomarkers of renal function (BUN and urea) over the 12-month follow-up period. At F3 and F12, 6% and 57% of patients lost more than 30% excess weight loss, respectively. At F12, more than of patients suffering from obesity-related disease had a remission, and among subjects with HbA1c > 6%, remission of type 2 diabetes mellitus was recorded in 8.73% of cases after LSG. Based on our results, a significant decrease in cytokine levels occurred after sleeve gastrectomy. This could be probably due to a reduction in the adipose tissue macrophages, which are a source of pro- and anti-inflammatory cytokines [5].
In the present study, the abundance of several major phyla and species increased after surgery. The total numbers of GM were significantly lower in patients with morbid obesity before LSG despite the fact that some bacterial were more abundant. The positive effect of the LSG could be explained by the proliferation of some beneficial intestinal flora after surgery [22]. In our samples, a high level of Bacteroidetes, Bifidobacterium, Akkermansia muciniphila, Roseburia spp., and Clostridium cluster IV appeared at F3 and remained high at F12. These bacteria are strongly associated with healthy metabolic status and negatively correlated with variables including BMI, glucose, leptin, triglycerides, insulin, and inflammatory markers [23,24,25,26]. Moreover, our findings confirm those previous studies showing that the Firmicutes/ Bacteroidetes ratio could be considered as a predictor of gut dysbiosis in patients with morbid obesity [27, 28]. A possible explanation for our findings is that Firmicutes are more efficient in harvesting energy from food than Bacteroidetes, thus promoting more absorption of calories and following weight gain [27]. Previous studies in humans and animal models have shown differences in GM composition after bariatric surgery [6, 9, 22]. These differences in abundance could be due to several factors, including study design, type of surgery, methodology, and subject characteristics such as ethnicity, age, and diet. In fact, our findings show the weight loss induced by LSG and the changes in life style affecting the GM abundance. Now, it has not yet been clarified whether the bacterial modification induces beneficial or harmful long-term effects on health state.
To date, this study has the biggest sample size showing GM changes, and a thorough analysis of the biochemical and cytokine parameters in patients undergoing LSG.
Conclusion
In conclusion, this study suggests that the LSG led to significant improvement in glucose, insulin, lipids, and many other metabolic and hormonal. Moreover, a reduction of potential pathogens and the onset of beneficial bacteria after LSG were recorded. However, further studies to assess the host metabolic-microbial cross talk after LSG may help us to get novel insights into new therapeutic strategies for obesity.
Limitations
Our study has several limitations. First, the relationship between the clinical impact of intestinal microbiota and host response is still unclear. Second, fecal microbiota analysis is limited to nine 16S rRNA-gene-targeted specific primers and cannot provide comprehensive results on the role of LSG on the GM alterations. Third, our questionnaires, as well as data on sociodemographic were collected on oral report and are thus prone to response biases.
Confirmation Statement
Each listed author is submitting the paper in their own personal, professional capacity, and are not employees for an US-sanctioned government.
Data Availability
All data generated or analyzed in this study are included in the present article.
References
Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism. 2019;92:6–10.
Sivamaruthi BS, Kesika P, Suganthy N, et al. A review on role of microbiome in obesity and antiobesity properties of probiotic supplements. Biomed Res Int. 2019;2019:1–20.
Organization WH. Obesity and overweight. Fact sheet no. 311. Updated January 2015. World Health Organization [Cited: 2015 November 20] Available from: http://www.who.int/mediacentre/factsheets/fs311/en 2015.
Hruby A, Hu FB. The epidemiology of obesity: a big picture. Pharmacoeconomics. 2015;33(7):673–89.
Hady HR, Olszewska M, Czerniawski M, Groth D, Diemieszczyk I, Pawluszewicz P, Kretowski A, Ladny JR, Dadan J. Different surgical approaches in laparoscopic sleeve gastrectomy and their influence on metabolic syndrome: a retrospective study. Medicine. 2018;97(4).
Tabasi M, Ashrafian F, Khezerloo JK, et al. Changes in gut microbiota and hormones after bariatric surgery: a bench-to-bedside review. Obes Surg. 2019;29(5):1663–74.
Rosenbaum M, Knight R, Leibel RL. The gut microbiota in human energy homeostasis and obesity. Trends in Endocrinology & Metabolism. 2015;26(9):493–501.
Rajoka MSR, Shi J, Mehwish HM, et al. Interaction between diet composition and gut microbiota and its impact on gastrointestinal tract health. Food Sci Human Wellness. 2017;6(3):121–30.
Graessler J, Qin Y, Zhong H, et al. Metagenomic sequencing of the human gut microbiome before and after bariatric surgery in obese patients with type 2 diabetes: correlation with inflammatory and metabolic parameters. The pharmacogenomics journal. 2013;13(6):514–22.
Magouliotis DE, Tasiopoulou VS, Sioka E, et al. Impact of bariatric surgery on metabolic and gut microbiota profile: a systematic review and meta-analysis. Obes Surg. 2017;27(5):1345–57.
Hasan N, Yang H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ. 2019;7:e7502.
Owen-Smith A, Kipping R, Donovan J, et al. A NICE example? Variation in provision of bariatric surgery in England. Bmj. 2013;346:f2453.
Bergström A, Licht TR, Wilcks A, et al. Introducing GUt low-density array (GULDA)–a validated approach for qPCR-based intestinal microbial community analysis. FEMS Microbiol Lett. 2012;337(1):38–47.
Matsuki T, Watanabe K, Fujimoto J, et al. Use of 16S rRNA gene-targeted group-specific primers for real-time PCR analysis of predominant bacteria in human feces. Appl Environ Microbiol. 2004;70(12):7220–8.
Hermann-Bank ML, Skovgaard K, Stockmarr A, et al. The gut microbiota assay: a high-throughput qPCR approach combinable with next generation sequencing to study gut microbial diversity. BMC Genomics. 2013;14(1):788.
Koliada A, Syzenko G, Moseiko V, et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017;17(1):120.
Queipo-Ortuño MI, Seoane LM, Murri M, et al. Gut microbiota composition in male rat models under different nutritional status and physical activity and its association with serum leptin and ghrelin levels. PLoS One. 2013;8(5):e65465.
Schelbert KB. Comorbidities of obesity. Prim Care. 2009;36(2):271–85.
Webber J. The comorbidities of obesity. Practical Diabetes International. 2001;18(8):293–6.
Ruiz-Tovar J, Oller I, Priego P, et al. Short-and mid-term changes in bone mineral density after laparoscopic sleeve gastrectomy. Obes Surg. 2013;23(7):861–6.
Mihmanli M, Isil RG, Isil CT, et al. Effects of laparoscopic sleeve gastrectomy on parathyroid hormone, vitamin D, calcium, phosphorus, and albumin levels. Obes Surg. 2017;27(12):3149–55.
Anhê FF, Varin TV, Schertzer JD, et al. The gut microbiota as a mediator of metabolic benefits after bariatric surgery. Can J Diabetes. 2017;41(4):439–47.
Johnson EL, Heaver SL, Walters WA, et al. Microbiome and metabolic disease: revisiting the bacterial phylum Bacteroidetes. J Mol Med. 2017;95(1):1–8.
Reyed M. The role of bifidobacteria in health. Res J Med Med Sci. 2007;2(1):14–24.
Schneeberger M, Everard A, Gómez-Valadés AG, et al. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci Rep. 2015;5:16643.
Tamanai-Shacoori Z, Smida I, Bousarghin L, et al. Roseburia spp.: a marker of health? Future Microbiol. 2017;12(2):157–70.
Koliada A, Syzenko G, Moseiko V, et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017;17(1):1–6.
Miranda VP, dos Santos Amorim PR, Bastos RR, de Faria ER, de Castro Moreira ME, do Carmo Castro Franceschini S, do Carmo Gouveia Peluzio M, de Luces Fortes Ferreira CL, Priore SE. Abundance of gut microbiota, concentration of short-Chain fatty acids, and inflammatory markers associated with elevated body fat, overweight, and obesity in female adolescents. Mediators of Inflammation. 2019.
Acknowledgments
This article was part of the project conducted by Mohsen Tabasi to fulfill the requirement for a Ph.D. degree. We would like to express our gratitude to the Pasteur Institute of Iran and Legal Medicine Research Center for providing financial support. We also would like to express our appreciation to Dr. Nader Shahrokhi, Dr. Sara Ahmadi Badi, Mr. Milad Kheirvari, Mr. Mohammad Reza Yazdannasab, and staff in Shariati Hospital of Tehran, Iran for their technical assistance.
Funding
This project was financially supported by the Pasteur Institute of Iran (grant no. TP-9567).
Author information
Authors and Affiliations
Contributions
MT and SE design the study and data analysis. SDS and FE data: collecting and writing paper. ARS and SB: conceptualization, investigation, formal analysis, writing-review and editing. All co-authors commented on the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
This study was conducted in accordance with the principles of the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All the participants were thoroughly informed about the study and procedures before signing consent forms. Participants were assured of anonymity and confidentiality. The Research Ethics Committee of the Pasteur Institute of Iran, Tehran, approved this study (IR.PII. REC.1397.029).
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tabasi, M., Eybpoosh, S., Siadat, S.D. et al. Modulation of the Gut Microbiota and Serum Biomarkers After Laparoscopic Sleeve Gastrectomy: a 1-Year Follow-Up Study. OBES SURG 31, 1949–1956 (2021). https://doi.org/10.1007/s11695-020-05139-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-020-05139-2