Abstract
Introduction
There is an ongoing debate on which procedure provides the best treatment for type 2 diabetes. Furthermore, the pathomechanisms of diabetes improvement of partly anatomically differing operations is not fully understood.
Methods
A loop duodenojejunostomy (DJOS) with exclusion of one third of intestinal length, a sleeve gastrectomy (SG), or a combination of DJOS + SG was performed in 8-week-old male ZDF rats. One, three, and six months after surgery, an oral glucose tolerance test and measurements of GLP-1, GIP, insulin, and bile acids were conducted.
Results
After an initial (4 weeks) equal glucose control, DJOS and DJOS + SG showed significantly lower glucose levels than SG 3 and 6 months after surgery. There was sharp decline of insulin levels in SG animals over time, whereas insulin levels in DJOS and DJOS + SG were preserved. GIP levels were significantly larger in both groups containing a sleeve at all three time points, whereas GLP-1 was equal in all groups at all time. Bile acid levels were significantly higher in the DJOS compared to the SG group at all time points. Interestingly, the additional SG in the DJOS + SG group led to lower bile acid levels 1 and 6 months postoperatively.
Conclusion
The effect of SG on glucose control was transient, whereas a duodenal exclusion was the more effective procedure in this model due to a sustained pancreatic function with a preserved insulin secretion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of type 2 diabetes (T2DM) and obesity is dramatically rising worldwide [1, 2]. Metabolic surgery clearly is the most effective treatment option for this disease and the only option prompting true diabetes remission in a relevant number of patients [3,4,5,6,7]. Despite this apparent clinical evidence, the physiological mechanisms have not been fully clarified yet.
Traditionally, the basic hypothesis explaining diabetes improvement is based on the idea that gastric bypass surgery leads to a diversion of the foregut (foregut theory) and an increased stimulation of the hindgut (hindgut theory). Which of these two theories has more validity is matter of an ongoing debate [8]. Although there is substantial evidence that hindgut stimulation has an effect, recently published manuscripts demonstrate that foregut exclusion is primarily responsible for the anti-diabetic effect seen after metabolic surgery and that the hindgut plays a subordinate role [9,10,11].
With the spread of laparoscopic sleeve gastrectomy (LSG) in the last decade, examinations of its effect on T2DM have ensued. Indeed, LSG exerts an impressive anti-diabetic effect similar to results after biliopancreatic diversion and Roux-en-Y-gastric bypass surgery (RYGB) [12,13,14]. The anti-diabetic effect occurs rapidly after surgery and before relevant weight loss, suggesting multiple driving mechanisms [15, 16]. Furthermore, LSG leads to typical modifications of gastrointestinal hormones [17, 18]. However, there is “increasing evidence that RYGB and LSG influence glycemic control through differential mechanisms” [19]. Hence, the results seen after LSG challenge the traditional idea of anti-diabetic mechanisms after metabolic surgery.
We therefore opposed a traditional metabolic model with a sleeve gastrectomy in diabetic rats in order to characterize the (possible) difference of the anti-diabetic effect of both operations aiming at a deeper understanding of diabetes improvement after metabolic surgery.
Material and Methods
Diets and Animals
Eight-week-old male obese Zucker diabetic fatty rats (ZDF-leprfa/CRL) were acquired from Charles River Breeding Laboratories (Wilmington, MA). Animal care was as described previously [11]. Rats were fasted 4 h before surgery, and 6 h before oral glucose tolerance test (OGTT) and hormone measurements. All animal experimental protocols were approved by the local animal welfare committee under the auspices of the responsible regional commission. All applicable institutional and national guidelines for the care and use of animals were followed.
Experimental Protocol
Rats were acquired and left to acclimatize with free access to food and water for at least 7 days. In one group of rats, we performed an OGTT 2 days prior to surgery. Rats were randomly assigned to the three operative groups: sleeve gastrectomy (SG), duodenojejunostomy (DJOS), and duodenojejunostomy with sleeve gastrectomy (DJOS + SG) using sealed envelopes. For evaluation of glucose metabolism, OGTTs were performed in all groups 1, 3, and 6 months after surgery. Hormone measurements were conducted 2 days after each OGTT and 20 min after glucose stimulation. Following the last procedure, the rats were euthanized with a lethal intracardial injection of potassium chloride (2 mmol/kg body weight) under general anesthesia. Body weight was recorded twice daily in the first week, twice a week for the remaining period. Food and water consumption were recorded in the first week after surgery only. Figure 1 illustrates the experimental protocol as well as numbers of animals operated and lost to follow-up.
Surgery
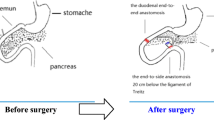
DJOS operations were performed as described previously [11]. In brief, after a midline incision of 3–4 cm, the total length of the small intestine was determined. The duodenum was then divided in pars 1. The remaining duodenal stump was closed using PDS 6/0 (Ethicon). The earlier defined jejunum was anastomosed via an end-to-side duodeno-enterostomy, excluding the duodenum and one third of total intestinal length. Mesenteric openings were closed with PDS 6/0 (Ethicon). For sleeve surgery, the great curvature of the stomach was exposed. The gastrocolic and gastrosplenic ligaments were divided using bipolar coagulation. Gastric resection was performed using an Endo GIA™ system (Universal Roticulator™ 60-2,5Stapling System, Covidien) beginning 5–8 mm above the level of pylorus. The staple line was reinforced using PDS 6/0 (Ethicon). For DJOS + SG, both steps were combined (Fig. 2).
Anesthesia was induced and maintained using isoflurane 2% (AbbVie Deutschland GmbH&Co.KG, Ludwigshafen, Germany) and oxygen flow at 2 l/min under spontaneous breathing [20]. Perioperative analgesia was conducted via subcutaneous carprofen (Rimadyl, Pfizer, Switzerland) injection (4 mg/kg body weight) at the beginning of the operation, and the next 3 days postoperatively. Additionally, buprenorphine (MSD SHARP & DOHME GmbH, Haar, Germany) was injected (0.05 mg/kg body weight) every 8 h for the first 24 h.
Animals were kept with free access to water and Fresubin energy drink (Fresenius Kabi Deutschland GmbH, Bad Homburg, Germany) on day 1. Oral food was continuously increased to free access until day 5 after operation.
Oral Glucose Tolerance Test
OGTTs were performed under general anesthesia and were initiated after placement of an orogastric tube (central venous catheter, Arrow Deutschland GmbH, Kernen, Germany) via infusion of a 70% glucose solution at the dosage of 1 g/kg body weight. Glucose was determined via tail tap at 0, 20, 60, 90, and 120 min using a glucose meter (Accu-Check Aviva, Roche Diagnostics Deutschland GmbH, Mannheim, Germany).
Hormone Measurement
The experimental setting was identical to the OGTT and as described previously. Twenty minutes after gastric glucose infusion, 400 μl blood were drawn via cannulization of the tail vein, using tubes containing 0.69 mg K3EDTA (Sarstedt AG & Co, Nümbrecht, Germany).
High-range rat insulin ELISA was a solid phase two-site enzyme immunoassay using HRP reaction for detection (DRG Instruments GmbH, Marburg, Germany). GLP-1 samples were added directly to a straptavidin-coated microtiter plate (EMD Millipore Corporation, Darmstadt, Germany). For GIP (1-42) and (3-42), a microtiter plate coated by a pre-titered amount of anti-GIP monoclonal antibodies was used (EMD Millipore Corporation, Darmstadt, Germany). Bile acids were measured with the total bile acids assay kit (Diazyme Laboratories, MDSS, Hannover, Germany).
Statistical Analysis
Statistical analyses were conducted using Prism 6 for Mac OS X (GraphPad Software, Inc.). The t test was used for group comparison. Curves were analyzed with two-way ANOVA. For glucose and body weight analysis, data from an earlier published SHAM group in an identical experimental setting were used [11]. For earlier SHAM surgery, an incision had been made and closed using PDS 6/0 (Ethicon) in the duodenum as well as small intestine. Applicable values of p < 0.05 were considered significant.
Results
Body Weight
Body weight developed largely similar between DJOS and SG animals until week 12, when SG animals reached their maximum body weight due to the natural course of ZDF pathology. DJOS animals in turn continued to gain body weight, reaching the maximum weight at the end of the study. Overall, the additional sleeve led to a temporarily slower weight gain (Fig. 3). Similar to the current SG, SHAM animals started to loose body weight within the observation period.
Glucose
DJOS and DJOS + SG led to a continuous and sustained improvement in glucose control towards earlier operated SHAM animals (two-way ANOVA p < 0.0005 for all time points) (Fig. 4). The additional sleeve in the DJOS + SG had no additive effect on glucose control at all time points (two-way ANOVA 1 month p = 0.9957, 3 months p = 0.2724 and 6 months p = 0.9977). SG alone could solely improve glucose tolerance temporarily, showing a similar effect to DJOS and DJOS + SG 1 month after surgery only (two-way ANOVA DJOS vs. SG p = 0.4337 and DJOS + SG vs. SG p = 0.7206). Preoperative levels could not be reached by either intervention.
Plot of blood glucose levels after OGTT with additional display of group means ± SD 1 (a), 3 (b), and 6 (c) months after surgery. As reference, preoperative glucose levels and SHAM-operated animals [11] are displayed. p values refer to results of a two-way ANOVA, markers in the graph reflect the significance level in relation to the SG group. a ***SG vs. SHAM p < 0.0001; SG vs. DJOS ± SG p = 0.7206 (n.s.), SG vs. DJOS p = 0.4337 (n.s.), SG vs. preOP p = 0.0217, DJOS vs. DJOS + SG p = 0.9957, DJOS vs. SHAM p = 0.0005, DJOS vs. preOP p < 0.0001, DJOS + SG vs. SHAM/preOP p = 0.0003. b ***SG vs. SHAM p < 0.0001, ###SG vs. DJOS ± SG p < 0.0001, πππSG vs. DJOS p < 0.0001; all other comparisons p < 0.0001 except DJOS vs. DJOS + SG p = 0.2724. c ππSG vs. DJOS p = 0.0005, ###SG vs. DJOS ± SG p < 0.0001, SG vs. SHAM p = 0.1341(n.s.); all other comparisons p < 0.0001 except DJOS vs. DJOS + SG p = 0.9977
Glucagon-Like-Peptide 1
The impact on stimulated GLP1-levels was not significantly different at all examined time points (Table 1, Fig. 5a). In the two groups with a duodenal exclusion (DJOS and DJOS + SG), there was a trend towards increased GLP-1 levels compared to SG alone. A pooled analysis of all three time points could confirm this difference (DJOS vs. SG Mann-Whitney p = 0.0078; DJOS + SG vs. SG p = 0.0097).
Glucose-Dependent Insulinotropic Polypeptide
Interestingly, both sleeve gastrectomy groups had significantly larger GIP levels than a duodenal exclusion alone. This effect was consistent at all three time points (Table 1). In turn, there was no difference between SG and DJOS + SG at all time points (Table 1, Fig. 5b).
Insulin
Three months postoperatively, there was a sharp decline of insulin levels in animals with a sleeve alone (SG insulin levels 1 vs. 3 months Mann-Whitney p = 0.0186 and 3 vs. 6 months p = 0.0371), whereas the insulin production in the DJOS and DJOS + SG groups was preserved (insulin DJOS/DJOS + SG 1 month vs. 3 months Mann-Whitney p = 0.5919 for DJOS and p = 0.9815 for DJOS + SG). This effect was sustained in the 6-month follow-up with again significantly decreasing insulin levels in the SG group alone SG insulin levels 3 vs. 6 months Mann-Whitney p = 0.0195). The additional DJOS in the DJOS + SG group seemed to preserve insulin secretion in DJOS + SG animals when directly compared to SG animals 6 months postoperatively (Table 1, Fig. 6a).
Box plot of insulin (a) and bile acid levels (b) with SD 20 min after oral glucose infusion at 1, 3, and 6 months postoperatively. a Ω: There is a significant decrease of insulin production over time in the SG group (1 vs. 3 months p = 0.0147, 3 vs. 6 months p = 0.0195). b π π SG vs. DJOS, #, ##SG vs. DJOS ± SG p ≤ 0.0001, & DJOS vs. DJOS + SG Mann-Whitney p < 0.05 and p < 0.01, respectively
Bile Acid
Serum bile acid levels were significantly higher in the DJOS compared to the SG group at all time points (Table 1, Fig. 6b). Interestingly, the additional sleeve in the DJOS + SG group largely led to lower bile acid levels, finally resulting in similar bile acid levels compared to SG alone 6 months after operation (Table 1).
Discussion
There is a debate on which surgery produces the best therapeutic results for T2DM. One reason for the ongoing debate is the fact that the effect of time is not fully understood [21]. Possibly, improvement of T2DM is more transient in some procedures than in others.
In the current study, 1 month after surgery all three interventions had a similar impact on glucose tolerance, improving it towards SHAM yet not leaving it within preoperative ranges. Total remission could not be achieved, which is the natural course of ZDF rats. SG and RYGB in diabetic rat models result in equal glucose control in a short-term follow-up (4–8 weeks) [22, 23]. The current analysis clearly demonstrated that the glucose improving effect with a sleeve alone (SG) was transient. Already 3 months postoperatively, both duodenal exclusion groups performed significantly better, 6 months after surgery SG glucose levels were similar to those of earlier operated SHAM animals at that time point [11]. This is a circumstance confirming the results of Wang et al. showing faster diabetes relapse after sleeve gastrectomy compared to RYGB after initially equal glucose control [24]. We interpret the fact that the add-on sleeve gastrectomy in the DJOS + SG group showed no additional benefit regarding glucose control to corroborate this hypothesis. This suggests that the major effect on glucose control was generated by the duodenal exclusion.
Regarding the time-course and mechanism of diabetes improvement, our 1-month results point in a similar direction. All procedures had a similar impact on glucose tolerance despite fundamentally different anatomic (re-)constructions. Perhaps a contributing factor of improved glucose tolerance is GLP-1. GLP-1 levels were equal in all three groups at that time despite fundamentally different anatomic reconstructions, hence possibly indicating a differential mechanism of GLP-1 stimulation [25]. This is a fact known from other studies [16, 22, 26]. In other words, GLP-1 either has an ameliorating effect in all or none of the groups. The latter hypothesis is supported by the fact that GLP-1 levels stayed on the same level in all three groups throughout the study despite significantly better glucose tolerance in both DJOS groups. In previous experiments, our group demonstrated a similar independence of GLP-1 in the same animal model [11]. Furthermore, Buchwald et al. concluded that glucose control was independent of GLP-1 in a model of ileal resection and exclusion [27]. Paradoxically, GLP-1 was even increased after resection of the L-cell-rich ileum in this study, moreover questioning the origin of GLP-1. Although GLP-1 was considered to be the key player of diabetes remission, there is an ongoing debate not only on its impact but also on its origin [28]. In the current study, there is an overall better stimulation of GLP-1 in both groups with duodenal exclusion, supporting at least a hindgut origin of this messenger.
Regarding GIP, it seems as if sleeve gastrectomy leads to significantly higher levels even in the group with duodenal exclusion. This is surprising because GIP is thought to originate from the duodenum and proximal jejunum. Possibly, paracrine mechanisms are responsible for this observation. Functionally, GIP seems to play a subordinate role in diabetes improvement in the current study [29, 30]. Three and six months postoperatively, GIP levels in SG and DJOS + SG groups were equal despite significantly better glucose control in the DJOS + SG group. Furthermore, GIP levels in the DJOS + SG group were significantly higher compared to DJOS alone despite similar glucose control. Raghavendra et al. similarly concluded that GIP is not the crucial “foregut factor” after duodenojejunal bypass [29, 31].
Bile acids remain a possible mediator of improved glucose control in the current study. Especially 1 month after surgery, when glucose control is equal in all groups, bile acid levels differed significantly between the groups, suggesting that they do not play a major role in ZDF rats. Furthermore, bile acids remained significantly lower in both sleeve groups 6 months postoperatively despite equal glucose control in DJOS and DJOS + SG groups. However, there is substantial evidence that bile acids might be a key regulator of glucose homeostasis after metabolic surgery [10, 31,32,33]. Possibly, lower bile acid levels in the sleeve gastrectomy groups could not outweigh the beneficial effect of duodenal exclusion in the current study.
One of the most relevant findings of the current study is a preservation of insulin secretion in both groups with duodenal exclusion and nicely reflects the glucose outcome. Preservation of β-cell function and decrease of β-cell loss are phenomena that have been observed following different types of intestinal rerouting in diabetic rat models [35, 36]. Sleeve gastrectomy may lead to elevated insulin levels compared to SHAM as demonstrated 12 weeks after surgery in ZDF rats [31]. Sun et al. furthermore showed insulin secretion comparable with a combination of sleeve gastrectomy and duodenojejunal bypass in a longer follow-up, hence also indicating sustained insulin secretion [31]. In turn, the sleeve’s impact on insulin secretion faded 24 weeks postoperatively in another examination. Similar to the current study, this group showed that the combination of sleeve gastrectomy and intestinal loop led to more sustained glucose control than sleeve gastrectomy alone [37]. The loss of insulin secretion in the SG group was functionally reflected by a stagnation of body weight gain 12 weeks after surgery, followed by weight loss in the SG group. After initial weight gain, the loss of weight in adolescent ZDF rats mirrors an increasing deficit of pancreatic function [38, 39].
Conclusion
There is a relevant amelioration of glucose control in all groups in short-term follow-up examinations. This effect most likely neither depends on GLP-1 or GIP nor is it majorly dependent on bile acid secretion. Sleeve gastrectomy alone leads to transient diabetic improvement only, reflected by significantly sinking insulin levels in consecutive follow-ups. In turn, the anti-diabetic effect after duodenal exclusion is sustained, carried by a persistent pancreatic function. Adding restriction to duodenojejunostomy does not create an added effect on glucose control. In this animal model, duodenal exclusion appears to have the more relevant anti-diabetic impact.
References
WHO | Diabetes [Internet]. WHO. [cited 2016 Apr 19]. Available from: http://www.who.int/mediacentre/factsheets/fs312/en/.
Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. https://doi.org/10.1371/journal.pmed.0030442.
Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222(3):339–52. https://doi.org/10.1097/00000658-199509000-00011.
Ribaric G, Buchwald JN, McGlennon TW. Diabetes and weight in comparative studies of bariatric surgery vs conventional medical therapy: a systematic review and meta-analysis. Obes Surg. 2014;24(3):437–55. https://doi.org/10.1007/s11695-013-1160-3.
Maggard-Gibbons M, Maglione M, Livhits M, et al. Bariatric surgery for weight loss and glycemic control in nonmorbidly obese adults with diabetes: a systematic review. JAMA. 2013 Jun 5;309(21):2250–61. https://doi.org/10.1001/jama.2013.4851.
Lee W-J, Hur KY, Lakadawala M, et al. Gastrointestinal metabolic surgery for the treatment of diabetic patients: a multi-institutional international study. J Gastrointest Surg. 2012;16(1):45–52. https://doi.org/10.1007/s11605-011-1740-2.
Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366(17):1577–85. https://doi.org/10.1056/NEJMoa1200111.
Chai J, Zhang G, Liu S, et al. Exclusion of the distal ileum cannot reverse the anti-diabetic effects of duodenal-jejunal bypass surgery. Obes Surg. 2016;26(2):261–8. https://doi.org/10.1007/s11695-015-1745-0.
Flynn CR, Albaugh VL, Cai S, et al. Bile diversion to the distal small intestine has comparable metabolic benefits to bariatric surgery. Nat Commun. 2015;6:7715. https://doi.org/10.1038/ncomms8715.
Kohli R, Setchell KD, Kirby M, et al. A surgical model in male obese rats uncovers protective effects of bile acids post-bariatric surgery. Endocrinology. 2013;154(7):2341–51. https://doi.org/10.1210/en.2012-2069.
Laessle C, Michelmichel S, Marjanovic G, Kuesters S, Seifert G, Hopt UT, et al. Common channel length in bypass surgery does not impact T2DM in diabetic Zucker rats. Obes Surg [Internet]. 2017 Mar 9 [cited 2017 Mar 27]; Available from: http://springerlink.bibliotecabuap.elogim.com/10.1007/s11695-017-2611-z.
Switzer NJ, Prasad S, Debru E, et al. Sleeve gastrectomy and type 2 diabetes mellitus: a systematic review of long-term outcomes. Obes Surg. 2016;26(7):1616–21. https://doi.org/10.1007/s11695-016-2188-y.
Li J, Lai D, Wu D. Laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy to treat morbid obesity-related comorbidities: a systematic review and meta-analysis. Obes Surg. 2016;26(2):429–42. https://doi.org/10.1007/s11695-015-1996-9.
Aminian A, Brethauer SA, Andalib A, et al. Can sleeve gastrectomy “‘cure’” diabetes? Long-term metabolic effects of sleeve gastrectomy in patients with type 2 diabetes. Ann Surg. 2016 Oct 4;264(4):674–81. https://doi.org/10.1097/SLA.0000000000001857.
Peterli R, Steinert RE, Woelnerhanssen B, et al. Metabolic and hormonal changes after laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy: a randomized, prospective trial. Obes Surg. 2012;22(5):740–8. https://doi.org/10.1007/s11695-012-0622-3.
Romero F, Nicolau J, Flores L, et al. Comparable early changes in gastrointestinal hormones after sleeve gastrectomy and Roux-en-Y gastric bypass surgery for morbidly obese type 2 diabetic subjects. Surg Endosc. 2012;26(8):2231–9. https://doi.org/10.1007/s00464-012-2166-y.
Farey JE, Preda TC, Fisher OM, et al. Effect of laparoscopic sleeve gastrectomy on fasting gastrointestinal, pancreatic, and adipose-derived hormones and on non-esterified fatty acids. Obes Surg. 2017;27(2):399–407. https://doi.org/10.1007/s11695-016-2302-1.
Mallipedhi A, Prior SL, Barry JD, et al. Temporal changes in glucose homeostasis and incretin hormone response at 1 and 6 months after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2014;10(5):860–9. https://doi.org/10.1016/j.soard.2014.02.038.
Batterham RL, Cummings DE. Mechanisms of diabetes improvement following bariatric/metabolic surgery. Diabetes Care. 2016;39(6):893–901. https://doi.org/10.2337/dc16-0145.
Marjanovic G, Holzner P, Kulemann B, et al. Pitfalls and technical aspects during the research of intestinal anastomotic healing in rats. Eur Surg Res. 2010;45(3–4):314–20. https://doi.org/10.1159/000320768.
Lee W-J, Chong K, Ser K-H, et al. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus a randomized controlled trial. Arch Surg. 2011;146(2):143–8. https://doi.org/10.1001/archsurg.2010.326.
Eickhoff H, Louro TM, Matafome PN, et al. Amelioration of glycemic control by sleeve gastrectomy and gastric bypass in a lean animal model of type 2 diabetes: restoration of gut hormone profile. Obes Surg. 2015;25(1):7–18. https://doi.org/10.1007/s11695-014-1309-8.
Xu B, Yan X, Shao Y, et al. A comparative study of the effect of gastric bypass, sleeve gastrectomy, and duodenal–jejunal bypass on type-2 diabetes in non-obese rats. Obes Surg. 2015;25(10):1966–75. https://doi.org/10.1007/s11695-015-1835-z.
Wang K, Zhou X, Quach G, et al. Effect of sleeve gastrectomy plus side-to-side Jejunoileal anastomosis for type 2 diabetes control in an obese rat model. Obes Surg. 2016;26(4):797–804. https://doi.org/10.1007/s11695-015-1811-7.
Chambers AP, Smith EP, Begg DP, et al. Regulation of gastric emptying rate and its role in nutrient-induced GLP-1 secretion in rats after vertical sleeve gastrectomy. AJP Endocrinol Metab. 2014;306(4):E424–32.
Jiménez A, Casamitjana R, Flores L, et al. Long-term effects of sleeve gastrectomy and Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus in morbidly obese subjects. Ann Surg. 2012;256(6):1023–9. https://doi.org/10.1097/SLA.0b013e318262ee6b.
Buchwald H, Menchaca HJ, Michalek VN, et al. Ileal effect on blood glucose, HbA1c, and GLP-1 in Goto-Kakizaki rats. Obes Surg. 2014;24(11):1954–60. https://doi.org/10.1007/s11695-014-1307-x.
Ye J, Hao Z, Mumphrey MB, et al. GLP-1 receptor signaling is not required for reduced body weight after RYGB in rodents. AJP Regul Integr Comp Physiol. 2014;306(5):R352–62.
Rao RS, Kini S. GIP and Bariatric surgery. Obes Surg. 2011;21(2):244–52. https://doi.org/10.1007/s11695-010-0305-x.
Kindel TL, Yoder SM, D’Alessio DA, et al. The effect of duodenal–jejunal bypass on glucose-dependent Insulinotropic polypeptide secretion in Wistar rats. Obes Surg. 2010;20(6):768–75. https://doi.org/10.1007/s11695-010-0095-1.
Sun D, Liu S, Zhang G, et al. Type 2 diabetes control in a nonobese rat model using sleeve gastrectomy with duodenal–jejunal bypass (SGDJB). Obes Surg. 2012;22(12):1865–73. https://doi.org/10.1007/s11695-012-0744-7.
Ryan KK, Tremaroli V, Clemmensen C, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509(7499):183–8. https://doi.org/10.1038/nature13135.
Prawitt J, Caron S, Staels B. Glucose-lowering effects of intestinal bile acid sequestration through enhancement of splanchnic glucose utilization. Trends Endocrinol Metab. 2014;25(5):235–44. https://doi.org/10.1016/j.tem.2014.03.007.
Trabelsi M-S, Lestavel S, Staels B, Collet X. Intestinal bile acid receptors are key regulators of glucose homeostasis. Proc Nutr Soc. 2017;76(3):192–202.
Speck M, Cho YM, Asadi A, et al. Duodenal-jejunal bypass protects GK rats from -cell loss and aggravation of hyperglycemia and increases enteroendocrine cells coexpressing GIP and GLP-1. AJP Endocrinol Metab. 2011;300(5):E923–32.
Zhou X, Qian B, Ji N, et al. Pancreatic hyperplasia after gastric bypass surgery in a GK rat model of non-obese type 2 diabetes. J Endocrinol. 2016;228(1):13–23.
Zhong M-W, Liu S-Z, Zhang G-Y, et al. Effects of sleeve gastrectomy with jejuno-jejunal or jejuno-ileal loop on glycolipid metabolism in diabetic rats. World J Gastroenterol. 2016;22(32):7332–41. https://doi.org/10.3748/wjg.v22.i32.7332.
Shimabukuro M, Zhou Y-T, Levi M, et al. Fatty acid-induced β cell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci. 1998;95(5):2498–502. https://doi.org/10.1073/pnas.95.5.2498.
Fed Glucose and Insulin Values for Obese Male ZDF Rats Fed Purina 5008 (January 2010 – July 2010) [Internet]. [cited 2016 Jun 3]. Available from: http://www.criver.com/files/pdfs/rms/zdf/rm_rm_r_glucose_insulin_zdf_july_2010.aspx.
Acknowledgements
The authors thank Silke Hempel for the outstanding work in assistance with ELISA measurements. Moreover, we thank Claudia Bravo and Monika Kolterjahn for the excellent work with animal care.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Laessle, C., Nenova, G., Marjanovic, G. et al. Duodenal Exclusion but Not Sleeve Gastrectomy Preserves Insulin Secretion, Making It the More Effective Metabolic Procedure. OBES SURG 28, 1408–1416 (2018). https://doi.org/10.1007/s11695-017-3045-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-017-3045-3