Abstract
We aimed to make a meta-analysis regarding the effect of bariatric surgery on female sexual function. PubMed, EMBASE, and CENTRAL were searched from database inception through August 2019. Articles were eligible for inclusion if they examined the effect of bariatric surgery on obese women’s sexual function assessed by the Female Sexual Functioning Index (FSFI) or/and the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12). Twenty articles were included into meta-analysis. Bariatric surgery was associated with significant increase in the total FSFI score. When parameters included in the FSFI scoring system were separately evaluated, significant improvements were observed in sexual desire, sexual arousal, lubrication, orgasm, sexual satisfaction, and sexual pain. However, the PISQ-12 and FSFI scores in women with pelvic floor disorders (PFDs) were not significantly changed postoperatively. Bariatric surgery improves female sexual function in obese patients, but not in women with PFD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity represents a major health problem worldwide, which is associated with the development of multiple comorbidities, including diabetes mellitus (DM), osteoarthritis, certain types of cancer, cardiovascular disease, depression, low self-esteem, as well as pelvic floor disorders (PFD), such as urinary incontinence (UI), pelvic organ prolapse (POP), and fecal incontinence (FI) [1,2,3,4,5,6]. Also, obesity can affect sexual function in both men and women [7, 8]. Compared with men, obese women experience more sexual difficulties [9]. The relationship between obesity and female sexual dysfunction (FSD) appears to be particularly true for individuals with PFD [10]. Relevant literature has demonstrated the prevalence of FSD between 78.3% and 91.7% in obese and overweight populations [11, 12].
Bariatric surgery is the most effective treatment for patients with obesity. Change in sexual function has been described postoperatively. Previous meta-analyses have indicated that bariatric surgery leads to significant increase in sexual function in men [13, 14]. However, a more limited degree of improvement is achieved in women, as reflected by non-significant change of Female Sexual Functioning Index (FSFI) score [14]. With regard to this aspect, Janik et al. [15] compared sexual function after weight loss with controls seeking bariatric surgery in a cross-sectional study, suggesting that there were no differences in the prevalence of FSD and the median FSFI score between groups. Not only that, no change in sexual function after bariatric surgery was observed in women with PFD [16]. Nevertheless, numerous studies have reported beneficial modifications in female sexual function following weight reduction [17, 18].
As yet, the literature on the effect of bariatric surgery on sexual function in obese women is considerable and has not been comprehensively meta-analyzed. Therefore, the aim of our study was to make a meta-analysis regarding the impact of bariatric surgery on female sexual function.
Methods
Search Strategy
A search was conducted in August 2019 of PubMed, EMBASE, and the Cochrane Central Register of Controlled Trails (CENTRAL), covering a period from inception to August 2019, with no language limitations, and using the search terms “bariatric surgery,” “metabolic surgery,” “weight loss surgery,” “obesity surgery,” “gastric bypass,” “sleeve gastrectomy,” “gastric banding,” “biliopancreatic diversion,” “duodenojejunal bypass,” “sexual function,” “sexual life,” “sexual functioning,” and “sexual dysfunction,” using the Boolean operators “AND” and “OR.” We also searched the references of published studies manually to ensure that relevant articles were not missed. This meta-analysis was completed following the Preferred Reporting items for Systematic Reviews and Meta-Analyses (PRISMA) [19].
Inclusion and Exclusion Criteria
Articles were enrolled in this meta-analysis if they met the following criteria: (1) obese women with BMI > 30 kg/m2, (2) studies regarding the effect of bariatric surgery on female sexual function, (3) provided the preoperative and postoperative data, (4) used at least one of the following outcome measures: the FSFI [20] and the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12) [21]. In terms of any lack of data, we would contact the authors by email for complete information if possible. Exclusion criteria were as follows: case reports, insufficient data, meta-analyses, comments, letters, conference abstracts, and review articles.
Selection Process and Data Abstraction
Two independent researchers screened the titles, abstracts, and full text following the inclusion and exclusion criteria. Discrepancies that occurred at the title and abstract screening stages were directly incorporated into the full-text assessment to ensure that all relevant papers were not omitted. Discrepancies at the full-text stage were resolved by discussion or with a third investigator. A standard data extraction form was used to collect the following information: the first author, publication year, country, study design, study population, type of bariatric surgery, study sample size, mean age at time of surgery, length of follow-up, mean BMI before surgery, and outcome indicators.
Outcomes Assessed and Risk of Bias Assessment
The primary outcomes of interest included the FSFI and the PISQ-12. In order to more accurately understand the effect of surgery on various aspects of sexual function, we conducted meta analysis on the six domains of FSFI (sexual desire, sexual arousal, lubrication, orgasm, sexual satisfaction, and sexual pain). Secondary outcomes were sex hormones (estradiol (E2), total testosterone (TT), luteinizing hormone (LH), follicle-stimulating hormone (FSH), sex hormone binding globulin (SHBG), and dehydroepiandrosterone sulfate (DHEA-S)). The quality of included study was accessed by two investigators independently using the Methodological Index for Non-Randomized Studies (MINORS) [22].
Statistical Analysis
All statistical analyses were performed on Review Manager (RevMan version 5.3) and Stata (version 12.0) with a level of significance set at p of < 0.05. Mean difference (MD) or standardized mean difference (SMD) with a corresponding 95% CI, when appropriate, were calculated for continuous outcomes. Mean and standard deviation were calculated for studies that only reported median and interquartile range using the computing method proposed by Wan et al. [23]. The homogeneity of different studies was measured using the I2 statistic, with a significance threshold of I2 > 50% [24]. A random effect model was used if the I2 statistic was significant. Otherwise, a fixed effect model would be used. Sensitivity analyses were conducted by using the one-study-out method and changing the effects model. In order to identify potential sources of heterogeneity, subgroup analyses were performed according to different types of study population (women with PFD vs. women without PFD) and follow-up time (≥ 12 months vs. < 12 months). Due to the most commonly used bariatric procedure being RYGB and SG, we also conducted a subgroup analysis of related outcomes with studies that conducted RYGB or/and SG. In addition, we did a meta-regression to analyze the relationship between change of sexual function and preoperative sexual function and amount of weight loss (% total weight loss) if necessary and feasible. The Egger test was used to assess publication bias for the included studies ≥ 10.
Results
Literature Retrieval Results and Basic Characteristics
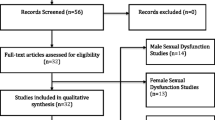
The initial literature search yielded 329 citations, and three other publications were added to the search result by manual search. After removing the duplicate investigations and screening the titles and abstracts, 249 articles were excluded. Eighty-three publications were identified for eligibility of inclusion criterion in full text. Ultimately, 20 studies were selected for inclusion. Of the included studies, one study had two arms, which were analyzed separately in this meta-analysis. The detailed process of study selection is shown in Fig. 1.
The characteristics of included studies are presented in Table 1. The preoperative mean age distribution of patients with obesity centralized from 41.3 to 48.8 years, and the mean BMI before surgery ranged from 39.7 to 52.2 kg/m2. The follow-up period ranged from 3 to 48 months. Most of the studies (13/21; 61.9%) had performed more than one type of bariatric surgery and did not report separate outcomes for specific operative methods. The study quality assessment is displayed in Table 2.
Primary Outcomes
FSFI
Sixteen trials reported the outcome of FSFI in 881 patients. Because of between-study heterogeneity (I2 = 94%), random effect model was used to pool results and showed that the total FSFI score significantly increased after surgery (MD = − 5.15, 95% CI − 7.66 to − 2.55, p = 0.0001) (Fig. 2).
Grouping the studies by type of study population, heterogeneity could only be resolved for the two studies focused on women with PFD (MD = 0.24, 95% CI − 0.85 to 1.33, p = 0.67), but the pooled result was statistically significant for the 14 focused on women without PFD (MD = − 6.02, 95% CI − 8.01 to − 4.03, p < 0.00001). Stratifying the researches by follow-up duration led to homogeneous result for six studies with short-term follow-up (< 12 months) (MD = − 5.70, 95% CI − 7.04 to − 4.36, p < 0.00001), but not for the 10 studies with long-term follow-up (≥ 12 months) (MD = 1.06, 95% CI 0.29 to 1.83, p = 0.007). When we looked at the subgroup of RYGB or/and SG, a significant pooled result (MD = − 6.84, 95% CI − 8.29 to − 5.39, p < 0.00001) was found. The I2 was 0, indicating no presence of heterogeneity (Table 3).
In sensitivity analysis, the pooled results did not markedly change when any one research was removed in sequence, with a range from − 5.58 (95% CI − 7.64 to − 3.52) to − 4.65 (95% CI − 6.53 to − 2.77). Additionally, the improvements in female sexual function after bariatric surgery were confirmed by changing the random effect model to the fixed effect model (MD = − 6.09, 95% CI − 6.63 to − 5.54, p < 0.00001). No significant publication bias was seen with Egger test (p = 0.065).
Meta-regression analysis (Table 4) indicated that the baseline FSFI score and the amount of weight loss were not significant predictors of the change in the total FSFI score after surgery (p > 0.05 for all).
Sexual Desire, Satisfaction, and Pain
Ten studies involving 593 patients were brought into the meta-analysis of sexual desire, sexual satisfaction, and sexual pain. I2 was 79%, 58%, and 58%, respectively, indicating the presence of heterogeneity. The random effect models showed that bariatric surgery was associated with prominent ameliorations in sexual desire (MD = − 0.79, 95% CI − 1.10 to − 0.49, p < 0.00001) (Fig. 3a), sexual satisfaction (MD = − 0.88, 95% CI − 1.21 to − 0.56, p < 0.00001) (Fig. 3b), and sexual pain (MD = − 0.50, 95% CI − 0.86 to − 0.14, p = 0.007) (Fig. 3c). Subgroup analysis with RYGB and/or SG did not change these significant outcomes (MD = − 1.12, 95% CI − 1.60 to − 0.63, p < 0.00001, MD = − 1.22, 95% CI − 1.66 to − 0.78, p < 0.00001 and MD = − 0.69, 95% CI − 1.34 to − 0.03, p = 0.04, respectively). In the meta-analysis of sexual desire, subgroup analysis showed either no significance or high heterogeneity. Stratifying the studies by follow-up time in the meta-analysis of sexual satisfaction, heterogeneity could be resolved (Table 3).
When using the one-study-out method separately, no pooled estimates were remarkably altered. What is more, the fixed effect models yielded similar results with the random effect analyses. However, we observed that I2 decreased from 58 to 33% in the meta-analysis of sexual pain after omitting the study by Olivera et al. [25]. No significant publication bias was found using Egger test for sexual desire (p = 0.127), satisfaction (p = 0.299), and sexual pain (p = 0.488).
Sexual Arousal, Lubrication, and Orgasm
Ten studies reported preoperative and postoperative scores of sexual arousal, lubrication, and orgasm, including 593 participants. No significant heterogeneity was seen between studies (I2 < 50% in all), and the fixed effect models showed that patients’ sexual arousal (MD = − 0.85, 95% CI − 1.06 to − 0.64, p < 0.00001) (Fig. 4a), lubrication (MD = − 0.66, 95% CI − 0.90 to − 0.42, p < 0.00001) (Fig. 4b), and orgasm (MD = − 0.70, 95% CI − 0.93 to − 0.47, p < 0.00001) (Fig. 4c) scores after bariatric surgery were significantly higher than before surgery. Subgroup analyses were not performed because of homogeneity. To verify the stability of pooled results, we conduct sensitivity analysis by using different effect models. The random effect model also indicated that bariatric surgery resulted in significant improvements in sexual arousal (MD = − 0.85, 95% CI − 1.13 to − 0.56, p < 0.00001), lubrication (MD = − 0.69, 95% CI − 1.02 to − 0.37, p < 0.0001), and orgasm (MD = − 0.70, 95% CI − 0.98 to − 0.43, p < 0.00001). The p values of the Egger test for sexual arousal (p = 0.299), lubrication (p = 0.280), and orgasm (p = 0.233) suggested that there were no significant publication bias.
PISQ-12
Seven studies examined the effect of bariatric surgery on PISQ-12 score, with a total of 509 participants, I2 = 94%, indicating significant heterogeneity. Based on a random effect model, meta-analysis showed no statistical significant change in PISQ-12 score (MD = − 0.21, 95% CI − 3.13 to 2.71, p = 0.89) (Fig. 5). Grouping these researches by type of study population did not resolve heterogeneity, but we found the unchanged PISQ-12 score only occurred in women with PFD. When we analyzed the subgroups classified by follow-up duration and specific surgical group, no homogeneous results were observed (Table 3).
When omitting any one study out in turn, the pooled results were not observably changed, with a range from − 0.73 (95% CI − 4.03 to 2.56) to 0.44 (95% CI − 2.64 to 3.51). Also, after changing the random effect model to the fixed effect model, the pooled result was still not statistically significant (MD = 0.18, 95% CI − 0.54 to 0.89, p = 0.63), so bariatric surgery had no significant effect on PISQ-12.
Secondary Outcomes
Sex Hormones
To explore the changes in sex hormone levels before and after bariatric surgery was our secondary purpose. Therefore, only two studies were included in the meta-analysis of estradiol, TT, LH, FSH, SHBG, and DHEA-S (Fig. 5a–f), with 200 patients overall. The fixed effect model analysis showed that bariatric surgery was associated with decreased TT (MD = 26.07, 95% CI 20.26 to 31.88, p < 0.00001, I2 = 0) and increased LH (MD = − 7.28, 95% CI − 0.90 to − 4.67, p < 0.00001, I2 = 0), FSH (MD = − 17.02, 95% CI − 22.97 to − 11.07, p < 0.00001, I2 = 0), and SHBG (SMD = − 1.27, 95% CI − 1.48 to − 1.05, p < 0.00001, I2 = 0). In addition, the random effect model analysis showed that bariatric surgery could significantly decrease DHEA-S (MD = 36.62, 95% CI 16.90 to 54.33, p = 0.0002, I2 = 52%), but did not affect estradiol (MD = − 4.53, 95% CI − 112.43 to 103.37, p = 0.93, I2 = 63%).
Discussion
Sexual health is an important element of quality of life (QOL). Previous studies investigated the influence of bariatric surgery on sexual function using the sexual life subscale of the Impact of Weight on Quality of Life-Lite (IWQOL-Lite) questionnaire and revealed positive effects [8, 43,44,45,46,47,48]. Nonetheless, almost all these investigations examined change in sexual function in both women and men without separate outcomes of different gender. In the current study, we used the FSFI and PISQ-12 to evaluate women’s sexual function.
The FSFI is a well-validated and widely used tool. Changes in FSFI domains have been described in obese women after bariatric surgery. However, the results of previous studies are not constant. Some reported that all of the six parameters improved markedly [17, 18, 26, 28]; some found no alteration in any of these parameters [25], while others observed remarkable improvements in all the domains, except for pain [27], desire [31], or orgasm [32]. To the best of our knowledge, this is the first meta-analysis reporting the influence of bariatric surgery on female sexual function across all sexual components; we found that bariatric surgery significantly improved all aspects of female sexual function, as reflected by positive change in both the total FSFI score and each individual domain score. These findings were in accordance with three studies included in the meta-analysis without providing the detailed data of all sexuality subscales [18, 26, 28]. In addition, our results suggested that both RYGB and SG had the favorable effects on sexual function improvements. As for obese women with FSD, a beneficial effect of bariatric surgery has been described in a majority of included studies, which could be supported by remission rate of FSD [17, 26, 28, 31, 36], together with the increased total FSFI score (MD = −7.86; 95% CI (−11.94 to −4.22), p < 0.00001, data not shown).
The underlying mechanisms responsible for increased women’s sexual function after bariatric surgery are not well understood yet and may be explained by several reasons. First, it is about weight reduction. Weight loss through sibutramine and behavioral therapy has been showed to be associated with sexual function improvements in women [49]. Bariatric surgery–induced weight loss could also make a contribution [8, 50, 51]. Second, the ameliorations were likely connected not only with weight loss but also with other mechanisms, including mediation of related sex hormone [51], especially increase in E2, FSH, LH, and SHGB, and decrease in TT and DHEA-S [33, 35, 52,53,54]. However, we found that postoperative E2 levels were not significantly changed as compared with that before surgery. Considering the heterogeneity and small patient sizes, the pooled result should be cautiously treated. Third, alterations in psychological and mental conditions after weight loss could bring benefits to sexual function. For with obesity, dissatisfaction with physical appearance and body image negatively impacts sexual behavior [8, 55, 56], but postsurgical weight reduction could greatly enhance their sexual satisfaction. Furthermore, the massive loss of adipose tissue boosted their self-esteem, alleviated their anxiety as well as reduced depressive symptoms [15, 18, 27, 32, 57], thus increasing their sexual response. Fourth, women with DM [58,59,60] were at increased risk of sexual dysfunction. DM remissions after bariatric surgery can explain the ameliorations as well. Another noteworthy reason is that improvements in health-related quality of life (HRQOL) after weight loss following bariatric surgery may also play a role [8, 29, 51].
Nevertheless, research has yielded contradictory results regarding the link between alteration in sex hormones, physical aspects of QOL, body image, and depressive symptoms and postsurgical enhancement of female sexual function[34]. Also of note is that the huge benefit to sexual function may not be dependent on the amount of weight reduction; this standpoint is supported by the evidence that change in the total FSFI score was not associated with percentage of excess weight loss [26] or BMI reduction [29]. Similar to previous reports, our meta-regression results indicated that the increased FSFI score after surgery was not related to percentage of total weight loss (β = − 0.00, p = 0.97). With the in-depth research, Oliveira et al. [25] found that the increase of FSFI score was negatively connected with the baseline FSFI score. Moreover, Bond et al. [26] observed that greater sexual function improvements were significantly correlated with worse preoperative sexual function. Another study by Efthymiou et al. [29] also reported a negative association between improvements in total sexual satisfaction and baseline sexual function. However, the pooled results showed that postoperative improvements in sexual function could not be independently predicted by preoperative sexual function (β = − 0.04, p = 0.46). In view of current evidence, we believe that elevated sexual function is more likely to be the result of a combination of factors that are modified by bariatric surgery (i.e., body image, psychological and mental status, sex hormone levels, health- and weight-related quality of life, and DM remissions).
The PISQ-12 is a valid and reliable questionnaire focused on sexual function in patients with POP and/or UI. In this meta-analysis, sexual function in obese women with PFD was assessed by the FSFI and PISQ-12. We found that both PISQ-12 and FSFI were not notably changed after surgery. Our findings agreed with previous studies suggesting that bariatric surgery could not lead to improvements in female sexual function in patients with PFD [61]. In the sexual pain analysis, heterogeneity was derived from the article focusing on patients with PFD. These may be explained by the fact that PFD such as UI, POP, or FI may cause embarrassment or pain during sexual intercourse [10].
This meta-analysis was not without limitations. First, both FSFI and PISQ-12 were the self-reported patient questionnaires, which can possibly cause embarrassment in certain female participants and influence the accuracy of their response regarding their sexual satisfaction. Furthermore, there was significant heterogeneity in the outcome analyses. This may be ascribed to the varying lengths of follow-up, different patient populations, and different types of bariatric surgery of included studies. Another limitation is that bariatric surgery might improve female sexual function rests on the outcome of improved levels of sex hormones. Although we investigated the impact of bariatric surgery on sex hormone levels, due to limited data, we were unable to further explore the relationship between change in sex hormones and FSFI and its six domains. Finally, the majority of the included studies were observational in nature with no comparators.
Conclusion
Bariatric surgery appears to be effective in improving female sexual function in obese patients. However, our study also suggests that bariatric surgery has no benefits on sexual function in women with PFD. Randomized prospective studies with larger samples and longer follow-up are needed to confirm these findings.
References
Guh DP, Zhang W, Bansback N, et al. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9(1):88.
Preiss K, Brennan L, Clarke D. A systematic review of variables associated with the relationship between obesity and depression. Obes Rev. 2013;14(11):906–18.
Ozmen D, Ozmen E, Ergin D, et al. The association of self-esteem, depression and body satisfaction with obesity among Turkish adolescents. BMC Public Health. 2007;7(1):80.
Cortese S, Cuzzolaro M, Maffeis C, et al. Depressive symptoms and low self-esteem in obese children and adolescents. Minerva Pediatr. 2005;57(2):65.
Andrzej P, Wojciech L, Maciej K, et al. Obesity and pelvic floor disorders: a review of the literature. Med Sci Monit. 2016;22:1880–6.
Chen CCG, Gatmaitan P, Koepp S, et al. Obesity is associated with increased prevalence and severity of pelvic floor disorders in women considering bariatric surgery. Surg Obes Relat Dis. 2008;5(4):411–5.
Rowland DL, McNabney SM, Mann AR. Sexual function, obesity, and weight loss in men and women. Sex Med Rev. 2017;5(3):323–38. https://doi.org/10.1016/j.sxmr.2017.03.006. Review
Sarwer DB, Hanson AJ, Voeller J, et al. Obesity and sexual functioning. Curr Obes Rep. 2018;7(4):301–7. https://doi.org/10.1007/s13679-018-0319-6. Review
Kolotkin RL, Crosby RD, Gress RE, et al. Two-year changes in health-related quality of life in gastric bypass patients compared with severely obese controls. Surg Obes Relat Dis. 2009;5:250–6.
Ramalingam K, Monga A. Obesity and pelvic floor dysfunction. Best Pract Res Clin Obstet Gynaecol. 2015;29(4):541–7.
Rabiepoor S, Khalkhali HR, Sadeghi E. What kind of sexual dysfunction is most common among overweight and obese women in reproductive age? Int J Impot Res. 2017;29(2):61–4. https://doi.org/10.1038/ijir.2016.46.
Martins e Silva B, Rêgo LM, Galvão MA, et al. Incidence of sexual dysfunction in patients with obesity and overweight. Rev Col Bras Cir. 2013;40(3):196–202. English, Portuguese
Lee Y, Dang JT, Switzer N, et al. Impact of bariatric surgery on male sex hormones and sperm quality: a systematic review and meta-analysis. Obes Surg. 2019;29(1):334–46. https://doi.org/10.1007/s11695-018-3557-5. Review
Wen J-P, Wen L-Y, Zhao Y-J, et al. Effect of bariatric surgery on sexual function and sex hormone levels in obese patients: a metaanalysis. J Endocr Soc. Oxford University Press. 2018;2:117–32.
Janik MR, Bielecka I, Paśnik K, et al. Female sexual function before and after bariatric surgery: a cross-sectional study and review of literature. Obes Surg. 2015;25(8):1511–7. https://doi.org/10.1007/s11695-015-1721-8. Review
Montenegro M, Slongo H, Juliato CRT, et al. The impact of bariatric surgery on pelvic floor dysfunction: a systematic review. J Minim Invasive Gynecol. 2019;26(5):816–25. https://doi.org/10.1016/j.jmig.2019.01.013.
Lechmiannandan S, Panirselvam M, Muninathan P, et al. Resolution of female sexual dysfunction (FSD) among the obese multiethnic Malaysian women now a reality with bariatric surgery: a prospective pilot study in Malaysia. Obes Surg. 2019;29(5):1571–5. https://doi.org/10.1007/s11695-019-03722-w.
Cherick F, Te V, Anty R, et al. Bariatric surgery significantly improves the quality of sexual life and self-esteem in morbidly obese women. Obes Surg. 2019;29(5):1576–82. https://doi.org/10.1007/s11695-019-03733-7.
Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–41.
Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26(2):191–208.
Rogers RG, Coates KW, Kammerer-Doak D, et al. A short form of the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12). Int Urogynecol J Pelvic Floor Dysfunct. 2003;14(3):164–8. discussion 168. Erratum in: Int Urogynecol J Pelvic Floor Dysfunct. 2004 May-Jun;15(3):219
Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ J Surg. 2015;73(9):712–6.
Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. https://doi.org/10.1186/1471-2288-14-135.
Higgins JP, Thompson SG. Quantifying heterogeneity in a metaanalysis. Stat Med. 2002;21(11):1539–58.
Olivera CK, Herron DM, Kini SU, et al. Long-term quality of life and pelvic floor dysfunction after bariatric surgery. Am J Obstet Gynecol. 2012;207(5):431.e1-4. https://doi.org/10.1016/j.ajog.2012.06.041.
Bond DS, Wing RR, Vithiananthan S, et al. Significant resolution of female sexual dysfunction after bariatric surgery. Surg Obes Relat Dis. 2011;7(1):1–7. https://doi.org/10.1016/j.soard.2010.05.015.
Akan S, Uruc F, Aydin MT, et al. The effect of sleeve gastrectomy technique on women’s sexual function: a prospective study. Rev Int Androl. 2018;16(4):167–73. https://doi.org/10.1016/j.androl.2017.12.003. Erratum in: Rev Int Androl. 2019 Apr - Jun;17(2):78
Hernández Hernández JR, López-Tomassetti Fernández E, Caballero Díaz Y, et al. Remission of female sexual dysfunction in morbidly obese female patients with the Scopinaro procedure. Surg Obes Relat Dis. 2013;9(6):987–90. https://doi.org/10.1016/j.soard.2013.02.007.
Efthymiou V, Hyphantis T, Karaivazoglou K, et al. The effect of bariatric surgery on patient HRQOL and sexual health during a 1-year postoperative period. Obes Surg. 2015;25(2):310–8. https://doi.org/10.1007/s11695-014-1384-x.
Pichlerova D, Bob P, Zmolikova J, et al. Sexual dysfunctions in obese women before and after bariatric surgery. Med Sci Monit. 2019;25:3108–14. https://doi.org/10.12659/MSM.913614.
Goitein D, Zendel A, Segev L, et al. Bariatric surgery improves sexual function in obese patients. Isr Med Assoc J. 2015;17(10):616–9.
Assimakopoulos K, Karaivazoglou K, Panayiotopoulos S, et al. Bariatric surgery is associated with reduced depressive symptoms and better sexual function in obese female patients: a one-year follow-up study. Obes Surg. 2011;21(3):362–6. https://doi.org/10.1007/s11695-010-0303-z.
Sarwer DB, Spitzer JC, Wadden TA, et al. Changes in sexual functioning and sex hormone levels in women following bariatric surgery. JAMA Surg. 2014;149(1):26–33. https://doi.org/10.1001/jamasurg.2013.5022.
Sarwer DB, Wadden TA, Spitzer JC, et al. 4-Year changes in sex hormones, sexual functioning, and psychosocial status in women who underwent bariatric surgery. Obes Surg. 2018;28(4):892–9. https://doi.org/10.1007/s11695-017-3025-7.
Legro RS, Dodson WC, Gnatuk CL, et al. Effects of gastric bypass surgery on female reproductive function. J Clin Endocrinol Metab. 2012;97(12):4540–8. https://doi.org/10.1210/jc.2012-2205.
Oliveira CFA, Dos Santos PO, de Oliveira RA, et al. Changes in sexual function and positions in women with severe obesity after bariatric surgery. Sex Med. 2019;7(1):80–5. https://doi.org/10.1016/j.esxm.2018.10.001.
Leshem A, Shimonov M, Amir H, et al. Effects of bariatric surgery on female pelvic floor disorders. Urology. 2017;105:42–7. https://doi.org/10.1016/j.urology.2017.03.003.
Leshem A, Groutz A, Amir H, et al. Surgically induced weight loss results in a rapid and consistent improvement of female pelvic floor symptoms. Scand J Urol. 2018;52(3):219–24. https://doi.org/10.1080/21681805.2018.1447600.
Shimonov M, Groutz A, Schachter P, et al. Is bariatric surgery the answer to urinary incontinence in obese women? Neurourol Urodyn. 2017;36(1):184–7. https://doi.org/10.1002/nau.22909.
Cuicchi D, Lombardi R, Cariani S, et al. Clinical and instrumental evaluation of pelvic floor disorders before and after bariatric surgery in obese women. Surg Obes Relat Dis. 2013;9(1):69–75. https://doi.org/10.1016/j.soard.2011.08.013.
Romero-Talamás H, Unger CA, Aminian A, et al. Comprehensive evaluation of the effect of bariatric surgery on pelvic floor disorders. Surg Obes Relat Dis Off. 2016;12(1):138–43.
Whitcomb EL, Horgan S, Donohue MC, et al. Impact of surgically induced weight loss on pelvic floor disorders. 2012.
Wee CC, Fleishman A, Mccarthy AC, et al. Decision regret up to 4 years after gastric bypass and gastric banding. Obes Surg. 2019.
Strain GW, Kolotkin RL, Dakin GF, et al. The effects of weight loss after bariatric surgery on health-related quality of life and depression. Nutr Diabetes. 2014;4(9):e132.
Conason A, McClure Brenchley KJ, Pratt A, et al. Sexual life after weight loss surgery. Surg Obes Relat Dis. 2017;13(5):855–61. https://doi.org/10.1016/j.soard.2017.01.014.
Dymek MP, Le GD, Neven K, et al. Quality of life after gastric bypass surgery: a cross-sectional study. Obes Res. 2012;10(11):1135–42.
Sarwer DB, Wadden TA, Moore RH, et al. Changes in quality of life and body image after gastric bypass surgery. Surg Obes Relat Dis. 2010;6(6):608–14. https://doi.org/10.1016/j.soard.2010.07.015.
Billy HT, Sarwer DB, Ponce J, et al. Quality of life after laparoscopic adjustable gastric banding (LAP-BAND): APEX interim 3-year analysis. Postgrad Med. 2014;126(4):131–40. https://doi.org/10.3810/pgm.2014.07.2791.
Kim KK, Kang HC, Kim SS, et al. Influence of weight reduction by sibutramine on female sexual function. Int J Obes. 2006;30(5):758–63.
Merhi ZO. Bariatric surgery and subsequent sexual function. Fertil Steril. 2007;87(3):710–1. Review
Sarwer DB, Lavery M, Spitzer JC. A review of the relationships between extreme obesity, quality of life, and sexual function. Obes Surg. 2012;22(4):668–76. https://doi.org/10.1007/s11695-012-0588-1. Review
Ernst B, Wilms B, Thurnheer M, et al. Reduced circulating androgen levels after gastric bypass surgery in severely obese women. Obes Surg. 2013;23(5):602–7. https://doi.org/10.1007/s11695-012-0823-9.
Bastounis EA, Karayiannakis AJ, Syrigos K, et al. Sex hormone changes in morbidly obese patients after vertical banded gastroplasty. Eur Surg Res. 1998;30(1):43–7.
Eid GM, Mccloskey C, Titchner R, et al. Changes in hormones and biomarkers in polycystic ovarian syndrome treated with gastric bypass. Surg Obes Relat Dis. 2014;10(5):787–91.
Sarwer DB, Steffen KJ. Quality of life, body image and sexual functioning in bariatric surgery patients. Eur Eat Disord Rev. 2015;23(6):504–8. https://doi.org/10.1002/erv.2412. Review
van den Brink F, Smeets MA, Hessen DJ, et al. Positive body image and sexual functioning in Dutch female university students: the role of adult romantic attachment. Arch Sex Behav. 2016;45(5):1217–26. https://doi.org/10.1007/s10508-015-0511-7.
Steffen KJ, King WC, White GE, et al. Changes in sexual functioning in women and men in the 5 years after bariatric surgery. JAMA Surg. 2019; https://doi.org/10.1001/jamasurg.2018.1162.
Elyasi F, Kashi Z, Tasfieh B, et al. Sexual dysfunction in women with type 2 diabetes mellitus. Iran J Med Sci. 2015;40(3):206–13.
Rutherford D, Collier A. Sexual dysfunction in women with diabetes mellitus. Gynecol Endocrinol. 2005;21(4):189–92. Review
Giraldi A, Kristensen E. Sexual dysfunction in women with diabetes mellitus. J Sex Res. 2010;47(2):199–211.
Lian W, Zheng Y, Huang H, et al. Effects of bariatric surgery on pelvic floor disorders in obese women: a meta-analysis. Arch Gynecol Obstet. 2017;296(2):181–9. https://doi.org/10.1007/s00404-017-4415-8. Review
Funding
The authors have no support or funding to report.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
For this type of study, formal consent is not required.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gao, Z., Liang, Y., Deng, W. et al. Impact of Bariatric Surgery on Female Sexual Function in Obese Patients: a Meta-Analysis. OBES SURG 30, 352–364 (2020). https://doi.org/10.1007/s11695-019-04240-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-019-04240-5