Abstract
Introduction
Bariatric surgery is increasingly performed. Since there are numerous surgical techniques, the effects of these on the esophageal function are still poorly understood. We aimed at assessing the effect of different techniques on esophagogastric junction (EGJ), esophageal peristalsis and reflux exposure using high-resolution manometry (HRM), and impedance-pH monitoring (MII-pH).
Methods
All obese patients underwent symptomatic questionnaires, endoscopy, HRM, and MII-pH before and 1 year after surgery. Esophageal function and EGJ were classified according to Chicago Classification V. 3.0. Intragastric pressure (IGP) and gastroesophageal pressure gradient (GEPG) were assessed. Total acid exposure time (AET %), total number of refluxes, and symptom association probability (SAP) were assessed. A group of healthy volunteers (HVs) served as control.
Results
One hundred and twelve obese subjects and 15 HVs (normal weight) were studied. Thirteen underwent endoscopic balloon placement (BIB), 12 gastric banding (GB), 26 sleeve gastrectomy (SG), 18 Roux-en-Y gastric bypass (RYGB), 15 mini-gastric bypass (MGB), 16 biliointestinal bypass (BIBP), and 12 biliopancreatic diversion (BPD). IGP and GEPG significantly decreased after RYGP, BPD, and BPBP, whereas they significantly increased after GB and SG. Post-operative greater AET (p < 0.05) and increased total number of reflux (p < 0.001) were present after GB and SG. RYGB and MGB showed a significant decrease in AET (p < 0.05) and total number of reflux (p < 0.001).
Conclusions
HRM verified that different bariatric techniques produced different modification of IGP and GEPG, leading to different reflux exposure. Only GB and SG can negatively impact on esophageal function and reflux exposure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is dramatically increasing worldwide. Bariatric surgery is considered to be the most effective and durable option for achieving a considerable weight loss.1 Since there are numerous surgical techniques, the effects of these on the esophageal function are still poorly understood.2 Furthermore, some bariatric techniques proved to be very effective as antireflux procedures, such as Roux-en-Y gastric bypass (RYGB), whereas “de novo” gastroesophageal reflux disease (GERD) and dysmotility were reported after some bariatric procedures, such as sleeve gastrectomy (SG).3
Because gastric and esophageal reflux can depend on proximal gastric pressure, we aimed to assess the effect of the most commonly performed bariatric techniques on esophagogastric junction (EGJ) function, esophageal peristalsis, and reflux exposure using current diagnostic gold standard tests, e.g., the high-resolution impedance manometry (HRiM) and impedance-pH monitoring (MII-pH). Secondary endpoint was to assess the relationship of post-operative reflux pattern with post-operative modification of intragastric pressure (IGP) and gastroesophageal pressure gradient (GEPG).
Materials and Methods
All obese (body mass index, BMI, > 35) patients underwent symptomatic questionnaires (GerdQ), endoscopy, HRM, and MII-pH before and 1 year after surgery.4 We enrolled only obese without dysmotility or any evidence of GERD, in order to verify the real incidence of de novo GERD. Esophageal motor function and EGJ were classified according to Chicago Classification V. 3.0.5 EGJ contractile integral (EGJ-CI) was also calculated.6 Intragastric pressure (IGP) and gastroesophageal pressure gradient (GEPG) were assessed. Total acid exposure time (AET %), total number of refluxes, and symptom association probability (SAP) were assessed.7 A group of healthy volunteers (HVs) served as control.
Study Design and Patient Selection
We enrolled adult obese subjects (18 years or older). All patients who underwent different bariatric procedures performed instrumental testing at our Bariatric Surgical Unit in Naples, Italy, between 2010 and 2017. Inclusion criteria were as follows: presence of class II or higher morbid obesity (defined as a body mass index, BMI, greater than 40 or higher than 35 in case of comorbidities), indications to undergo bariatric surgery according to international guidelines,4 and absence of any pre-operative signs of pathological GERD, such as clinically relevant GER symptoms, hiatal hernia, esophagitis, or Barrett’s esophagus. Exclusion criteria were as follows: absence of any clinical or instrumental examination required by the study protocol, psychiatric illness, loss to follow-up, and declining consent to participate. Our Institutional Review Board approved the study protocol, and informed consent was obtained from each subject. The study was conducted according to the Helsinki declaration.
Study protocol was designed to obtain data before and 1 year following different bariatric procedure. Only for intragastric balloon positioning (BIB) data were obtained before and at 6 months, at the end of the procedure, the day before the removal (with BIB in place).
Assessment of anthropometrics (weight, height, BMI) was done in all subjects; typical reflux-related symptoms were investigated using the GerdQ score, a validated questionnaire incorporating a Likert visual analog scale (0–3, where 0 = absent, 1 = mild, 2 = moderate, and 3 = severe).8 Furthermore, atypical symptoms like cough, hoarseness, asthma, wheezing, and dysphonia were investigated by means of a 4-point Likert scale (0–3, where 0 = absent, 1 = mild, 2 = moderate, and 3 = severe). Then, patients underwent upper endoscopy, HRiM, and combined 24-h pH-impedance monitoring (multichannel intraluminal impedance-pH monitoring, MII-pH).
During upper gastrointestinal endoscopy, the presence of esophagitis and/or gastric inflammation was recorded and graded according to the Los Angeles classification9 and to the Sidney system,10 for esophagitis and gastritis respectively. Biopsies were obtained and histological examination performed when visible inflammation was present.
Pathophysiological Esophageal Evaluation
HRiM and MII-pH were performed off medication (any antacid medication or prokinetic was stopped at least 14 days prior to testing), after an overnight fasting. After automatic analysis, tracings were reviewed manually by a blinded single expert investigator (ST).
HRiM was performed with a trans-nasal 32-channel probe (Sandhill-HRiM catheter InSight, Sandhill Scientific, Highlands Ranch, CO, USA) according to our and international guidelines.11 After thermal compensation, acquisition and data analysis were performed using Sandhill Bioview, Sandhill Sci. software. With the patient supine, the catheter was introduced transnasally, placed to record from hypopharynx to stomach, with at least 5 intragastric sensors to optimize EGJ and intragastric recording. The HRiM protocol included a 5-min period to assess EGJ pressure at resting and 10 5-mL swallows (0.3% saline) to evaluate esophageal body function. After LES identification, its resting pressure and relaxation response to swallow (integrated relaxation pressure over 4 s, IRP) were recorded. EGJ contractile integral (EGJ-CI) was also calculated. Crural diaphragm (CD) was discernable as the axial point with the maximal inspiratory pressure augmentation. Patients were then classified to have normal EGJ (with LES and CD superimposed) or hiatal hernia (with a presence of axial separation, measured in cm, between LES and CD).12, 13 Proximal intragastric pressures (IGP) and distal intraesophageal pressures (IEP) were recorded; then the gastroesophageal pressure gradient (GEPG) was calculated using the average values of the simultaneous IEP and IGP.
HRiM motility patterns were categorized according to the Chicago Classification V 3.05; thus, patients were graded to have “normal” or “abnormal” motility, by means of calculation of the IRP, distal contractile integral (DCI), and distal latency (DL).5
MII-pH was performed with a dedicated single-use catheter placed transnasally (with impedance sensors located at 3, 5, 7, 9, 15, and 17 cm above the LES) (Sandhill Scientific). Patients were asked to record every meal, changes in body position (i.e., from upright to reclining and vice versa), and symptoms occurrence during the monitoring day. After excluding meal periods, MII-pH data were analyzed with the Bioview GERD Analysis Software (Sandhill Scientific). At MII-pH, the following features were evaluated: percentage distal acid exposure time (AET %) with pH < 4; abnormal AET %, defined as > 6.0% for total time, > 4.2% for upright time, and > 1.2% for recumbent period; number of total reflux episodes identified at MII (normal value < 80); and symptom association probability (SAP), as described elsewhere.7, 14
Bariatric Procedures
Patients underwent the following procedures: intragastric balloon positioning (BIB), during an upper endoscopy; laparoscopic gastric banding (GB); laparoscopic SG (along a 38 Fr bougie, starting 6 cm from pylorus, to the his angle, 1 cm laterally to the EGJ); laparoscopic mini-gastric/single anastomosis bypass (MGB) (with a 15–18-cm long gastric pouch and a 4–4.5 cm vertical latero-lateral anastomosis between the gastric pouch and the jejunal loop 150–200 cm distal to Treitz’ ligament); laparoscopic RYGB (with a 60-cc gastric pouch and a R-e-Y limb 100 cm); laparoscopic classic Scopinaro’s biliopancreatic diversion (BPD), and laparoscopic biliointestinal bypass (BIBP, only two anastomosis between jejunum 40 cm distally to Treitz to ileus 40 cm proximally to ileocecal valve and between gallbladder and proximal jejunum15).
Control Group
A group of adult patients without pathology and normal BMI (20–25) (healthy volunteers, HV) who previously underwent HRiM and MII-pH in our motility lab for other study protocol.
Statistical Analysis
Statistical analysis was performed using SPSS for MAC OSX (version 22; SPSS Inc., Chicago, IL, USA). Continuous data are expressed as median and interquartile (25th–75th) range, unless otherwise stated. The Wilcoxon signed-rank test for paired data was used for comparison of means in the same patients pre- and post-operatively. Unpaired t test was used for comparison of means in different procedures and vs the HVs. A two-sided p value of 0.05 was considered statistically significant. A power calculation for two independent groups for comparing differences in continuous data (means) with alpha set at 0.05 and power set at 80% was performed; a sample size of at least 8 patients for each group was needed.
Results
One hundred and twelve obese subjects (39 ± 12 years old, mean weight 135 (97–202) kg, mean BMI 42 (37–69) kg/m2, and 15 HVs (normal weight)) were studied. The proportion on overall obese population we operated was 1:5.3. About these latter, 1:3 were excluded because hiatal hernia or esophagitis >grade B at endoscopy or for a positive reflux testing.
Thirteen underwent endoscopic balloon placement (BIB), 12 gastric banding (GB), 26 sleeve gastrectomy (SG), 18 Roux-en-Y gastric bypass (RYGB), 15 mini-gastric bypass (MGB), 16 biliointestinal bypass (BIBP), and 12 biliopancreatic diversion (BPD). All patients showed a significant decrease of weight and BMI 1 year after surgery (mean weight pre-op 135 kg (97–202), mean BMI pre-op 42 kg/m2 (37–69) vs. mean weight post-op 81.2 kg (72–111) and mean BMI post-op 30 kg/m2 (27–42), p < 0.05 and p < 0.05, respectively).
“De Novo” GERD symptoms were observed in 1 (3.8%) SG and 2 (16%) GB.
At baseline endoscopy, none of the subjects had esophagitis. After 1 year, esophagitis grade A was detected in 6 (23%) SG and in 4 (33%) GB. MGB and RYGB showed 46% and 33% of perianastomotic inflammation, respectively.
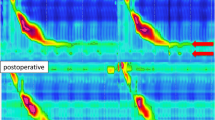
At HRM, IGP, and GEPG significantly decreased after RYGP, BPD, and BIBP, whereas they significantly increased after GB and SG (Table 1). EGJ morphology changed after GB, with 6 patients showing type III morphology, and in 1 SG passing from type I to type II. LESp (Fig. 1), EGJ-CI, IRP, and DCI increased significantly (p < 0.001) only after GB. Hypercontractile and premature contractions waves were present in 40% of patients after GB, whereas ineffective motility (36%) waves were present after SG (Fig. 2).
Changing in lower esophageal sphincter pressure (LESp, in mmHg) before and after endoscopic balloon placement (BIB), gastric banding (GB), sleeve gastrectomy (SG), Roux-en-Y gastric bypass (RYGB), mini-gastric bypass (MGB), biliointestinal bypass (BIBP), and biliopancreatic diversion (BPD). HV, healthy volunteers
Frequency (as percentage) in normal and ineffective peristalsis before and after mini-gastric bypass (MGB), Roux-en-Y gastric bypass (RYGB), biliopancreatic diversion (BPD), biliointestinal bypass (BIBP), and endoscopic balloon placement (BIB) (all of these are merged together because of same results), vs. sleeve gastrectomy (SG)
At MII-pH, post-operative greater AET (p < 0.05) (Fig. 3) and increased total number of reflux (p < 0.001) were present after GB and SG. RYGB and MGB showed a significant decrease in AET (p < 0.05) and total number of reflux (p < 0.001), whereas BIBP showed a non-significant reduction in AET and reflux events but similar to HVs patterns (Fig. 4).
Changing in acid exposure total (AET) with pH < 4, %, before and after endoscopic balloon placement (BIB), gastric banding (GB), sleeve gastrectomy (SG), Roux-en-Y gastric bypass (RYGB), mini-gastric bypass (MGB), biliointestinal bypass (BIBP), and biliopancreatic diversion (BPD). HV, healthy volunteers
Changing in total number of reflux detected at impedance-pH monitoring before and after endoscopic balloon placement (BIB), gastric banding (GB), sleeve gastrectomy (SG), Roux-en-Y gastric bypass (RYGB), mini-gastric bypass (MGB), biliointestinal bypass (BIBP), and biliopancreatic diversion (BPD). HV, healthy volunteers
Discussion
Bariatric surgery is increasingly performed worldwide, with optimal results about weight loss and comorbidity resolution. However, an ideal technique that fits for all patients is still lacking, with a variety of procedures ranging from gastric restriction to malabsorption. Despite large comorbidity resolution, GERD after bariatric surgery is still a matter of concern.2, 3 This is because obesity and GERD share a strong link; thus, it should be conceivable that weight loss can result in GERD remission.16 On the other hand, almost all bariatric procedures provide an anatomical change of the stomach that can create a new physiology of the gastric accommodation and of its internal pressure. RYGB demonstrated optimal reduction of GERD, because of its small gastric pouch and its rapid passage of gastric content into jejunum through the anastomosis.17 On the contrary, SG in several reports seems to produce an increase of reflux up to “de novo” GERD.18 Of note, there are no studies comparing esophageal function pre- and post-operatively in the most widely used bariatric techniques. This is the first study, to our knowledge, in which objective data are provided about pathophysiological outcomes after these operations. In particular, we provided data about the effects of bariatric surgery on changing pattern of intragastric pressures, EGJ efficacy, and reflux features, using the current gold standard instruments for their assessment: high-resolution impedance manometry and 24-h impedance-pH monitoring. The most noticeable finding of this study was that the most of bariatric procedure obtained a reduction in GERD patterns without altering EGJ function and esophageal peristalsis. Only GB and SG strongly increased their IGP and GEPG, with a subsequent increase in AET and number of total reflux. The mechanism underlying this modification in GB should be intuitive; the presence of the band creates an outflow obstruction in the proximal gastric part; thus, the proximal IGP is increased and reflux is more likely to happen. Also the presence of hypercontractile activity of the esophageal body seems to be consistent with the mechanical slow down offered by the band. Our data are also consistent with previous study in which GB caused in some cases a pseudo-achalasia syndrome.19, 20 In the SG, instead, the increased IGP and GEPG should be explained by the narrowing of the gastric tube and the loss of gastric accommodation, normally provided by the fundus.21 The lack of fundus and the sleeved-shape makes the stomach now adaptable to Laplace’s law, in which the pressure in a poorly dilatable cylinder (like the new shaped stomach) is inversely related to its diameter. Thus, when the stomach reach sooner its full capability of food, reflux can easily flow back into the esophagus. The esophageal motility impairing should now be explained by the possible initial damage offered by increased reflux contact. Our group previously showed similar data, confirming actual analysis.22, 23
On the contrary, the reduction of reflux in RYGB seems to be dependent by the reduction of IGP and GEPG; this phenomenon was previously found also in a previous study, where a large anastomosis allowing creation of a common cavity has been documented by HRiM.17
The modification after MGB instead stands in between SG and RYGB. The sleeved-shape stomach mildly increased its IGP, but the presence of the anastomosis allows the bile to flow through the anastomosis, but it is forced by the increased IGP to continue its flow down into jejunum. Also, a decrease in total reflux number is consistent with two previous reports by our team,24 with further confirmation of these results in MGB patients even followed for 5 years and even compared with classic Billroth II patients.25
BPD did not show a particular changing of IGP, GEPG, and thus of the reflux pattern. We tried to explain these results with the fact that in classical BPD, the proximal stomach and fundus are intact; thus, the accommodation mechanism is still normal, and the lowered number of reflux can be dependent from the gastric outflow and from the weight loss. Finally, the BIBP showed interesting results that can be an initial confirm about the interplay of weight loss and reflux control. This technique is the solely that do not alter gastric and duodenal anatomy,15 so we could speculate that the changing on esophageal physiology is linked only to weight loss and eating habits.
Our study has some limitations. First, the number of patients in this study was relatively small, due to the invasiveness of the pre- and post-operative procedures required for the study protocol—upper GI endoscopy, HRiM, and impedance-pH monitoring. Secondly, patients were enrolled prospectically but not consecutively, because of the difficulty to follow-up obese patients after surgery. Because of these issues in the enrollment, this study could be under-powered to determine if a technique is protective against reflux. Also, up to date, we are offering more SG, RYGB, and MGB than BPD, BIBP, or GB; thus, in these latter, three subgroups data were older than other techniques. Finally, a possible bias could be represented by the influence that central obesity accounts on reflux exposure and increased intragastric pressure; thus, a markedly weight reduction could confuse the post-operative reflux exposure. However, in order to try to reduce this possible bias, we selected only patients without pre-operative GERD, highlighting EGJ adaption only in this setting. We still need data on EGJ physiology after different techniques in patients with pre-existing GERD.
Conclusion
HRM verified that different bariatric techniques produced different modifications of IGP and GEPG, leading to different reflux exposures. Only GB and SG seem to have the possibility to negatively impact on esophageal function and reflux exposure, and they should be avoided in obese patients with pre-existing GERD.
Further studies with a conspicuous number of patients are needed to confirm these results.
References
Angrisani L, Santonicola A, Iovino P, Vitiello A, Zundel N, Buchwald H, Scopinaro N. Bariatric Surgery and Endoluminal Procedures: IFSO Worldwide Survey 2014. Obes Surg 2017;27:2279–2289
Savarino E, Marabotto E, Savarino V. Effects of bariatric surgery on the esophagus. Curr Opin Gastroenterol. 2018;34:243–248
Tolone S, Savarino E, Yates RB. The impact of bariatric surgery on esophageal function. Ann N Y Acad Sci. 2016;1381:98–103.
American Society for Metabolic and Bariatric Surgery Clinical Issues Committee. American Society for Metabolic and Bariatric Surgery position statement on global bariatric healthcare. Surg Obes Relat Dis. 2011;7:669–71
Kahrilas PJ, Bredenoord AJ, Fox M, Gyawali CP, Roman S, Smout AJ, Pandolfino JE; International High Resolution Manometry Working Group. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil. 2015;27:160–74
Tolone S, De Bortoli N, Marabotto E, de Cassan C, Bodini G, Roman S, Furnari M, Savarino V, Docimo L, Savarino E. Esophagogastric junction contractility for clinical assessment in patients with GERD: a real added value? Neurogastroenterol Motil. 2015;27:1423–31
Roman S, Gyawali CP, Savarino E, Yadlapati R, Zerbib F, Wu J, Vela M, Tutuian R, Tatum R, Sifrim D, Keller J, Fox M, Pandolfino JE, Bredenoord AJ; GERD consensus group. Ambulatory reflux monitoring for diagnosis of gastro-esophageal reflux disease: Update of the Porto consensus and recommendations from an international consensus group. Neurogastroenterol Motil. 2017;29:1–15.
Gyawali CP, de Bortoli N, Clarke J, Marinelli C, Tolone S, Roman S, Savarino E. Indications and interpretation of esophageal function testing. Ann N Y Acad Sci. 2018;1434:239–253.
Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, Galmiche JP, Johnson F, Hongo M, Richter JE, Spechler SJ, Tytgat GN, Wallin L. Endoscopic assessment of esophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45:172–80.
Stolte M, Meining A. The updated Sydney system: Classification and grading of gastritis as the basis of diagnosis and treatment. Can J Gastroenterol. 2001;15:591–8.
Gyawali CP, Roman S, Bredenoord AJ, Fox M, Keller J, Pandolfino JE, Sifrim D, Tatum R, Yadlapati R, Savarino E; International GERD Consensus Working Group. Classification of esophageal motor findings in gastro-esophageal reflux disease: conclusions from an international consensus group. Neurogastroenterol Motil. 2017;29. https://doi.org/10.1111/nmo.13104.
Tolone S, Savarino E, de Bortoli N, Frazzoni M, Furnari M, d'Alessandro A, Ruggiero R, Docimo G, Brusciano L, Gili S, Pirozzi R, Parisi S, Colella C, Bondanese M, Pascotto B, Buonomo N, Savarino V, Docimo L. Esophagogastric junction morphology assessment by high resolution manometry in obese patients candidate to bariatric surgery. Int J Surg. 2016;28 Suppl 1:S109–13.
Tolone S, Savarino E, Zaninotto G, Gyawali CP, Frazzoni M, de Bortoli N, Frazzoni L, Del Genio G, Bodini G, Furnari M, Savarino V, Docimo L. High-resolution manometry is superior to endoscopy and radiology in assessing and grading sliding hiatal hernia: A comparison with surgical in vivo evaluation. United European Gastroenterol J. 2018;6:981–989.
Frazzoni M, de Bortoli N, Frazzoni L, Furnari M, Martinucci I, Tolone S, Farioli A, Marchi S, Fuccio L, Savarino V, Savarino E. Impairment of chemical clearance and mucosal integrity distinguishes hypersensitive esophagus from functional heartburn. J Gastroenterol. 2017;52:444–51.
Del Genio G, Gagner M, Limongelli P, Tolone S, Pournaras D, le Roux CW, Brusciano L, Licia Mozzillo A, Del Genio F, Docimo L. Remission of type 2 diabetes in patients undergoing biliointestinal bypass for morbid obesity: a new surgical treatment. Surg Obes Relat Dis. 2016;12:815–821.
De Bortoli N, Tolone S, Savarino EV. Weight Loss Is Truly Effective in Reducing Symptoms and Proton Pump Inhibitor Use in Patients With Gastroesophageal Reflux Disease. Clin Gastroenterol Hepatol. 2015;13:2023.
Björklund P, Lönroth H, Fändriks L. Manometry of the upper gut following Roux-en-Y gastric bypass indicates that the gastric pouch and Roux limb act as a common cavity. Obes Surg. 2015;25:1833–41.
Sebastianelli L, Benois M, Vanbiervliet G, Bailly L, Robert M, Turrin N, Gizard E, Foletto M, Bisello M, Albanese A, Santonicola A, Iovino P, Piche T, Angrisani L, Turchi L, Schiavo L, Iannelli A. Systematic Endoscopy 5 Years After Sleeve Gastrectomy Results in a High Rate of Barrett's Esophagus: Results of a Multicenter Study. Obes Surg. 2019;29:1462–1469
Naef M, Mouton WG, Naef U, van der Weg B, Maddern GJ, Wagner HE. Esophageal dysmotility disorders after laparoscopic gastric banding--an underestimated complication. Ann Surg. 2011;253:285–90.
Roman S, Kahrilas PJ. Pseudoachalasia and laparoscopic gastric banding. J Clin Gastroenterol. 2011;45:745–7.
Lind JF, Duthie HL, Schlegel JF, Code CF. Motility of the gastric fundus. Am J Physiol. 1961;201:197–202
Del Genio G, Tolone S, Limongelli P, Brusciano L, D'Alessandro A, Docimo G, Rossetti G, Silecchia G, Iannelli A, del Genio A, del Genio F, Docimo L. Sleeve gastrectomy and development of “de novo” gastroesophageal reflux. Obes Surg. 2014;24:71–7.
Mion F, Tolone S, Garros A, Savarino E, Pelascini E, Robert M, Poncet G, Valette PJ, Marjoux S, Docimo L, Roman S. High-resolution impedance manometry after sleeve gastrectomy: Increased intragastric pressure and reflux are frequent events. Obes Surg. 2016;26:2449–56.
Tolone S, Cristiano S, Savarino E, Lucido FS, Fico DI, Docimo L. Effects of omega-loop bypass on esophagogastric junction function. Surg Obes Relat Dis. 2016;12:62–9.
Tolone S, Musella M, Savarino E, Cristiano S, Docimo L, Deitel M. Esophagogastric junction function and gastric pressure profile after minigastric bypass compared with Billroth II. Surg Obes Relat Dis. 2019;15:567–574
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Statements
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
This article does not contain any studies with animals performed by any of the authors.
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tolone, S., Savarino, E., de Bortoli, N. et al. Esophageal High-Resolution Manometry Can Unravel the Mechanisms by Which Different Bariatric Techniques Produce Different Reflux Exposures. J Gastrointest Surg 24, 1–7 (2020). https://doi.org/10.1007/s11605-019-04406-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-019-04406-7