Abstract
Demand for edible insects, especially crickets, for human consumption has trended increasingly as a result of their high nutrients. Apart from containing high protein, cricket also has a high fat content consisting of essential fatty acids, which can be produced as a valuable edible oil source. This study aimed to investigate the effect of green extraction methods on the chemical, physical, and biological properties of cricket oils. The full-fat cricket powder from Gryllus bimaculatus and Acheta domesticus was extracted using ultrasound extraction (UAE) with water as a green solvent, hot stirrer with water (SW), and mechanical hot press oil machine (HP). The Soxhlet extraction with n-hexane servesd as a control. The oil yield from both cricket species obtained by the UAE process was 72–75 g/100 g of total lipid content found by Soxhlet extraction, which was higher than SW and HP. The oils from both crickets extracted using the UAE showed significantly stronger antioxidant activity (DPPH and FRAP) and higher TPC compared to the oils obtained from other extraction methods. Similarly, L*, a*, and b* in the oils obtained from UAE were also found to have higher values. For the chemical quality of the oils, the FFA, PV, and TBA values of the oils obtained by UAE were slowler than other methods over 28 days of storage. The concentrations of α-linolenic (omega 3) and total mono-unsaturated fatty (MUFA) acids were also higher in both of the obtained cricket oils. The results suggested that ultrasound-assisted extraction is a potential green process for oil extraction to improve the quality of the cricket oil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The demand for edible insects for human consumption has trended increasingly around the world because of their highly valuable nutrients and their source of food security. Compared to conventional livestock, the production of edible insects could reduce land use, water consumption, greenhouse gas emissions, and feed conversion [1]. Crickets are one of the most popular edible insects, belonging to the family Gryllid, which can be divided into several different subfamilies. Among these families, field (Gryllus bimaculatus (Orthoptera: Gryllidae)) and house crickets (Acheta domesticus (Orthoptera: Gryllidae)) are two of the most popular edible crickets and are farmed economically in many countries. The nutritional data of these cricket species were most studied to assess the possibility of consuming insects as a source of health food [1,2,3]. These crickets contain high protein, essential fatty acids, and vitamins. Previous studies reported that edible cricket powder showed a high content of protein (42.0–45.8 g/100 g of dry matter) [3]. A. domesticus and G. bimaculatus cricket powder also contained significant levels of lipids (10–23 g/100 g of dry matter) with a rich source of essential fatty acids, such as linoleic and linolenic acids [2]. Moreover, the production of edible cricket ingredients such as high-protein powder and protein concentrates is challenging due to their high lipid content, which interferes with protein extraction, resulting in a decrease in protein yield. Therefore, the extraction of lipid or defatted ingredients needs to include the production of both functional oils and defatted cricket powder containing high protein content [4, 5]. Consequently, cricket oil may be used as an alternative food ingredient in the food industry in the future. The possibility of producing edible cricket oil is also interesting as an alternative food ingredient and functional oil containing omega-3 fatty acids and essential fatty acids [6, 7].
In general, the most conventional techniques used for the extraction of lipids from insects are Soxhlet combined with organic solvents such as n-hexane, methanol, and ethanol [4, 5]. In particular, n-hexane is most commonly used solvent to extract oils due to its easy evaporation, low energy cost, and high selectivity for oils. However, hexane is flammable, volatile, has green effects, and has consequential health problems [6, 7]. Therefore, safety, environmental, and economic aspects force industry to use green extraction. Aqueous oil extraction eliminates the problems associated with the use of n-hexane solvent and possibly improves the quality of the protein and oil obtained [8]. Nonetheless, the polarity and solubility of oil in an aqueous system are critical problems impacting its diffusivity and extractability [9]. Hence, extraction techniques to assist water as a solvent to increase mass transfer and obtain higher yields and quality of the oils is necessary.
Recently, ultrasound has become a popular technique for extracting bioactive substances from food materials with low process time, high penetration depth, and improved product quality and extraction yields. During the ultrasound extraction process, mechanical and cavitation phenomena induce rupture of the cell walls and particle size reduction, resulting in enhanced mass transfer across the cell membrane and, consequently, increased recovery yield of oils and bioactive compounds [7, 9]. Ultrasound extraction efficiency depends on several factors, including the chemical compounds and physical structures of the food materials, the types of solvent used, pretreatment techniques, and extraction conditions [6, 10]. Therefore, the application of ultrasound-assisted extraction (UAE) of oils from edible crickets is possible to improve product quality and increase recovery yield.
To our knowledge, there is a lack of published reports regarding green solvent-base extraction methods such as water extraction and mechanical extraction of the oils from crickets. However, using water as a solvent to extract the oil is unsuccessful since the oil and water are immiscible, therefore, this study was carried out using ultrasound treatment to assist oil extraction. The objectives of this study were to determine the chemical, physical, and biological properties of cricket oils extracted from Gryllus bimaculatus and Acheta domesticus powders as affected by different extraction methods, including ultrasound extraction with water as a green solvent (UAE), hot stirrer with water (SW), and mechanical hot press oil machines (HP). Soxhlet extraction with n-hexane was used as a control.
Materials and methods
Sample preparation
The cricket species in this study were field cricket (Gryllus bimaculatus) and house cricket (Acheta domesticus). Two frozen crickets of G. bimaculatus and A. domesticus were purchased from a local farm in Maha Sarakham, Thailand, that rearing by following the Standard Agricultural Practices (GAP, no. 8202/2562). Both cricket species were fed a feed consisting of fish meal, soybean meal, crushed Acacia leaves, maize meal, and rice bran until they reached full maturity (42–45 days) as adult crickets, which is the ideal age for cricket harvesting, processing, and consumption to avoid natural attrition and lower feed costs because feed conversion efficiency starts to decrease beyond this age.
The frozen cricket samples were thawed in a refrigerator (5 °C) and blanched for 10 min at 80 °C with a cricket-to-water ratio of 1:10 (w/w) before placing them in cold water (10 ± 1 °C). Blanched cricket samples were promptly dried using tray drying at 80 °C (Memmert, OLM-500, Germany) until the moisture content and water activity (aw) reached less than 0.6 and 10 ± 1%, wet basis, respectively. To obtain cricket powder samples, the whole dried cricket was finely ground in a coffee grinder and filtered through a 100-mesh sieve (less than 149 μm). Two cricket samples’ chemical composition, including moisture content, protein, ash, fat, and fiber, was assessed using the approved AOAC (2019) [11] procedures. The content of vitamin A (Retinol), α-tocopherol, and β-carotene in the cricket powder was also determined using a high-performance liquid chromatographic (HPLC) technique following the method of AOAC (2019) [11] before experimentation.
Cricket oil extraction using different methods
Four different methods were used for the extraction of the oils from G. bimaculatus and A. domesticus cricket powders. The yields of crude cricket oil was calculated by the following formula [12]:
where mo and mf are the masses of the extracted oils (g) and cricket powder (g), respectively.
Soxhlet extraction with n-hexane
The Soxhlet extraction was carried out by the method of AOAC (2019) [11]. Ten grams of cricket powder were placed into a thimble paper cone, and n-hexane was used to extract the cricket power in a Soxhlet apparatus for 4 h. Following that, hexane was removed using a hot air dryer (Binder Ovens, Germany) set to 105 °C until no solvent was detectable. The crude cricket oil obtained was stored in brown vials and kept at 4 °C until required for analysis.
Mechanical hot pressing (HP)
For hot-pressing, the cricket powders were directly pressed in an oil extraction expeller (Lab scale cricket oil extractor) at 115–120 °C to obtain the oil. The crude cricket oil was passed through a cooking oil filter bag (10 µ). Finally, clear oils were collected and stored in brown glass bottles at 4 °C for further analysis. Each extraction was repeated in triplicate.
Hot stirrer with water (SW)
The cricket powder was immersed and stirred in distilled water (ratio, 1:20 (w/v)), and the extraction was performed at 100 °C for 2 h under a hot plate magnetic stirrer (Daihan Scientific Co., Ltd., Korea) before centrifuging at 6000 g for 15 min at 4 °C. The upper layer was collected as crude cricket oil. All experiments were conducted in three repetitions.
Ultrasound extraction (UAE)
Ultrasound extraction with water as the solvent was applied to extract the oils from G. bimaculatus and A. domesticus cricket powder, following the method of our previous study [10] with small modifications. Cricket powder was added to distilled water at a ratio of 1:20 (w/v) and continuously mixed with a hot plate stirrer for 5 min. The mixture was then immersed in an ultrasound (VCX 500 Vibra-CellTM, Sonics and Materials Inc., USA) equipped with a titanium alloy probe (length 136 mm, weight 340 g) (Model CV334, USA). The ultrasound extraction was done at a maximum frequency and power of 20 kHz and 500 W for 20 and 30 min at a 40% amplitude level and ambient temperature. After ultrasonic pretreatment, the sample was centrifuged at 6000 g for 15 min (4 °C), and the oil layer was collected. All experiments were conducted in three repetitions.
Color measurement
The colors of the oils obtained from the different extraction methods were determined using a colorimeter (Minolta, model CR400, Japan) and calibrated before each analysis with white and black standard tiles. Color readings were expressed following the CIELAB system for L* (darkness to lightness), a* (green to red), and b* (blue to yellow).
Antioxidant activity determination
Clude cricket oil samples were treated with methanol and hexane before analysis following Loypimai et al. (2015) [13] with slight modifications. Briefly, 1.0 g of the oil sample was dissolved in 5 mL of mixed solvent (methanol and hexane, 3:2) by sonication (Vibra cell, 130 W, 20 kHz) for 5 min. Methanolic extracts were evaporated in a rotary evaporator, and the residue was dissolved in 2 mL of methanol and stored at -20 °C before antioxidant activity analysis. All extractions were performed in triplicate.
DPPH assay
DPPH radical scavenging activity was evaluated using a UV-Vis spectrophotometer (G10S UV-Vis model, Thermo Fisher Scientific, China). Results were compared with a reference standard (Trolox) and expressed as µg Trolox equivalent/g sample [14].
FRAP assay
The FRAP assay of the extracted sample, as the reduction of Fe3+-TPTZ to blue-colored Fe2+-TPTZ, was determined following Benzie and Strain (1996) [15] with slight modifications. The colored product reading (ferrous tripyridyltriazine complex) was measured at 539 nm, with results expressed in µM FeSO4 equivalent per gram (g) of the sample.
Total phenolic content (TPC) analysis
The TPC of the crude cricket oils was determined by the reaction of the extract to the Folin-Ciocalteu reagent following Iqbal et al. (2005) [16]. The absorbance at 765 nm was recorded using a UV-visible spectrophotometer (Shimadzu, Japan). A calibration curve was made with a standard solution of gallic acid (0–250 µg/mL). The data was calculated by comparing between the standard curve of gallic acid with the absorbance of each sample. TPC was expressed as mg gallic acid equivalents (GAE) per g of dry matter of the oil sample.
Changes in the chemical properties of crude cricket oils during storage
The changes in free fatty acids (FFA), peroxide value (PV), and thiobarbituric acid (TBA) of crude oil samples obtained from different extraction methods were measured using the AOCS (1998) standard methods [17]. The 10 g oil samples were placed into 15 mL brown glass vials and sealed with a plastic cover to prevent the air-sensitive chemicals from oxidizing. The samples were kept in a thermoelectric incubator at room temperature (25 ± 2 °C) for 28 days. During storage, 12 vials (three for each sample) were randomly picked on the first day and thereafter at seven-day intervals (7, 14, 21, and 28 days) and examined with three different assays. The FFA was measured by titrating the sample (1.0 g) with alkali and calculating the result (AOCS Cd 3a-63). The PV was measured by oil titration (0.5 g) with sodium thiosulfate solution (AOCS Cd 8–53). The measurement of the TBA value was done by heating a 5 mL aliquot of a solution of the sample (50–200 mg) in 25 mL 1-butanol with a 5 mL TBA reagent at 95 °C for 120 min and reading the absorbance at 530 nm. All determinations were carried out in triplicate.
Fatty acid composition analysis
The fatty acid porfiles of crude oils from both cricket species obtained from UAE and Soxhlet extraction methods was compared following the method of AOAC (2019) using a gas chromatograph (GC) [11]. The oil sample (2.0 g) was added to 50 mL of a chloroform-methanol (2:1) mixture solution and placed in a vibration shaker for 30 min. The fatty acids in the oil samples were then esterified to fatty acid ethyl esters (FAME) using 2.0 mL of boron trifluoride-methanol (14% BF3) reagent and heated at 100 °C for 15 min. After cooling, 200 µL of n-heptane was added to the mixture, agitated manually for a minute, and then filtered through a 0.45 μm nylon syringe filter before injection into the GC. The GC apparatus (Agilent 7890B, Agilent Technologies, Inc., Santa Clara, CA) was equipped with a Supelco SPTM 2560 capillary column (100 m × 0.25 mm × 0.2 μm) and a flame ionization detector (FID). The temperature of the injector was set at 225 °C, whereas that of the detector was 240 °C. The carrier gas used was nitrogen, with a flow rate of 0.18 m/s. Fatty acids were measured and expressed as the quantity of each fatty acid in the total acids present in the sample. Omega-3 (n-3) fatty acids, omega-6 (n-6) fatty acids, total saturated fatty acids (SFA), total mono-unsaturated fatty acids (MUFA), and polyunsaturated fatty acids (PUFA) were also determined.
Statistical analysis
The results were reported as mean values and standard deviations from triplicate samples of each treatment for all experiments. The data regarding the chemical, physical, and biological properties of oil extracted from both cricket species were analyzed using a one-way ANOVA using the SPSS trial version. Duncan’s Multiple Range Test was performed to identify significant differences between extraction methods for each cricket species. The student t-test was used to compare significant differences in the chemical, physical, and biological properties among cricket species and between UAE and Soxhlet fatty acid extraction methods. Statistical significance was determined at p < 0.05.
Results and discussion
Nutritional compositions of cricket powders
Table 1 shows the nutritional compositions of cricket powders from G. bimaculatus and A. domisticus before being oil extracted. The results demonstrated a statistically significant difference in the protein, fat, ash, vitamin A, vitamin E, and β-carotene content between the two cricket species. Compared to A.domesticus, cricket powder from G. bimaculatus had significantly (p < 0.05) higher content of fat (23.5 ± 1.40 g/100 g), vitamin A (14.7 ± 0.20 µg/100 g), and β-carotene (161 ± 2.52 µg/100 g), whereas protein (62.2 ± 1.68 g/100 g), ash (3.67 ± 0.22 g/100 g), and vitamin E (620 ± 14.1 µg/100 g) were found to have lower content. These findings agreed with the report by Udomsil et al. (2019) [2], who found that thermally dried cricket powder from A. domesticus had significantly higher protein content (71.1 0.5 g/100 g dry matter) and lower lipid content (10.4 0.1 g/100 g dry matter) than cricket powder from G. bimaculatus. The protein content varies between 7 and 91 g/100 g dry matter, depending on the insect species [1]. Therefore, the cricket powder obtained from two cricket species was considered a high protein food (56.46–72.34 g/100 g) according to WHO/FAO and is a 10 g/100 g edible part [1]. Interestingly, in addition to high protein content (ranging from 62.2 to 69.3 g/100 g), fat content (ranging from 11.2 to 23.5 g/100 g) was also the second main component for both cricket powders. These results were similar to those reported by Yi et al. (2013) [5], who reported that lipids are the second largest component in insects behind only protein content. However, the fat and protein content of edible insects depended strongly on species, developmental stage at the time of harvest, and feed or de novo syntheses [18]. Many studies have confirmed the value of crickets as a vitamin source [19]. The levels of vitamin A in A.domesticus (1.51 µg/100 g) and G. bimaculatus (14.7 µg/100 g) in this investigation differed significantly. These vitamin A levels are comparatively lower than those found in earlier studies [19]. In the meantime, both cricket species’ vitamin E concentration findings are consistent with other earlier investigation studies [20]. Conversely, the two types of crickets under investigation had β-carotene levels (111–161 µg/100 g) that were significantly higher than those stated by Magara et al. (2021) [20]. Crickets collected from different areas have varied vitamin levels, which are influenced by feed and rearing conditions [20]. On the other hand, an insignificant difference in moisture and fiber content between two cricket species was present. The result was comparable to Khatun et al. (2021) [21], who reported that A. domesticus and Gryllus assimilis cricket powders prepared using freeze-drying and oven-drying methods had low aw ranging from 0.26 to 0.32. In addition, the powder with a lower moisture content is more hygroscopic, which is related to the greater water concentration gradient between the powder and the surrounding air [22].
Extraction yield of crude cricket oil
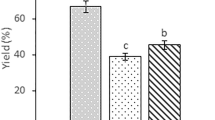
The extraction methods had an impact on the yield of the crude oil, as presented in Fig. 1. The oils from G. bimaculatus extracted using the Soxhlet method gave the highest yield of 23.21 g/100 g, followed by the oils from G. bimaculatus extracted using UAE for 30 and 20 min (16.95 and 16.67 g/100 g), whereas the lowest yield (11.92 g/100 g) was found in the oils from G. bimaculatus obtained using water extraction without ultrasound treatment. The oil yield from G. bimaculatus was significantly (p < 0.05) two-fold higher than that of A.domesticus. This was due to the Soxhlet extraction with n-hexane being the standard method for determining a fat content of food or related materials approved by the Association of Official Analytical Chemicals (AOAC) [9, 10], . Results were similarly reported by Tzompa-Sosa et al. (2014) [4], who reported that the lipid yield of Soxhlet extraction was close to 100 g/100 g for T. molitor, A. diaperinus, and A. domesticus. Relative to the lipid content of the Soxhlet extraction as a total fat content of cricket, a high percentage of 72–75 g/100 g of total fat content was found for both cricket species extracted using UAE, which offered a higher yield of the oils with a shorter extraction time (20 min) than that of the oils extracted using HP and SW. This incident may be due to the mechanical and cavitation effects of ultrasound treatment, which could generate pressure and temperature as micro-jets toward the contact surface between the food matrix and liquid solvent phase, resulting in rupture of the cell membranes, particle size reduction, and enhanced mass transfer across the cell membrane [9, 10, 23]. The propagation of a strong ultrasound wave induced compression and shearing of the solvent molecules [9]. In addition, these effects lead to swelling and hydration, causing the expansion of the pores in the cell membranes [23]. As a result, the cell membranes of crickets are destroyed, and, consequently, intercellular lipids and free lipids are released into the solvent, resulting in an increased extraction yield of the oils. On the contrary, Otero et al. (2020) [24] reported that ultrasound extraction with ethanol as a solvent for A. domesticus and Tenebrio molitor showed higher extraction yields compared with a mixture of ethanol and water solvent. However, the oil from both crickets extracted by ultrasound-assisted extraction with these solvents (around 19 g/100 g) had a comparable yield to pressurized-liquid extraction (24 g/100 g) [24]. The suitable period for industrial ultrasonic extraction of natural products was 60 min or less [10].
Etraction yield of crude oils obtained from G. bimaculatus and A. domisticus extracted using different methods (HP = hot pressing; SW = hot stirrer with water; UAE20 = ultrasound-assisted extraction for 20 min; UAE30 = ultrasound-assisted extraction for 30 min). Each observation is the mean ± SD of replicate experiments (n = 3). The same small letter above each column of each cricket species indicates there is no significant difference between extraction methods (p < 0.05). The same capital letter above each column of each extraction method indicates there is no significant difference between cricket species (p < 0.05). The vertical bars on each column indicate the standard deviation
Color of cricket oil
Color values in terms of L*, a*, and b* of the oils from two cricket species extracted using different methods are displayed in Fig. 2. The L*, a*, and b* values of the extracted oils were significantly affected by extraction methods. Among the extraction processes, the oils from both types of cricket extracted using HP, SW, and UAE had significantly (p < 0.05) higher values of L*, a*, and b* than those extracted using the Soxhlet method. The highest L*, a*, and b* were observed in the oils from A.domesticus extracted using HP, UAE20, and UAE30, whereas the oils obtained from the Soxhlet extraction showed significantly (p < 0.05) the lowest values. Compared to A.domesticus, the oils extracted from G. bimaculatus extracted using UAE showed lower b* and a*, whereas L* was found to be comparable. This may be due to the face that the Soxhlet method used a higher temperature and a longer extraction time (4 h) than other methods. As a result, UAE, as a non-thermal process, could reduce the degradation of thermally sensitive substances and other compounds, such as carotenoids in the extracted samples (Table 1), resulting in an increase in bright yellow pigments. These results concurred with Kutlu et al. (2021) [25], who reported that an increase in L* and a* values of cornelian cherry extracts obtained by ohmic heating-assisted ultrasound extraction correlated with an increase in total anthocyanins. In addition to heating and degradation impacts, color changes were related to the enzymatic browning reaction between cricket phenolic acids and exit enzymes such as phenoloxidase, laccase, tyrosine hydroxylase, decarboxylase, and peroxidase [26]. Most phenolic compounds in insects are derived from l-tyrosine through the shikimic acid pathway using enzymatic reactions [27, 28]. Phenolic compounds can react with proteins and amino acids, forming dark compounds (melanins) [29]. Enzymatic browning in three insect species (Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens) has been reported [30]. These are some of the reasons for color changes in the oils obtained from different cricket species and extraction methods.
Color values of crude oils obtained from G. bimaculatus and A. domisticus extracted using different methods (HP = hot pressing; SW = hot stirrer with water; UAE20 = ultrasound-assisted extraction for 20 min; UAE30 = ultrasound-assisted extraction for 30 min). Each observation is the mean ± SD of replicate experiments (n = 3). The same small letter above each column of each cricket species indicates there is no significant difference between extraction methods (p < 0.05). The same capital letter above each column of each extraction method indicates there is no significant difference between cricket species (p < 0.05). The vertical bars on each column indicate the standard deviation
Total phenolic content (TPC) of cricket oil
Cricket species and extraction methods showed a significant effect (p < 0.05) on TPC in the extracted oils (Table 2.). The oils from both cricket species extracted using UAE at 20 and 30 min among the extraction processes had significantly (p < 0.05) the highest TPC, whereas the lowest value was observed in the oils obtained from the Soxhlet extraction. Compared to G. bimaculatus, the oils from A. domesticus obtained from all extraction methods had significantly (p < 0.05) higher TPC. The highest TPC of 257.4 ± 9.31 and 261.6 ± 8.87 µg GAE/g was observed in the oils from A. domesticus extracted using UAE for 20 and 30 min, respectively, whereas the oil from G. bimaculatus extracted with the Soxhlet method had the lowest TPC of 121.9 ± 5.45 µg GAE/g. This may be due to the phenolic acids in the extracted cricket oils being destroyed by extraction methods with high temperatures and long extraction times (the Soxhlet extraction and HP). The results were a similar study by Anuduang et al. (2020) [31], who reported that phenolic compounds in silkworm pupae decreased after immersion in hot water (90 °C). Increasing heating times caused phenolic acids to leach into the hot water [32]. Therefore, the increase in TPC can be obtained from ultrasound extraction with water as a solvent.
Antioxidant activity of cricket oil
The cricket species and extraction methods had a significant impact (p < 0.05) on antioxidant activity in DPPH and FRAP assays in extracted oils (Table 2). Overall, among the extraction processes, the oils from two crickets extracted using UAE had significantly (p < 0.05) the strongest antioxidant activity in both tests, followed by the oils extracted by SW, respectively, whereas the lowest antioxidant activity was observed in the oils extracted using the Soxhlet method. The oils from A. domesticus showed significantly (p < 0.05) higher antioxidant activity as compared to the oils from G. bimaculatus. The highest DPPH and FRAP were observed in the oils from A. domesticus extracted using UAE for 20 min (121.8 ± 9.67 µg Trolox /g and 98.04 ± 6.11 µM FeSO4/g) and 30 min (198.5 ± 5.43 µg Trolox/g and 105.3 ± 5.49 µM FeSO4/g), respectively, whereas the oils from G. bimaculatus obtained using the Soxhlet extraction were found to have the lowest values for DPPH (65.62 ± 4.32 µg Trolox) and FRAP (25.65 ± 4.51 µM FeSO4/g), respectively. This may be because higher temperatures and longer exposure times cause the degradation of heat-sensitive antioxidant compounds. Using higher temperatures (100 °C, 15 min) resulted in a lower antioxidant capacity of cricket powder due to the leaching of antioxidants into the boiling water [32]. Shorter times in ultrasound extraction could reduce the degradation of sensitive bioactive compounds against chemical alterations like hydrolysis, isomerization, and oxidation [33]. Thermal oxidation caused by longer exposure times and higher temperatures can result in increased PV [34, 35]. The cavitation effects under ultrasound treatment have induced disruption of the cricket cell walls and membranes, causing the release of intercellular antioxidant compounds into the extracted oil. In addition, ultrasound extraction can enhance lipid antioxidant properties, which are probably due to differences in the chemical composition of extracted lipids such as aldehyde-active and hydroxyl-active groups [35]. As results, the reduced extraction time and temperature in UAE improved the lipid quality. Similarly, the oils from two crickets obtained by UAE also contained higher TPC among extraction methods. These are the reasons for the increase in antioxidant activity in the oils obtained from UAE.
Changes in cricket oil quality during storage
Extraction methods had a significant effect (p < 0.05) on the FFA, PV, and TBA of the extracted oils during 28 days of storage (Fig. 3.). FFA, PV, and TBA of the extracted oils increased as storage time increased during the 28 days of storage. Among the extraction processes, the oils from both crickets extracted using UAE showed significantly the lowest levels of FFA, PV, and TBA (p < 0.05) during storage, while the highest levels were observed in the oil obtained from the Soxhlet extraction. Interestingly, the increased concentrations of FFA over 28 days of storage in the extracted oils from G. bimaculatus (0.15–3.34 g/100 g) and A. domesticus (0.16–4.20 g/100 g) obtained from ultrasound-assisted extractions were still suitable for human consumption, as they were less than 5 g/100 g FFA, according to the report of Orthoefer and Eastman (2003) [36]. At 28 days of storage, higher FFA (5.58–5.68 g/100 g) was found for the extracted oils from A. domesticus extracted with the Soxhlet method and hot pressing. This result is in agreement with the data documented by Khoei and Chekin (2016) [37], who reported that rice bran oil extracted by an aqueous process had a lower content of FFA and lower color-imparting components than the hexane-extracted oil. Similarly, for the extracted oils from UAE, the PV for G. bimaculatus and A. domesticus were found to be in the range of 0.16–4.38 and 0.08–3.68 meqO2/kg oil, respectively.
Changes in free fatty acid (FFA), thiobarbituric acid (TBA), and peroxide value (PV) of crude oils from G. bimaculatus (Gb.) and A. Domisticus (Ad.) extracted using different methods during storage at room temperature for 28 days. (HP = hot pressing; SW = hot stirrer with water; UAE20 = ultrasound-assisted extraction for 20 min; UAE30 = ultrasound-assisted extraction for 30 min). Each observation is the mean ± SD of replicate experiments (n = 3)
According to Codex Alimentarius (2017) [38], the maximum PV for fats and oils was 10 meqO2/kg oil. This result is consistent with the findings of Ugur et al. (2021) [39], who discovered that PV for Tenebrio molitor (yellow mealworm) and A.domesticus (house cricket) ranged between 1.61 and 2.21 meqO2/kg oil. This may be a result of the large concentrations of antioxidative compounds in both cricket varieties (data shown in Tables 1 and 2), which can scavenge free radicals such as lipid alkyl hydroxyl or lipid peroxyl radicals, increasing the oxidative stability of the oils.
Comparison of fatty acid profiles in cricket oil
The fatty acid composition of cricket oil obtained from the optimal extraction method (UAE20) was determined, and the result was compared with the Soxhlet extraction with n-hexane, as displayed in Table 3. The extraction methods had a significant impact (p < 0.05) on the saturated (lauric, myristic, arachidic, and total saturated fatty acids) and unsaturated (α-,inolenic acid and MUFA) acids in the obtained cricket oils. Cricket oils extracted from UAE20 had significantly higher (p < 0.05) concentrations of α-linolenic (omega 3) and MUFA compared to Soxhlet extraction. For A. domesticus, the concentrations were 22.4 and 27.2 g/100 g, while for G. bimaculatus, they were 18.3 and 28.2 g/100 g. In contrast, UAE20 oils contained a lower concentrations of lauric, myristic, and arachidic acids, as well as SFA. For both crickets, linoleic (omega 6), cis-9-oleic (omega 9) acids had a comparable concentration to the oils obtained from the Soxhlet extraction. Similarly, there was no significant difference in palmitic, heptadecanoic acid and stearic acid extraction techniques. Among the fatty acids, linoleic acid, or omega 6 (28.8–32.2 g/100 g), was the majority of fatty acids in both cricket oils, followed by palmitic acid (25.7–28.9 g/100 g) and cis-9-oleic acid, or omega 9 (20.5–21.7 g/100 g), respectively. The results are in line with those of Udomsil et al. (2019) [2], who found that essential linoleic acid and α-linolenic acid were present in the powders of G. bimaculatus and A. domesticus. A. domesticus cricket contained linoleic acid (30–40 g/100 g), oleic acid (23–27 g/100 g), palmitic acid (24–30 g/100 g), and stearic (7–11 g/100 g), with minor levels of palmitoleic (3–4 g/100 g), myristic (1 g/100 g), and linolenic acid (less than 1 g/100 g) [40]. Similarly, palmitic acid (C16:0), followed by stearic acid (C18:0), had the highest concentration of saturated fatty acids in the lipids of insects [41]. The crude cricket oil obtained from ultrasound with water extraction had a higher MUFA and a lower SFA content. This was due to a higher content of α-linolenic acid (omega 3) and a lower content of lauric, myristic, and arachidic acids. Low polarity of hexene yielded less affinity for MUFA than high polarity solvents, such as water solvents [42]. In addition, the use of ultrasound improved mass transfer and yielded higher mono-unsaturated fatty acids i.e. α-linolenic acid. Internal glandular lipids can be successfully liberated when tissues and cells have been ultrasonically deconstructed [35]. However, the fatty acid profiles of both cricket oils were similar to those of other studies [4, 24, 43]. Therefore, both types of cricket oils obtained from ultrasound-assisted extraction have a high level of α-linolenic fatty acid (omega 3) and MUFA.
Conclusions
The UAE (20 and 30 min) was an effective method to assist in the oil extraction from both cricket species. It could give a higher yield and exhibit significantly higher antioxidant activity (DPPH and FRAP), TPC, and color values (L*, a*, and b*) than other methods applied in this study. Both cricket oils extracted with UAE were more stable, with slower progressive increases in FFA, PV, and TBA over 28 days of storage than the oil from the Soxhlet extraction. The crude cricket oils from both species also contained high levels of α-linolenic acid (omega 3) and MUFA. The results suggested that ultrasound extraction using water as a solvent had potential as an alternative process to improve the properties of the crude oil extracted without using chemicals.
Data availability
The data that supports the findings of this study is available from the corresponding author upon reasonable request.
References
A. Van Huis, J. Van Itterbeeck, H. Klunder, E. Mertens, A. Halloran, G. Muir, P. Vantomme, Edible Insects: Future Prospects for food and feed Security (Food and agriculture organization of the United Nations, 2013)
N. Udomsil, S. Imsoonthornruksa, C. Gosalawit, M. Ketudat-Cairns, Nutritional values and functional properties of house cricket (Acheta domesticus) and field cricket (Gryllus Bimaculatus). Food Sci. Technol. Res. 25, 597–605 (2019). https://doi.org/10.3136/fstr.25.597
M. Montowska, P.Ł. Kowalczewski, I. Rybicka, E. Fornal, Nutritional value, protein and peptide composition of edible cricket powders. Food Chem. 289, 130–138 (2019). https://doi.org/10.1016/j.foodchem.2019.03.062
D.A. Tzompa-Sosa, L. Yi, H.J.F. van Valenberg, M.A.J.S. van Boekel, C.M.M. Lakemond, Insect lipid profile: aqueous versus organic solvent-based extraction methods. Food Res. Int. 62, 1087–1094 (2014). https://doi.org/10.1016/j.foodres.2014.05.052
L. Yi, C.M.M. Lakemond, L.M.C. Sagis, V. Eisner-Schadler, A. van Huis, M.A.J.S. van Boekel, Extraction and characterisation of protein fractions from five insect species. Food Chem. 141, 3341–3348 (2013). https://doi.org/10.1016/j.foodchem.2013.05.115
F. Chemat, N. Rombaut, A.-G. Sicaire, A. Meullemiestre, A.-S. Fabiano-Tixier, M. Abert-Vian, Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 34, 540–560 (2017). https://doi.org/10.1016/j.ultsonch.2016.06.035
P. Loypimai, A. Moongngarm, K. Sittisuanjik, T. Wongsadee, Enhancement of bioactive compounds and oxidation stability of soybean oil by enrichment with tocols and γ-oryzanol extracted from rice bran using ultrasound and ohmic heating. J. Food Process. Preserv. 46 (2022). https://doi.org/10.1111/jfpp.16991
P. Loypimai, A. Moongngarm, P. Chottanom, Impact of stabilization and extraction methods on chemical quality and bioactive compounds of rice bran oil. Emir J. Food Agric. 849–856 (2015). https://doi.org/10.9755/ejfa.2015.09.738
A.M. Goula, M. Ververi, A. Adamopoulou, K. Kaderides, Green ultrasound-assisted extraction of carotenoids from pomegranate wastes using vegetable oils. Ultrason. Sonochem. 34, 821–830 (2017). https://doi.org/10.1016/j.ultsonch.2016.07.022
P. Loypimai, A. Moongngarm, A. Sittisuanjik, S. Khamanan, Optimization of tocols and γ-oryzanol extraction from rice bran using ultrasound and soybean oil as a green solvent. Food Res. 4, 2322–2332 (2020). https://doi.org/10.26656/fr.2017.4(6).360
G.W. Latimer, Official Methods of Analysis of AOAC International, 21st edn. (Association of Official Analytical Chemists, 2019)
J. Xiao, J. Wu, Y. Chao, R. Liu, C. Li, Z. Xiao, Evaluation of yields and quality parameters of oils from Cornus Wilsoniana fruit extracted by subcritical n-butane extraction and conventional methods. Grain Oil Sci. Technol. 5, 204–212 (2022). https://doi.org/10.1016/j.gaost.2022.09.003
P. Loypimai, A. Moongngarm, P. Chottanom, Impact of stabilization and extraction methods on chemical quality and bioactive compounds of rice bran oil. Emir J. Food Agric. 27, 849–856 (2015). https://doi.org/10.9755/ejfa.2015.09.738
N. Dasgupta, B. De, Antioxidant activity of Piper betle L. leaf extract in vitro. Food Chem. 88, 219–224 (2004). https://doi.org/10.1016/j.foodchem.2004.01.036
I.F.F. Benzie, J.J. Strain, The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP Assay. Anal. Biochem. 239, 70–76 (1996). https://doi.org/10.1006/abio.1996.0292
S. Iqbal, M.I. Bhanger, F. Anwar, Antioxidant properties and components of some commercially available varieties of rice bran in Pakistan. Food Chem. 93, 265–272 (2005). https://doi.org/10.1016/j.foodchem.2004.09.024
American Oil Chemists’ Society, D. Firestone, Official Methods and Recommended Practices of the AOCS, American Oil Chemists’ Society, 1998. https://books.google.co.th/books?id=YnnsvwEACAAJ
D. Tzompa-Sosa, V. Fogliano, Potential of insect-derived ingredients for food applications. Insect Physiol. Ecol. 215–231 (2017). https://doi.org/10.5772/67318
D.K. Murugu, A.N. Onyango, A.K. Ndiritu, I.M. Osuga, C. Xavier, D. Nakimbugwe, C.M. Tanga, From farm to fork: crickets as alternative source of protein, minerals, and vitamins. Front. Nutr. 8, 704002 (2021). https://doi.org/10.3389/fnut.2021.704002
H.J.O. Magara, S. Niassy, M.A. Ayieko, M. Mukundamago, J.P. Egonyu, C.M. Tanga, E.K. Kimathi, J.O. Ongere, K.K.M. Fiaboe, S. Hugel, M.A. Orinda, N. Roos, S. Ekesi, Edible crickets (Orthoptera) around the world: distribution, nutritional value, and other benefits—A review. Front. Nutr. 7 (2021). https://doi.org/10.3389/fnut.2020.537915
H. Khatun, J. Claes, R. Smets, A. De Winne, M. Akhtaruzzaman, M. Van Der Borght, Characterization of freeze-dried, oven-dried and blanched house crickets (Acheta domesticus) and Jamaican field crickets (Gryllus assimilis) by means of their physicochemical properties and volatile compounds. Eur. Food Res. Technol. 247, 1291–1305 (2021). https://doi.org/10.1007/s00217-021-03709-x
R.V. Tonon, C. Brabet, M.D. Hubinger, Influence of process conditions on the physicochemical properties of açai (Euterpe Oleraceae Mart.) Powder produced by spray drying. J. Food Eng. 88, 411–418 (2008). https://doi.org/10.1016/j.jfoodeng.2008.02.029
C. Ashokkumar, Vibration control for structural damage mitigation. J. Vib. Control. 21, 2995–3006 (2014). https://doi.org/10.1177/1077546313519283
P. Otero, A. Gutierrez-Docio, J. Navarro del Hierro, G. Reglero, D. Martin, Extracts from the edible insects Acheta domesticus and Tenebrio molitor with improved fatty acid profile due to ultrasound assisted or pressurized liquid extraction. Food Chem. 314, 126200 (2020). https://doi.org/10.1016/j.foodchem.2020.126200
N. Kutlu Kantar, A. Isci, Ö. Şakıyan, A. Yilmaz, Effect of ohmic heating on ultrasound extraction of phenolic compounds from cornelian cherry (Cornus mas). J. Food Process. Preserv. 45 (2021). https://doi.org/10.1111/jfpp.15818
S.O. Andersen, Cuticular sclerotization and tanning. Insect Molecular Biology and Biochemistry, Elsevier, 2012: 167–192. https://doi.org/10.1016/B978-0-12-384747-8.10006-6
G.W. Morrow, The Shikimate Pathway: Biosynthesis of Phenolic Products from Shikimic acid, in: Bioorganic Synthesis (Oxford University Press, 2016)
N.F. Santos-Sánchez, R. Salas-Coronado, B. Hernández-Carlos, C. Villanueva-Cañongo, Shikimic acid pathway in biosynthesis of phenolic compounds, in: Plant physiological aspects of phenolic compounds, IntechOpen London, UK, 2019: pp. 1–15. https://doi.org/10.5772/intechopen.83815
Y. Qi, J. Liu, Y. Liu, D. Yan, H. Wu, R. Li, Z. Jiang, Y. Yang, X. Ren, Polyphenol oxidase plays a critical role in melanin formation in the fruit skin of persimmon (Diospyros kaki Cv. ‘Heishi’). Food Chem. 330, 127253 (2020). https://doi.org/10.1016/j.foodchem.2020.127253
R. Janssen, J.-P. Vincken, L. Broek, V. Fogliano, C. Lakemond, Nitrogen-to-protein Conversion factors for three Edible insects: Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens. J. Agric. Food Chem. 65 (2017). https://doi.org/10.1021/acs.jafc.7b00471
A. Anuduang, Y.Y. Loo, S. Jomduang, S.J. Lim, W.A. Wan, Mustapha, Effect of thermal processing on physico-chemical and antioxidant properties in mulberry silkworm (Bombyx mori L.) powder. Foods. 9, 871 (2020). https://doi.org/10.3390/foods9070871
F.-J. Kao, Y.-S. Chiu, W.-D. Chiang, Effect of water cooking on antioxidant capacity of carotenoid-rich vegetables in Taiwan. J. Food Drug Anal. 22, 202–209 (2014). https://doi.org/10.1016/j.jfda.2013.09.010
S.M.T. Gharibzahedi, S.M. Jafari, Fabrication of nanoemulsions by ultrasonication, in: S.M. Jafari, D.J. McClements (Eds.), Nanoemulsions, Academic Press, 2018: pp. 233–285. https://doi.org/10.1016/B978-0-12-811838-2.00009-6
P. Juliano, F. Bainczyk, P. Swiergon, M.I.M. Supriyatna, C. Guillaume, L. Ravetti, P. Canamasas, G. Cravotto, X.-Q. Xu, Extraction of olive oil assisted by high-frequency ultrasound standing waves. Ultrason. Sonochem. 38, 104–114 (2017). https://doi.org/10.1016/j.ultsonch.2017.02.038
Y. Deng, W. Wang, S. Zhao, X. Yang, W. Xu, M. Guo, E. Xu, T. Ding, X. Ye, D. Liu, Ultrasound-assisted extraction of lipids as food components: mechanism, solvent, feedstock, quality evaluation and coupled technologies–A review. Trends Food Sci. Technol. 122, 83–96 (2022). https://doi.org/10.1016/j.tifs.2022.01.034
F.T. Orthoefer, J. Eastman, Rice bran and oil, in Rice: Chemistry and Technology, 3rd edn., ed. by E.T. Champagne (Southern Regional Research Center, New Orleans, 2004), pp. 569–593. https://api.semanticscholar.org/CorpusID:82606642
M. Khoei, F. Chekin, The ultrasound-assisted aqueous extraction of rice bran oil. Food Chem. 194, 503–507 (2016). https://doi.org/10.1016/j.foodchem.2015.08.068
Codex, Alimentarius, Standard for edible fats and oils not covered by individual standards., 2017
A.E. Ugur, B. Bolat, M.H. Oztop, H. Alpas, Effects of high hydrostatic pressure (HHP) processing and temperature on physicochemical characterization of insect oils extracted from Acheta domesticus (House Cricket) and Tenebrio molitor (Yellow Mealworm). Waste Biomass Valori. 12, 4277–4286 (2021). https://doi.org/10.1007/s12649-020-01302-z
R.F.N. Hutchins, M.M. Martin, The lipids of the common house cricket, Acheta domesticus LI lipid classes and fatty acid distribution. Lipids. 3, 247–249 (1968). https://doi.org/10.1007/BF02531195
A. Orkusz, Edible insects versus meat—nutritional comparison: knowledge of their composition is the key to good health. Nutrients. 13, 1207 (2021). https://doi.org/10.3390/nu13041207
V.P. Dole, H. Meinertz, Microdetermination of long-chain fatty acids in plasma and tissues. J. Biol. Chem. 235, 2595–2599 (1960). https://doi.org/10.1016/S0021-9258(19)76920-8
A. Paul, M. Frederich, R.C. Megido, T. Alabi, P. Malik, R. Uyttenbroeck, F. Francis, C. Blecker, E. Haubruge, G. Lognay, Insect fatty acids: a comparison of lipids from three orthopterans and Tenebrio molitor L. larvae. J. Asia Pac. Entomol. 20, 337–340 (2017). https://doi.org/10.1016/j.aspen.2017.02.001
Acknowledgements
This work was supported by the National Research Council of Thailand (NRCT); Mahasarakham.
University; and Bansomdejchaopraya Rajabhat University.
Author information
Authors and Affiliations
Contributions
Thorung Pranil: Methodology; Data curation; Formal analysis; Writing – review and editing. Anuchita Moongngarm: Conceptualization; Data curation; Funding acquisition; Supervision; Visualization; Writing – original draft; Writing – review and editing. Tanongsak Moontree: Conceptualization; Data curation; Funding acquisition. Patiwit Loypimai: Conceptualization; Data curation; Formal analysis; Methodology; Writing – original draft; Writing – review and editing.
Corresponding author
Ethics declarations
Ethics approval
Ethics approval was not required for this research.
Conflict of interest
The authors declare no conflict of interest.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pranil, T., Moontree, T., Moongngarm, A. et al. Ultrasound-assisted extraction of edible cricket oils from Gryllus bimaculatus and Acheta domesticus. Food Measure 18, 6145–6155 (2024). https://doi.org/10.1007/s11694-024-02634-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-024-02634-3