Abstract
Introduction and Purpose
Sleeve gastrectomy (SG) is gaining ground in the field of bariatric surgery. Data are scarce on its impact on esophagogastric physiology. Our aim was to evaluate the impact of SG on esophagogastric motility with high-resolution impedance manometry (HRIM) and to assess the usefulness of HRIM in patients with upper gastrointestinal (GI) symptoms after SG.
Methods
A retrospective analysis of 53 cases of HRIM performed after SG was conducted. Upper GI symptoms at the time of HRIM were scored. HRIM was analyzed according to the Chicago classification v3.0. A special attention was devoted to the occurrence of increased intragastric pressure (IIGP) after water swallows and reflux episodes as detected with impedance. A measurement of sleeve volume and diameter was performed with CT scan in a subgroup of patients.
Results
IIGP occurred very frequently in patients after SG (77 %) and was not associated with any upper GI symptoms, specific esophageal manometric profile, or impedance reflux. Impedance reflux episodes were also frequently observed after SG (52 %): they were significantly associated with gastroesophageal reflux (GER) symptoms and ineffective esophageal motility. The sleeve volume and diameters were also significantly smaller in patients with impedance reflux episodes (p < 0.01).
Conclusion
SG significantly modified esophagogastric motility. IIGP is frequent, not correlated to symptoms, and should be regarded as a HRIM marker of SG. Impedance reflux episodes were also frequent, associated with GER symptoms and esophageal dysmotility. HRIM may thus have a clinical impact on the management of patients with upper GI symptoms after SG.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Sleeve gastrectomy (SG) is gaining ground in the field of bariatric surgery [1, 2]. Digestive comfort is thought to be much better than after gastric banding [3]. However, digestive symptoms such as gastroesophageal reflux disease (GERD), vomiting, regurgitations, food intolerance, or epigastric pain are also reported after this type of surgery [4–6].
Sleeve gastrectomy reduces gastric volume and decreases the gastric ability to relax during meals (accommodation) due to gastric fundus removal. This leads in turn to increased gastric pressure when the remaining stomach is full and thus may induce various upper gastrointestinal (GI) symptoms [7–9].
High-resolution manometry (HRM) has greatly helped to understand the functional gastric and esophageal complications occurring after laparoscopic gastric banding [10, 11]. The combination of HRM and intraluminal impedance monitoring (HRIM) allows the assessment of pressure as well as bolus clearance and reflux episodes within the esophagus and the proximal stomach [12–14].
Our aim was thus to describe the esophageal HRIM patterns after SG, in order to understand the impact of SG on esophagogastric physiology, and to evaluate the diagnostic impact of this technique in patients suffering from upper GI symptoms after SG.
Methods
A retrospective search of prospectively established databases of HRM in two tertiary care centers was performed to identify cases of HRIM done after SG. All cases were included provided they did not undergo any additional surgical procedure between SG and the HRIM. Anthropometric measures were noted at the time of HRIM, as well as the frequency of bothersome heartburn, regurgitations, epigastric pain, nausea and vomiting, and dysphagia, ranked on a 4-points Lickert scale: 0 = never, 1 = once a week, 2 = less than four times a week, and 3 = at least four times a week. Symptoms scoring 2 or 3 were considered significant.
HRIM was performed with a Manoscan™ (Medtronic France, Boulogne-Billancourt, France, 36 pressure sensors plus 9 electrode rings for impedance, 27 patients in France) or a Sandhill-HRiM Insight catheter (Sandhill Scientific Inc, Highlands Ranch, CO, USA, 32 pressure sensors plus 9 electrode rings, 26 patients in Italy) passed through the nose after local anesthesia. A 30-s period without swallowing was analyzed for baseline esophagogastric junction (EGJ) pressure and morphology, and 10 water swallows (5 ml) were performed in supine position for the analysis of esophageal peristalsis and EGJ relaxation. Impedance manometry studies were retrospectively reviewed by four investigators unaware of the symptoms of the patients. Analyses were performed with the Manoview 3.0.1 software or the Sandhill Bioview 5.6.3.0 software with the usual standardized metrics, including the Chicago classification of esophageal motility disorders with HRM (version 3.0, CC3) [15–17]. EGJ morphology was classified based on the presence of axial cranial separation between lower esophageal sphincter (LES) and crural diaphragm (CD), measured in centimeters, as follow: Type I, no separation between the LES and the CD; Type II, minimal separation (>1 and <2 cm); Type III (hiatal hernia), ≥2 cm of separation [18]. A special attention was devoted to the increase of intragastric pressure after water swallows: an increased pressure of more than 30 mmHg was considered as significant, if not associated with a simultaneous intrathoracic pressure (indicating an episode of cough or nausea); duration and maximal pressure were recorded. Intraesophageal variations of impedance after each water swallow were analyzed to identify complete or incomplete bolus clearance and reflux episodes [19]. In order to evaluate the potential role of the size of the gastric sleeve on upper GI symptoms, 15 patients also underwent a specific abdominal CT scan at the time of manometry, after ingestion of effervescent salts (tartric acid and calcium carbonate in less than 10 ml of water) and oral administration of 320 mg of phloroglucinol as previously described [20], the volumes of the gastric remnant, the sleeved part of the stomach and of the antrum, as well as the diameter of the sleeve were computed (Fig. 1).
Statistical Analyses
Data were analyzed using JMP (version 11.0, SAS institute Inc., Cary, NC, USA). Continuous data were expressed as mean (SD) unless otherwise indicated. Parametric or non-parametric tests were used as appropriate to compare groups. A p value <0.05 was considered statistically significant.
Results
Patients
Fifty-three consecutive cases of esophageal HRIM performed after SG in two centers from March 2012 to April 2015 were reviewed. Six of these patients (11 %) had a laparoscopic gastric banding before sleeve gastrectomy. Esophageal testing was performed as part of a systematic follow-up, or because of unexplained upper GI symptoms. There were 37 females, mean age 37 years (SD 12), with a mean body mass index (BMI) of 32 kg/m [2] (SD 5) at the time of HRIM. The median delay between SG and HRIM was 11 months (range 1 to 50 months), and the mean percentage total weight loss between SG and HRIM was 19 (range 10–34). The results of upper GI endoscopy performed at the time of manometry were available in 42 patients (81 %): 37 were normal, and there were three cases of endoscopic hiatal hernia, three cases of erosive esophagitis (two grade A and one grade B according to the Los Angeles classification), and one case of mild gastric stenosis. Twenty-nine patients (55 %) had significant upper GI symptoms at the time of HRIM: epigastric pain was the most frequent symptom (18/53, 34 %), followed by typical symptoms of GERD (heartburn and/or regurgitation) in 15 (28 %), dysphagia in 14 (26 %), nausea and/or vomiting in 9 (17 %), and belching in 2 (4 %). Post-prandial fullness was present in the majority of patients (39/53, 74 %) but was not counted as a symptom, since it is the direct result and objective of SG.
Esophageal HRIM Results
Esophageal HRM was normal in 30 patients, disclosed ineffective esophageal motility (IEM) in 20, and hypercontractile (jackhammer) esophagus in 3 patients. There was no case of outflow obstruction of the EGJ or achalasia (median integrated relaxation pressure, IRP = 4 mmHg (range 0–13)). A hiatal hernia, defined as the presence of EGJ type III morphology, was present in 14 cases. Significant bolus pressurization between both components of the EGJ was present in 7 cases (Fig. 2).
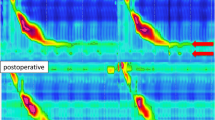
A typical aspect of increased intragastric pressure (IIGP) occurring after 5 ml water swallow (during the lower esophageal sphincter (LES) after contraction) was present in 41 cases (77 %), and the percentage of single swallows associated with IIGP for each subject ranged from 10 to 100 %, with a median of 30 % (Fig. 3). Esophageal bolus clearance during single swallows was similar whether IGP was present or not. Impedance reflux episodes were detected during the manometric protocol in 53 % of patients, ranging from 1 reflux out of 10 single swallows to 10 out 10 per patient (median 20 %) (Fig. 4).
Esophageal high-resolution manometry after sleeve gastrectomy. Typical increased intragastric pressure (IIGP) after single swallows of 5 ml of water. The gastric pressure clearly increased above 30 mmHg during the after-contraction of LES occurring after a swallow. In this case, this phenomenon was associated with epigastric pain
Esophageal high-resolution manometry with impedance after sleeve gastrectomy. Impedance reflux episodes are observed in pink after each single swallow (white arrow indicating the retrograde progression of intra-luminal esophageal impedance variation). The yellow arrows indicate the position of the lower esophageal sphincter (LES)
Comparisons Between Clinical and HRIM Data
Tables 1 and 2 summarized the differences of HRIM results between patients with and without epigastric pain and GERD, respectively; there were no significant differences for the other clinical symptoms (data not shown). Interestingly, patients with typical GER symptoms had significantly more water swallows followed by IIGP (although the percebtage of patients with this phenomenon was similar in the groups with and without typical GER symptoms) and more water swallows followed by impedance reflux. Using ROC curve analysis, we found that 20 % or more of reflux episodes occurring after water swallows was the best cut-off to identify subjects with GER symptoms (AUC = 0.918 (95 % CI 0.8–0.977), p < 0.0001).
There was no difference in terms of symptoms, results of esophageal manometry, CT scan sleeve volume and diameter between patients with and without IIGP. The occurrence of IIGP was similar whatever the delay between HRIM and SG. However, in two cases, a clear and definite relationship could be established between epigastric pain felt by the patients during water swallows and IIGP. These two cases were then operated on to transform the SG into gastric bypass, with resolution of the epigastric pain syndrome afterwards.
With regard to impedance reflux episodes, the demographics were similar between patients with and without (data not shown). Forty percent of patients with impedance reflux episodes had typical symptoms of GERD versus only 5 %, (p = 0.0022) of those without impedance reflux episodes. Among the HRIM variables, the mean IRP and the distribution of esophageal motility disorders as defined by the CC3 were significantly different between the two groups, while the mean distal contractile integral (DCI) tended to be lower in patients with impedance reflux episodes (Table 3). The percentage of IIGP after water swallows was higher in the group of patients with reflux episodes, but the difference in terms of percentage of patients with IIGP after water swallows, although higher in the reflux group, did not reach statistical significance. On CT scan, the measured sleeve volume and the sleeve diameter were significantly smaller in the group of patients with impedance reflux (N = 8) than in those without impedance reflux (N = 4) (141 (23) ml vs 247 (32) ml, p = 0.022; 36 (2) mm vs 47 (3) mm, p = 0.017, respectively).
Discussion
This study points out the potential usefulness of esophagogastric HRIM for the diagnostic work-up of patients with upper GI symptoms after SG. The compliance of the gastric sleeve is clearly reduced, as evidenced by the increased intragastric pressure after 5 ml water swallows. Interestingly, this IIGP was present only after the completion of the swallows, during the LES after-contraction, and not during the relaxation period of the EGJ. This may explain why this phenomenon was not associated with symptoms (dysphagia or GER). Thus, it should be considered more as a manometric marker of SG, rather than a pathological finding with clinical implications, except in cases when painful symptoms clearly occur associated with IIGP. Vidal et al. recently suggested that sleeve volume increased between 1 and 12 months after SG, and the more this volume increased the less body weight decreased [21]. It would thus be expected to see IIGP after swallows decrease as the delay between SG and HRM increases, but our results did not confirm this hypothesis. The prevalence of IIGP was similar in early HRM performed less than 6 months after surgery and late HRM performed more than 12 months after SG. Del Genio et al. [8] described that reflux episodes after SG were linked to increased intragastric pressure (“bounded” reflux): our results did not confirm convincingly this observation, although the percentage of water swallows followed by IIGP was significantly higher in the group of patients with impedance reflux episodes. Indeed, we did not find a significant correlation between the occurrence of IIGP and reflux impedance episodes. In some instances, a shortening of the esophagus with enlarged EGJ could be detected, similarly to the description made initially by Burton et al. after gastric banding [11, 22]. In these cases, the pressurization of the bolus within the supradiaphragmatic enlargement appears as a clear indicator of the impact of the SG on esophageal bolus clearance and transit. Thus, the reduced compliance of the gastric sleeve may impact on the anatomy and the physiology of the EGJ in some cases. However, in our series, the pressurization of the enlarged EGJ (or hiatal hernia) present in seven cases was not associated with dysphagia or other upper GI symptoms. Toro et al. have recently shown that the sleeve volume and compliance may vary greatly between subjects and that these variables do not appear to be associated with the efficacy of the bariatric procedure on weight loss [23]. They did not find a correlation between the sleeve volume and its compliance. Our small series comparing the sleeve volume as measured with CT scan and compliance as measured as the percentage of increased intragastric pressure after single swallows showed the same absence of correlation between these two variables.
With regard to reflux, our study demonstrated the usefulness of HRIM for a rapid assessment of GER occurrence after SG. Despite conflicting results in the literature, it seems rather clear that SG may induce GERD “de novo,” or increase GER symptoms already present before surgery [4, 24–28]. Our results based on the evaluation of impedance reflux episodes after 10 single swallows of 5 ml water showed that this test may be sufficient to confirm the absence of reflux: indeed only 5 % of the patients without reflux episodes at HRIM reported GER symptoms. These data are confirmed by the fact that patients with impedance reflux episodes had more frequently manometric abnormalities observed in patients with proven GERD, such as low baseline EGJ pressure, decreased DCI, and ineffective esophageal motility [29–32]. Manometric hiatal hernia was also more frequently observed in the group of patients with GER symptoms: these small hiatal hernias (or enlargement of the EGJ) were probably the consequence of the SG, as the presence of a significant hiatal hernia was regarded as a contraindication to SG in our series [33].
There are some limitations to this study: its retrospective nature is one of them. HRIM was performed as part of a routine work-up after SG in most patients, and as a diagnostic tool in patients with unexplained upper GI symptoms: this may create a bias in the interpretation of our data. However, as previously mentioned, the delay between SG and HRIM was not correlated with IIGP or the prevalence of impedance reflux episodes. Furthermore, this approach allowed us to compare HRIM results in asymptomatic and symptomatic patients, and thus to describe manometric abnormalities such as IIGP not correlated to clinical symptoms. Finally, we did not compare the results of HRIM with the gold standard for GERD diagnosis, i.e., prolonged esophageal pH-impedance monitoring.
In conclusion, this retrospective series describe typical results of HRIM after SG. Increased intragastric pressure after water single swallows is very common and most of the time not associated to clinical symptoms. Impedance reflux episodes are also very frequent after water single swallows and associated with GERD symptoms and manometric abnormalities usually found in GERD patients. HRIM may thus be useful to explore upper digestive symptoms occurring after SG, and outcomes data are needed to confirm this hypothesis. Meanwhile, we propose that HRIM should be performed after SG whenever bothersome upper GI symptoms are not explained by upper GI endoscopy and/or barium swallow.
References
Keren D, Matter I, Rainis T. Sleeve gastrectomy in different age groups: a comparative study of 5-year outcomes. Obes Surg. 2016;26(2):289–95.
Jammu GS, Sharma R. A 7-year clinical audit of 1107 cases comparing sleeve gastrectomy, roux-en-y gastric bypass, and mini-gastric bypass, to determine an effective and safe bariatric and metabolic procedure. Obes Surg 2015.
Buwen JP, Kammerer MR, Beekley AC, et al. Laparoscopic sleeve gastrectomy: The rightful gold standard weight loss surgery procedure. Surg Obes Relat Dis. 2015;11(6):1383–5.
Melissas J, Braghetto I, Molina JC, et al. Gastroesophageal reflux disease and sleeve gastrectomy. Obes Surg. 2015;25(12):2430–5.
Burgerhart JS, van de Meeberg PC, Mauritz FA, et al. Increased belching after sleeve gastrectomy. Obes Surg. 2016;26(1):132–7.
Biter LU, Gadiot RP, Grotenhuis BA, et al. The sleeve bypass trial: a multicentre randomized controlled trial comparing the long term outcome of laparoscopic sleeve gastrectomy and gastric bypass for morbid obesity in terms of excess BMI loss percentage and quality of life. BMC Obes. 2015;2:30.
Preissler C, Krieger-Grubel C, Borovicka J, et al. The contribution of intrabolus pressure to symptoms induced by gastric banding. J Gastrointestin Liver Dis. 2014;23:13–7.
Del Genio G, Tolone S, Limongelli P, et al. Sleeve gastrectomy and development of “de novo” gastroesophageal reflux. Obes Surg. 2014;24:71–7.
Burgerhart JS, Schotborgh CA, Schoon EJ, et al. Effect of sleeve gastrectomy on gastroesophageal reflux. Obes Surg. 2014;24:1436–41.
Burton PR, Brown WA, Laurie C, et al. Mechanisms of bolus clearance in patients with laparoscopic adjustable gastric bands. Obes Surg. 2010;20:1265–72.
Burton PR, Brown WA, Laurie C, et al. Pathophysiology of laparoscopic adjustable gastric bands: analysis and classification using high-resolution video manometry and a stress barium protocol. Obes Surg. 2010;20:19–29.
Lin Z, Carlson DA, Dykstra K, et al. High-resolution impedance manometry measurement of bolus flow time in achalasia and its correlation with dysphagia. Neurogastroenterol Motil. 2015;27:1232–8.
Park EJ, Lee JS, Lee TH, et al. High-resolution impedance manometry criteria in the sitting position indicative of incomplete bolus clearance. J Neurogastroenterol Motil. 2014;20:491–6.
Lin Z, Imam H, Nicodeme F, et al. Flow time through esophagogastric junction derived during high-resolution impedance-manometry studies: a novel parameter for assessing esophageal bolus transit. Am J Physiol Gastrointest Liver Physiol. 2014;307:G158–63.
Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil. 2015;27:160–74.
do Carmo GC, Jafari J, Sifrim D, et al. Normal esophageal pressure topography metrics for data derived from the Sandhill-Unisensor high-resolution manometry assembly in supine and sitting positions. Neurogastroenterol Motil. 2015;27:285–92.
Herregods TV, Roman S, Kahrilas PJ, et al. Normative values in esophageal high-resolution manometry. Neurogastroenterol Motil. 2015;27:175–87.
Pandolfino JE, Kim H, Ghosh SK, et al. High-resolution manometry of the EGJ: an analysis of crural diaphragm function in GERD. Am J Gastroenterol. 2007;102:1056–63.
Tutuian R, Castell DO. Combined multichannel intraluminal impedance and manometry clarifies esophageal function abnormalities: study in 350 patients. Am J Gastroenterol. 2004;99:1011–9.
Cansu A, Ahmetoglu A, Kul S, et al. Diagnostic performance of using effervescent powder for detection and grading of esophageal varices by multi-detector computed tomography. Eur J Radiol. 2014;83:497–502.
Vidal P, Ramon JM, Busto M, et al. Residual gastric volume estimated with a new radiological volumetric model: relationship with weight loss after laparoscopic sleeve gastrectomy. Obes Surg. 2014;24:359–63.
Cruiziat C, Roman S, Robert M, et al. High resolution esophageal manometry evaluation in symptomatic patients after gastric banding for morbid obesity. Dig Liver Dis. 2011;43:116–20.
Toro JP, Patel AD, Lytle NW, et al. Observed variability in sleeve gastrectomy volume and compliance does not correlate to postoperative outcomes. Surg Laparosc Endosc Percutan Tech. 2015;25:324–30.
Sucandy I, Chrestiana D, Bonanni F, et al. Gastroesophageal reflux symptoms after laparoscopic sleeve gastrectomy for morbid obesity. the importance of preoperative evaluation and selection. N Am J Med Sci. 2015;7:189–93.
Stenard F, Iannelli A. Laparoscopic sleeve gastrectomy and gastroesophageal reflux. World J Gastroenterol. 2015;21:10348–57.
Sheppard CE, Sadowski DC, de Gara CJ, et al. Rates of reflux before and after laparoscopic sleeve gastrectomy for severe obesity. Obes Surg. 2015;25:763–8.
Oor JE, Roks DJ, Unlu C, et al. Laparoscopic sleeve gastrectomy and gastroesophageal reflux disease: a systematic review and meta-analysis. Am J Surg. 2016;211(1):250–67.
Tolone S, Cristiano S, Savarino E, et al. Effects of omega-loop bypass on esophagogastric junction function. Surg Obes Relat Dis. 2016;12(1):62–9.
Hoshino M, Sundaram A, Mittal SK. Role of the lower esophageal sphincter on acid exposure revisited with high-resolution manometry. J Am Coll Surg. 2011;213:743–50.
Chen CL, Yi CH, Liu TT. Relevance of ineffective esophageal motility to secondary peristalsis in patients with gastroesophageal reflux disease. J Gastroenterol Hepatol. 2014;29:296–300.
Manabe N, Haruma K. Pathophysiology of gastroesophageal reflux disease from the viewpoint of esophageal motility. Nihon Shokakibyo Gakkai Zasshi. 2014;111:1923–32.
Martinucci I, de Bortoli N, Giacchino M, et al. Esophageal motility abnormalities in gastroesophageal reflux disease. World J Gastrointest Pharmacol Ther. 2014;5:86–96.
Rosenthal RJ, International Sleeve Gastrectomy Expert P, Diaz AA, et al. International Sleeve Gastrectomy Expert Panel Consensus Statement: best practice guidelines based on experience of >12,000 cases. Surg Obes Relat Dis. 2012;8:8–19.
Author Contributions
FM, ST, and SR were responsible for the study concept and design, acquisition of the data, analysis and interpretation of the data, drafting the manuscript, and approval of the final version of the manuscript.
SM, EP, MR, GP, PJV, and AG were responsible for the acquisition of the data, analysis and interpretation of the data, drafting the manuscript, and approval of the final version of the manuscript.
ES and LD were responsible for the analysis and interpretation of the data, drafting of the manuscript, and approval of the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
FM was a consultant for Medtronic; ES and SR were consultants for Medtronic, Sandhill; and ST, LD, AG, SM, MR, GP, EP, and PJV declare no conflict of interest.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Statement of Human Rights
All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Mion, F., Tolone, S., Garros, A. et al. High-resolution Impedance Manometry after Sleeve Gastrectomy: Increased Intragastric Pressure and Reflux are Frequent Events. OBES SURG 26, 2449–2456 (2016). https://doi.org/10.1007/s11695-016-2127-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-016-2127-y







