Abstract
Background
A considerable number of patients require revisional surgery after laparoscopic adjustable gastric banding (LAGB). Studies that compared the outcomes of revisional sleeve gastrectomy (r-SG) and revisional Roux-en-Y gastric bypass (r-RYGB) after failed LAGB are scarce in the literature. Our objective was to determine whether significant differences exist in outcomes between r-SG and r-RYGB after failed LAGB.
Methods
From 2005 to 2012, patients who underwent laparoscopic r-SG and r-RYGB after failed LAGB were retrospectively compared and analyzed. Data included demographics, indication for revision, operative time, hospital stay, conversion rate, percentage excess weight loss (%EWL), and morbidity and mortality.
Results
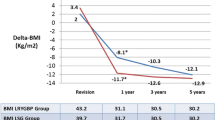
Out of 693 bariatric procedures, 42 r-SG and 53 r-RYGB were performed. The median preoperative weight (107.7 and 117.7 kg, respectively, p = 0.02) and body mass index (BMI) (38.5 vs. 43.2 kg/m2, respectively, p = 0.01) were statistically significantly lower in r-SG than in r-RYGB. The mean operative time and median hospital stay were significantly shorter in r-SG than in r-RYGB (108.4 vs. 161.2 min, p < 0.01) (2 vs. 3 days, p = 0.02), respectively. One patient underwent conversion to open surgery after r-RYGB (p = 0.5). The reoperation rate was lower in r-SG than in r-RYGB (0.0 vs. 3.8 %, p = 0.5). There was one postoperative leak in the r-RYGB, and the overall complication rate was significantly lower in r-SG patients than in r-RYGB patients (7.1 vs. 20.8 %, p = 0.05). The mean follow-up was significantly shorter in the r-SG group (9.8 vs. 29.3 months, p < 0.01). However, the mean postoperative BMI was not different at 1 year (32.3 vs. 34.7, p = 0.29) as well as mean %EWL was (47.4 vs. 45.6 %, p = 0.77).
Conclusions
Both r-SG and r-RYGB are safe procedures with similar outcomes in terms of %EWL. As a result of the long-term potential nutritional complication of r-RYGB, r-SG may be a better option in this group of patients. Longer follow-up is needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Obesity surgery is expanding all over the world. There are many surgical options for weight loss. Laparoscopic adjustable gastric banding (LAGB), introduced in the early 1990s [1], has been demonstrated to be safe and associated with low morbidity and mortality [2–4]. The long-term follow-up, however, has been unsatisfactory, both in terms of weight loss and the need for revision due to complications; the long-term failure rate after LAGB ranges from 30 to 50 % [5, 6]. Furthermore, LAGB complications including slippage, erosion, and pouch or esophageal dilatation may require revision in up to 25 % of patients [7, 8].
Parallel to the increasing acceptance of surgical treatment for morbid obesity, there is also a growing demand for revisional bariatric surgery.
Repeated banding has been demonstrated to be an ineffective option in many studies [9, 10]. Other procedures that have been demonstrated to be safe and feasible include gastric bypass [10–12], sleeve gastrectomy [13], and biliopancreatic diversion (BPD) and duodenal switch (DS) [14]. These procedures can be done by laparoscopy with low conversion and mortality rates [15]. However, revisional surgery is more demanding and is associated with higher complication (especially leakage) rates and longer hospital stay than primary procedures [16].
Sleeve gastrectomy or RYGB have been proposed as single-stage procedures and have been demonstrated to be safe and associated with adequate weight loss. Conversely, laparoscopic conversion to BPD or DS is technically demanding procedure with long operative times and a complication rate of up to 62 % [14].
It is not clear which procedure, restrictive or malabsorptive, is optimal after failed gastric banding. Few studies have compared the safety and weight loss after band removal followed by SG or RYGB. In our study, we retrospectively compared weight loss and complication rates between the two procedures.
Materials and methods
All records of patients undergoing revisional bariatric surgery from May 2005 to May 2012 were reviewed. Of 693 patients (340 SG, 353 RYGB), 95 patients underwent revisional procedures after LAGB. Band removal and r-SG (n = 42) or r-RYGB (n = 53) were performed because of failure of weight loss or because of complications from previous bariatric operations and form the population of this study.
Demographics, comorbid conditions, indications for revision, preoperative weight and body mass index (BMI), conversion, operative time, hospital stay, complications, overall mean follow-up, and mean BMI and percentage excess weight loss (%EWL) at 1 year were reviewed and compared.
Preoperative assessment
All patients underwent preoperative routine blood tests, ultrasound examination of the liver and gallbladder, barium upper gastrointestinal (GI) contrast studies, and gastroscopy to exclude gallstone disease and band complications (slippage, erosions). If pouch dilatation or slippage were detected, the band was deflated 1 month before revision. If gallstones were found, cholecystectomy was performed at the time of revision for both symptomatic and asymptomatic patients. Furthermore, patients with fatty liver found on US examination were put on a high-protein, low-carbohydrate diet 2–4 weeks before surgery to help reduce liver size. If band erosion was discovered at the time of gastroscopy, the band was removed with revision planned at a later stage. All patients who were positive for Helicobacter pylori were treated preoperatively with appropriate antibiotics. Sleep apnea was searched for routinely.
Surgical technique
All patients received preoperative low-molecular-weight heparin in addition to continuous pneumatic compression stocking application during surgery. Prophylactic antibiotics were provided preoperatively and continued until patient discharge. Patients were positioned in the French position (patient supine, legs apart), inclined in anti-Trendelenburg position, with the surgeon standing between the legs in r-SG or on the right side of the patient in r-RYGB. Pneumoperitoneum was established with a Veres needle inserted in the left subcostal area. The left liver lobe was retracted by a 5 mm trocar inserted in the subxiphoid position.
r-RYGB
Laparoscopic r-RYGB was performed using a five-port technique (5–12 mm, Excell, Ethicon Endosurgery, Cincinnati, OH, USA). Adhesions around the band were dissected completely, and the band was identified, divided, and removed. The lesser sac was entered from the lesser curvature, and all adhesions on the posterior wall of the stomach were dissected free in the direction of the left crus to avoid leaving a large pouch on the posterior wall. The stomach was transected below the band scar to avoid staple line disruption, and green loads (45 mm, Ethicon Endosurgery, Cincinnati, OH, USA) were used to create a 25–30 ml vertical proximal gastric pouch. The staple line was then oversewn with a continuous absorbable suture. We chose to perform a 150 cm alimentary limb if the patient’s BMI was >50 kg/m2 and a 100 cm limb if the BMI was <50 kg/m2. A gastrojejunostomy was performed side to side with a linear stapler (45 mm, blue load, Ethicon Endosurgery, Cincinnati, OH, USA), and the anterior wall defect was closed in two layers by hand-sewn continuous absorbable sutures. Enteroenterostomy was performed with a linear stapler (45 mm, white load, Ethicon Endosurgery, Cincinnati, OH, USA), and the anterior wall defect was closed in one layer by hand-sewn continuous absorbable sutures.
r-SG
Laparoscopic r-SG was performed using 3–4 port technique (5–12 mm, Excell, Ethicon Endosurgery, Cincinnati, OH, USA). After the scar covering the band was dissected, the band was left in place, and the omentum adjacent to the greater curvature and the short gastric vessels were divided with a Harmonic scalpel (Ethicon Endosurgery, Cincinnati, OH, USA) until reaching the left crus of the diaphragm. The band was dissected further until the whole fundus was mobilized from the posterior abdominal wall. The gastrogastric sutures were taken down to restore previous anatomy. The band was then cut and removed before division of the stomach. A 36F gastric bougie was inserted beyond the pylorus. r-SG was done using green loads (60 mm, Ethicon Endosurgery, Cincinnati, OH, USA) starting 3–4 cm from the pylorus up to the gastroesophageal junction. The staple line was then oversewn with continuous absorbable sutures.
The methylene blue test was performed to check for intraoperative leakage in all r-RYGB and r-SG patients. A closed suction drain was used routinely.
Postoperative management
All patients underwent an upper GI contrast study on postoperative day 2 to rule out any leak or stomal obstruction before oral intake and were discharged on the same day if there was no evidence of leak. Starting in 2011, we stopped performing routine postoperative GI contrast study, and patients were discharged on the second postoperative day if there was no evidence of sepsis and if they tolerated oral intake. Nasogastric tubes were not used in our patients.
Postoperatively, patients were placed on clear fluids for 2 weeks followed by pureed food for another 2 weeks and were allowed solid food after 4 weeks.
All patients were requested to receive proton pump inhibitor treatment postoperatively for 3 months. One week after surgery, all patients were also provided multivitamins, calcium, iron, and vitamin B12.
Study design
All patients who underwent laparoscopic band removal and r-SG or r-RYGB were compared. Data were collected and statistically analyzed by SPSS 17.0 for windows (SPSS, Chicago, IL, USA). Data were expressed as mean (range) ± SD. The chi-square test or Fisher’s exact test was used for categorical variables, as appropriate. The unpaired t test was used to assess the significance between means of two continuous variables. A p value of 0.05 or less was considered statistically significant.
Results
There was no statistical difference found in mean age (35.6 ± 10.4 vs. 39.0 ± 8.3 years, p = 0.08) or gender (F/M, 36/6 vs. 46/7, p = 0.5) between the r-SG and r-RYGB groups. Furthermore, there was no significant difference found in preoperative comorbidities or indications for surgery between the two groups (Table 1).
The median preoperative weight (107.7 vs. 117.7 kg) and BMI (38.5 vs. 43.2 kg/m2) was found to be significantly lower in the r-SG patients (p = 0.02, p = 0.01, respectively). The mean operative time (108.4 ± 20.1 vs. 161.2 ± 41.2 min) and the median hospital stay (2 vs. 3 days) were significantly longer for r-RYGB patients (p < 0.01 and p = 0.02, respectively) (Tables 1, 2).
Only one patient (1.9 %) required conversion after r-RYGB due to uncontrolled bleeding (p = 0.5), and two patients (3.8 %) required reoperation due to postoperative intra-abdominal bleeding and leaking (p = 0.5). Both patients were reoperated laparoscopically. The leak was found to be from the site of stapler line above the gastrojejunal anastomosis. The patient was reexplored, and the leak was sutured and the drains were left. However, the site continued to leak, and the patient’s condition was unstable. A stent was inserted at the site of the leak, and she improved. The stent was removed 6 weeks later without complications.
The overall postoperative complication rate was significantly higher among r-RYGB patients (7.1 vs. 20.8 %, p = 0.05) (Table 2). Postoperative superficial surgical site hematoma and ecchymosis was observed in two patients (4.8 %) after r-SG, and postoperative intra-abdominal bleeding requiring blood transfusion was present in one patient (2.4 %).
One r-RYGB patient developed a wound ecchymosis (1.9 %), and three patients developed postoperative bleeding (5.7 %). One patient required reoperation by laparotomy and splenectomy due to iatrogenic injury of the spleen, while two patients with melena and intra-abdominal bleeding were managed with blood transfusion only. An internal hernia was observed in two patients after r-RYGB, and both patients required surgical intervention (3.8 %). Postoperative adhesive intestinal obstruction was observed in one patient (1.9 %) who did not improve on conservative therapy and required laparotomy. Marginal ulcer was observed in one patient (1.9 %) who required resection of gastroenterostomy anastomosis with the ulcer, pouch reduction, and reanastomosis after failure to heal with medical treatment for more than 6 months. There was no mortality in either group (Table 2).
The overall mean follow-up among the r-RYGB was significantly longer because r-SG was introduced after r-RYGB (9.8 ± 8.7 vs. 29.3 ± 21.9, p < 0.01). The overall mean follow-up weight (90.0 ± 23 vs. 87.4 ± 13.8 kg, p = 0.5) and mean BMI (33.3 ± 8.1 vs. 32.6 kg/m2, p = 0.6) did not differ significantly. Furthermore, the overall mean %EWL was statistically significantly higher among r-RYGB patients (37.4 ± 22.8 vs. 49.1 ± 17.7, p = 0.01). However, the mean follow-up BMI (32.3 ± 6.4 vs. 34.7 ± 5.5, p = 0.2) and %EWL at 1 year (47.4 ± 21.6 vs. 45.6 ± 14.0, p = 0.7) was not significantly different between the two groups of patients (Table 3)
Compliance with postoperative vitamin intake was observed in 58.1 % after r-SG and in 69.8 % after r-RYGB patients (p = 0.2) (Table 2)
Discussion
Failure, which can follow any kind of procedure, can be related to many reasons, including complications or lack of compliance. In our center, the second-line option after failed gastric banding is either r-SG or r-RYGB.
At the beginning of our experience, RYGB was the procedure of choice after failure of the band. As SG became more popular, our patients preferred to have SG rather than RYGB because of the long-term complications after RYGB. Furthermore, because of lower morbidity and mortality, many of our patients were keen to have the band converted to another procedure to avoid frequent follow-up visits and reservoir filling constraints. This may explain the lower BMI observed in the r-SG group.
Laparoscopic sleeve gastrectomy (LSG) was first described as a staged procedure for super obesity, but it resulted in good weight loss with low morbidity [17, 18]. Patients experienced adequate weight loss, and because of its efficiency, technical simplicity, and low morbidity, surgeons adopted this procedure widely as a stand-alone procedure [18–20].
LSG has been demonstrated to be effective as a revisional bariatric procedure after failed gastric banding [21–23]. The leak rate, however, has been reported to be higher after revision of LAGB into LSG because of stapling the stomach in scared tissue, and dissection at the left crus can jeopardize the blood supply at the gastroesophageal junction [11–13].
Although it is debated whether performing another restrictive procedure after a failed restrictive procedure would help in reducing weight, it may be due to hormonal effects and reduction of ghrelin obtained by excision of the gastric fundus [24, 25].
Karamanakos et al. have demonstrated that the %EWL after LSG and RYGB was not statistically different at 1 year. However, ghrelin levels were statistically lower after LSG, and appetite suppression was greater at 1 year [25].
LSG does not alter bowel continuity, and therefore there are no mineral and vitamin deficiencies, except vitamin B12 because of the gastric resection [23]. Uglioni et al. [26] compared primary and secondary sleeve gastrectomy and found no difference in terms of morbidity, reoperation, or insufficient weight loss. However, Goitein et al. found significantly higher %EWL at 17 months in the group of patients who had primary sleeve than in patients who had synchronous band removal and sleeve gastrectomy. There were two leaks among the patients who underwent repeated procedures [27].
Laparoscopic revision to gastric bypass has been demonstrated to be feasible and effective for failed LAGB [10–12, 28–30]. Comparing primary treatment with r-RYGB was found to have similar safety profiles and weight loss [31, 32], although in other reports, it was demonstrated that in primary RYGB, the weight loss was more significant [33]. A major advantage of conversion to RYGB is the disappearance of esophageal motility disorders and the cure of gastroesophageal reflux symptoms [34]. In a case-matched case-control study, Martin et al. compared primary bypass and repeat resection bypass. In their series, revisional procedures were performed after different procedures, including bypass, vertical banded gastroplasty, and gastric banding. The operative time and hospital stay were longer among the repeat procedures. However, there was no mortality in either group, and morbidity was similar [32]. Cadiere et al. have also found similar results, with longer hospital stay and operative time in patients who underwent primary RYGB than secondary RYGB. Furthermore, the complications and reoperation rates were higher with the secondary RYGB patients. However, the follow-up %EWL was similar in both groups [35].
Band removal and revision to a different procedure in the same setting is still a controversial issue [36]. Because of the dense, fibrous tissue at the site of the band, the potential risks of leaks and bleeding are thought to be higher. However, there are no strong data to support this, which is in accordance with our results as well. We have adopted a policy of a single-step procedure in all our patients, unless there is evidence of slippage or erosion of the band. We think this option is better as long as it is feasible and safe to perform. It avoids another operation and avoids weight regain. After removal of the band, the pouch was calibrated using a 36F bougie to prevent occlusion of the gastroesophageal junction. Smaller bougies were used if the 36F did not pass easily through the band capsule. We performed our anastomosis in virgin tissue to avoid stricture or leak. We did not observe any strictures among our patients with the use of the linear stapler for the gastrojejunostomy, in accordance with the literature, which reported that linear stapling was associated with a lower stricture rate compared to circular stapled anastomosis [37].
The risk of postoperative complications are higher in patients who had revisional surgery and even higher after multiple revisions [16, 38]. The risk of leak after band removal and LSG might be due to the longer stapler line in LSG. Hallowell et al. [16] have demonstrated a ninefold increase in the leak rate, a 1.5-fold increase in the length of hospital stay, and a 2- to 5-fold increase in intensive care unit stay. However, all repeated procedures were done via an open technique. We had only one patient who had postoperative leak after band removal and RYGB at the staple line above the gastrojejunostomy. The patient underwent reexploration, but an attempt at drainage and laparoscopic closure of the leakage site failed, and the patient continued to experience leakage. A stent was inserted, and the leak was controlled. Six weeks later, the stent was removed and the patient recovered. Since then, we have adopted oversewing the stapler line on the proximal pouch in all patient after r-RYGB and after r-LSG. Our complication rate after r-SG was statistically lower than after r-RYGB (p = 0.05). Internal hernia and marginal ulcer can be serious complications after RYGB; such complications are not observed after SG. One could argue that the sleeve patients had fewer complications because their mean BMI was lower. However, most of the complications observed after RYGB were specifically related to the procedure itself (marginal ulcer, internal hernia, and intestinal obstruction). Furthermore, the leak after occurred early in our experience.
It has been demonstrated that %EBMI loss after revisional LSG is 42–46 % at 12 months’ follow-up [13, 26]. We began performing SG after RYGB, which explains the difference in the mean follow-up and the significant difference in %EWL between the two groups. However, when we compared the mean %EWL at 6 months and 1 year in either group, we found no significant difference (p = 0.4 and p = 0.7, respectively). We still need to find out what would be the long-term follow-up %EWL in patients who underwent r-SG so we can perform a better comparison.
Our results must be interpreted with caution until long-term results are available. Our series is relatively small and retrospective, with incomplete follow-up. Many of our patients were lost to follow-up, and at 1 year, only nine patients from the r-SG group and 25 patients from the r-RYGB patients were evaluated.
Although our study was retrospective, nonrandomized, and monocentric, we believe that both r-SG and r-RYGB are safe procedures with similar outcomes in terms of %EWL. Because of the complexity of r-RYGB and long-term potential nutritional complications, r-SG may be a better option in this group of patients. Further confirmation and longer follow-up are needed.
References
Belachew M, Jacqet P, Lardinois F, Karler C (1993) Vertical banded gastrosplasty vs adjustable silicone gastric banding in the treatment of morbid obesity: a preliminary report. Obes Surg 3(3):275–278
Weiner R, Blanco-Engert R, Weiner S, Motkowitz R, Schaefer L, Pomhoff I (2003) Outcome after laparoscopic adjustable gastric banding: 8 years experience. Obes Surg 13(3):427–434
Favretti F, Segato G, Ashton D, Busetto L, De Luca M, Mazza M, Ceoloni A, Banzato O, Calo E, Enzi G (2007) Laparoscopic adjustable gastric banding in 1,791 consecutive obese patients: 12-year results. Obes Surg 17(2):168–175
O’Brien PE, Macdonald L, Anderson M, Brennan L, Brown WA (2013) Long-term outcomes after bariatric surgery: fifteen-year follow-up of adjustable gastric banding and a systematic review of the bariatric surgical literature. Ann Surg 257(1):87–94
Suter M, Calmes JM, Paroz A, Giusti V (2006) A 10-year experience with laparoscopic gastric banding for morbid obesity: high long-term complication and failure rates. Obes Surg 16(7):829–835
DeMaria EJ, Sugerman HJ, Meador JG, Doty JM, Kellum JM, Wolfe L, Szucs RA, Turner MA (2001) High failure rate after laparoscopic adjustable silicone gastric banding for treatment of morbid obesity. Ann Surg 233(6):809–818
Morino M, Toppino M, Garrone C (1997) Disappointing long-term results of laparoscopic adjustable silicone gastric banding. Br J Surg 84(6):868–869
Tucker O, Sucandy I, Szomstein S, Rosenthal RJ (2008) Revisional surgery after failed adjustable gastric banding. Surg Obes Relat Dis 4(6):740–747
Gagner M, Gumbs AA (2007) Gastric banding: conversion to sleeve, bypass, or DS. Surg Endosc 21(11):1931–1935
Weber M, Muller MK, Michel JM, Belal R, Horber F, Hauser R, Clavien PA (2003) Laparoscopic Roux-en-Y gastric bypass, but not rebanding, should be proposed as rescue procedure for patients with failed laparoscopic gastric banding. Ann Surg 238(6):827–833
Spivak H, Beltran OR, Slavchev P, Wilson EB (2007) Laparoscopic revision from LAP-BAND to gastric bypass. Surg Endosc 21(8):1388–1392
van Wageningen B, Berends FJ, Van Ramshorst B, Janssen IF (2006) Revision of failed laparoscopic adjustable gastric banding to Roux-en-Y gastric bypass. Obes Surg 16(2):137–141
Iannelli A, Schneck A, Ragot E, Liagre A, Anduze Y, Msika S, Gugenheim J (2009) Laparoscopic sleeve gastrectomy as revisional procedure for failed gastric banding and vertical banded gastroplasty. Obes Surg 19:1216–1220
Topart P, Becouarn G, Ritz P (2007) Biliopancreatic diversion with duodenal switch or gastric bypass for failed gastric banding: retrospective study from two institutions with preliminary results. Surg Obes Relat Dis 3(5):521–525
Jones KB Jr (2005) Revisional bariatric surgery: potentially safe and effective. Surg Obes Relat Dis 1(6):599–603
Hallowell PT, Stellato TA, Yao DA, Robinson A, Schuster MM, Graf KN (2009) Should bariatric revisional surgery be avoided secondary to increased morbidity and mortality? Am J Surg 197(3):391–396
Silecchia G, Boru C, Pecchia A, Rizzello M, Casella G, Leonetti F, Basso N (2006) Effectiveness of laparoscopic sleeve gastrectomy (first stage of biliopancreatic diversion with duodenal switch) on co-morbidities in super-obese high-risk patients. Obes Surg 16(9):1138–1144
Brethauer S, Hammel J, Schauer P (2009) Systematic review of sleeve gastrectomy as staging and primary bariatric procedure. Surg Obes Relat Dis 5(4):469–475
Lalor PF, Tucker ON, Szomstein S, Rosenthal RJ (2008) Complications after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis 4(1):33–38
Roa PE, Kaider-Person O, Pinto D, Cho M, Szomstein S, Rosenthal RJ (2006) Laparoscopic sleeve gastrectomy as treatment for morbid obesity: technique and short-term outcome. Obes Surg 16(10):1323–1326
Berende CA, de Zoete JP, Smulders JF, Nienhuijis SW (2012) Laparoscopic sleeve gastrectomy feasible for bariatric revision surgery. Obes Surg 22(2):330–334
Jacobs M, Gomez E, Romero R, Jorge I, Fogel R, Celaya C (2011) Failed restrictive surgery: Is sleeve gastrectomy a good revisional procedure? Obes Surg 21(2):157–160
Acholonu E, McBean E, Court I, Bellorin O, Szomstein S, Rosenthal RJ (2009) Safety and short-term outcomes of laparoscopic sleeve gastrectomy as a revisional approach for failed laparoscopic adjustable gastric banding in the treatment of morbid obesity. Obes Surg 19(12):1612–1616
Cohen R, Uzzan B, Bihan H, Khochtali I, Reach G, Catheline JM (2005) Ghrelin levels and sleeve gastrectomy in super-super-obesity. Obes Surg 15(10):1501–1502
Karamanakos SN, Vagenas K, Kalfarentzos F, Alexandrides TK (2008) Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg 247(3):401–407
Uglioni B, Wölnerhanssen B, Peters T, Christoffel-Courtin C, Kern B, Peterli R (2009) Midterm results of primary versus secondary laparoscopic sleeve gastrectomy (LSG) as an isolated operation. Obes Surg 19:401–406
Goitein D, Feigin A, Segal-Leiberman G, Goitein O, Papa MZ, Zippel D (2011) Laparoscopic sleeve gastrectomy as a revisional option after gastric band failure. Surg Endosc 25:2626–2630
Brolin RE, Cody RP (2008) Weight loss outcome of revisional bariatric operations varies according to the primary procedure. Ann Surg 248(2):227–232
Nesset EM, Kendrick ML, Houghton SG, Mai JL, Thompson GB, Que FG, Thomsen KM, Larson DR, Sarr MG (2007) A two-decade spectrum of revisional bariatric surgery at a tertiary referral center. Surg Obes Relat Dis 3(1):25–30
Khoursheed M, Al-Bader I, Al-Asfar F, Mohammad A, Shukkur M, Dashti H (2011) Revision of failed bariatric procedures or Roux-en-Y gastric bypass. Obes Surg 21:1157–1160
Owens BM, Owens ML, Hill CW (1996) Effect of revisional bariatric surgery on weight loss and frequency of complications. Obes Surg 6(6):479–484
Martin MJ, Mullenix PS, Steele SR, Steele SR, See CS, Cuadrado DG, Carter PL (2004) A case-match analysis of failed prior bariatric procedures converted to resectional gastric bypass. Am J Surg 187(5):666–670
Zingg U, McQuinn A, DiValentino D, Kinsey-Trotman S, Game P, Watson D (2010) Revisional vs primary Roux-en-Y gastric bypass: a case-matched analysis: less weight loss in revisions. Obes Surg 20(12):1627–1632
Merrouche M, Sabate JM, Jouet P, Harnois F, Scaringi S, Coffin B, Msika S (2007) Gastro-esophageal reflux and esophageal motility disorders in morbidly obese patients before and after bariatric surgery. Obes Surg 17(7):894–900
Cadiere GB, Himpens J, Bazi M, Cadiere B, Vouche M, Capelluto E, Dapri G (2011) Are laparoscopic gastric bypass after gastroplasty and primary laparoscopic gastric bypass similar in terms of results? Obes Surg 21(6):692–698
Van Nieuwenhove Y, Ceelen W, Van Renterghem K, Van de Putte D, Henckens T, Pattyn P (2011) Conversion from band to bypass in two steps reduces the risk for anastomotic strictures. Obes Surg 21(4):501–505
Giordano S, Salminen P, Biancari F, Victorzon M (2011) Linear stapler technique may be safer than circular in gastrojejunal anastomosis for laparoscopic Roux-en-Y gastric bypass: a meta-analysis of comparative studies. Obes Surg 21(12):1958–1964
Roller JE, Provost DA (2006) Revision of failed gastric restrictive operations to Roux-en-Y gastric bypass: impact of multiple prior bariatric operations on outcome. Obes Surg 16(7):865–869
Acknowledgments
The authors thank Dr. Joseph Longenecker for his help and support with the statistical analysis.
Disclosures
Dr. Mousa Khoursheed, Dr. Ibtisam Al-Bader I, Dr. Ali Mouzannar, Dr. Abdulla Al-Haddad, Dr. Ali Sayed, Dr. Ali Mohammad, and Dr. Abe Fingerhut have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khoursheed, M., Al-Bader, I., Mouzannar, A. et al. Sleeve gastrectomy or gastric bypass as revisional bariatric procedures: retrospective evaluation of outcomes. Surg Endosc 27, 4277–4283 (2013). https://doi.org/10.1007/s00464-013-3038-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-013-3038-9