Abstract
Oil portion of Tenebrio molitor (yellow mealworm) and Acheta domesticus (house cricket) were examined and it was investigated how the physicochemical properties of the oils changed with High Hydrostatic Pressure Assisted Extraction (HHP-E) and conventional solvent extraction (CE) with hexane. The effect of HHP-E at 500 MPa and 30 and 40 °C for 15 min on the properties of oils was compared with the CE. Following the extraction of oil, fatty acid composition, peroxide value, crystallization and melting points, total phenolic content and antioxidant activities were determined. Oil yield was found in the range of 22.75–24.22% for mealworm and 16.17–18.09% for cricket with significant amount of Ω-3 and Ω-6 fatty acids. Fatty acid composition of insect oils was significantly affected from HHP-E and extraction temperature (p < 0.05). The difference between crystallization and melting point of mealworm were found to be higher than cricket (p < 0.05). HHP-E insect oil had desirable characteristics to be used as a food ingredient.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Statement of Novelty
HHP-E was successful over CE of oils obtained from Tenebrio molitor (yellow mealworm) and Acheta domesticus (house cricket). Oil yield, fatty acid composition, amount of myristic, palmitoleic and linolenic acids in mealworm and cricket oils with HHP-E were significantly high (p < 0.05). Mealworm and cricket oil are potential sources of essential fatty acids and antioxidants. Extraction with HHP can be an alternative method against conventional extractions when the optimum parameters are chosen. However, for industrial applications, a feasibility study between two extraction methods is required.
Introduction
Currently, two billion people from over 113 countries consume some 2000 recorded edible insects as part of their traditional diets. Many of these insects contain adequate amounts of protein, fat, vitamins, and minerals that are comparable to the commonly eaten livestock [1]. In that regard, introducing and increasing the use of insect-based foods as an alternative source of protein has recently increased for both academic and commercial interest in Europe [2].
The homeland of Tenebrio molitor is Europe; however, it is now found in many different parts of the world [3]. The consumption of Tenebrio molitor is very popular in Africa, Asia, America and Australia [4]. Oil content of yellow mealworm is in the range of 27.25 to 38.26% [5]. The major fatty acids that yellow mealworm includes are oleic (37.7 to 43.17%), linoleic (27.4 to 30.23%) and palmitic (16.72 to 21.1%) acids [6, 7].
The origin of Acheta domesticus is Southeastern Asia; however, it is also found in Europe, North Africa, Western Asia, Indian Subcontinent, Australasia, Mexico and North America [3]. Although house crickets are known as important sources of protein, they include undeniable amount of high-quality lipids (Ω-3 and Ω-6 fatty acids) [8]. The oil content is in the range of 18.6 to 22.8%. The major fatty acids that adult house cricket oil includes are linoleic (30 to 40%), oleic (23 to 27%), palmitic (24 to 30%) and stearic (7 to 11%) acids [9]. Acheta domesticus is often used as human food, but selection of species may depend on several factors such as legislation, resistance to disease, but also taste [10].
High Hydrostatic Pressure (HHP) is a technique where the pressure of a liquid is isostatically increased via the liquid compression, and is considered as a non-thermal treatment method in food processing within the pressure range of 100 to 1000 MPa [11,12,13]. During the process, the temperature and time is also specified in the range of 1 °C to 95 °C and a few seconds to 20 min respectively depending on the type of and the process [14]. The pressure is distributed rapidly and quasi-instantaneous uniformly through the sample of both liquid and water-containing solid [15, 12]. One of the significant attention-grabbing feature of HHP method is that the treatment causes no damage and no distortion on the food compound so long as the product does not include any emptiness inside [11].
The application HHP on food has increased with the increasing health consciousness and advanced technological development due to its eco-friendly no waste generating nature [16, 17]. HHP prevents thermal degradation of compounds of the food thanks to being non-thermal application. On the contrary to the conventional treatment applications; the color, aroma, nutritional value, flavor and the texture characteristics like desired attributes of the food compound is preserved and is enhanced in HHP treated food compounds [18]. There are many works about the effects of HHP on proteins and carbohydrates; however, the works are limited oils [19].
The goal of this study is to investigate the effect of high hydrostatic pressure assisted extraction on the physicochemical properties of oils extracted from Acheta domesticus and Tenebrio molitor. For that purpose, fatty acid composition, crystallization and melting points, peroxide value, total phenolic content and antioxidant activity of the insect oils were determined.
Materials and Methods
Materials
Freeze-dried larvae of yellow mealworm and house cricket powder were supplied from Tasty Worms Nutrition Inc. (Florida, USA) and JR Unique Foods Ltd. (Udon Thani, Thailand), respectively. Hexane, ammonium acetate (CH3CO2NH4), trolox (TR), ethanol (C2H5OH), acetic acid (CH3CO2H), DPPH·(2,2-diphenyl-1-picrylhydrazyl), methanol (CH3OH), Folin-Ciocalteu’s phenol reagent, chloroform (CHCl3), starch, sodium thiosulfate (Na2S2O3), potassium iodide (KI), pure nitrogen (N2), capric acid (C10H20O2), indium, n-dodecane, methanol sodium hydroxide, boron trifluoride methanol was obtained from Sigma Aldrich Chemical Co. (St. Louis, MO, USA).
Extraction of Oil from Insect Powders
High Hydrostatic Pressure Assisted Extraction (HHP-E)
Insect powders were separately mixed with hexane at a proportion of 1:15 (w/v) and completely dissolved solutions were poured into 25 ml sterile polyethylene cryotubes (LP Italiana SPA) and pressurized at 500 MPa for 15 min at 30 and 40 °C (HHP-E) (760.0118 type pressure equipment, SITEC-Sieber Engineering AG, Zurich, Switzerland). Since pressurization temperature is significant for HHP experiments, 2 temperatures were examined to see the effect of the process better. Selected pressure, temperature and time values were based on our preliminary experimental results (data not shown). Vessel volume of equipment was 100 ml. Internal diameter and length of pressure equipment were 24 mm and 153 mm, respectively. Distilled water was the aqueous medium inside the equipment. A built in heating and cooling system was used to keep inside temperature of the system constant (Huber Circulation Thermostat, Germany). The products obtained from HHP-E were centrifuged at 9,500 rpm for 20 min at both 30 °C and 40 °C. Following centrifugation hexane was removed by evaporation. As a control a conventional extraction (CE) procedure was also followed at 30 and 40 °C for 15 min (0.01 MPa). In the CE process; the powder of yellow mealworm and house cricket were separately mixed with hexane at the proportion of 1:15 (w/v), and the extraction was performed at 30 °C for 15 min under hot plate magnetic stirrer (Daihan Scientific Co., Ltd., Korea); then, the centrifugation was applied at 9,500 rpm for 20 min at 30 °C to obtain the oil-hexane mixture (Sigma 2-16PK, SciQuip Ltd., UK). Hexane in the oil-hexane mixture was removed with the evaporation for 24 h in a drying oven at 40 °C; thus, the pure oil portion of the two edible insects were obtained in liquid form [20].
Physicochemical Characterization of Insect Oils
Determination of Fatty Acid Composition with Gas Chromatography (GC)
Method described by Jeon et al. [21] was used with some modifications. 0.25 g of oil and 6 ml of 0.5 N of methanol sodium hydroxide were mixed and heated in water bath at 80 °C for 10 min. After cooling oil on ice for 3 min, 7 ml of 14% boron trifluoride methanol was added to the solution and mixture was heated 80 °C for 2 min; then the solution was cooled in an ice bath for 3 min before 5 ml of n-hexane addition. After preparation of the solution, oil was heated for 1 min and layer on the top was separated and transferred to a vial. Thermo Scientific Trace GC Ultra with CP-Sil 88 capillary column (50 m × 0.25 mm ID., 0.20 µm film; Chrompak, Middelburg, the Netherlands; catalog no: 7488) and ionization detector (260 flames) was used for measurements. As carrier gas, Helium was used at a rate of 1.3 ml/min. 1 µl of solution was injected with a split ratio of 50:1.
Determination of Crystallization and Melting Point with Differential Scanning Calorimeter (DSC)
DSC analysis described by Tomaszewska-Gras [22] was used with modifications. Perkin Elmer DSC 4000 (Turkey) DSC was used for measurements. Pure nitrogen was used as purge gas with a flow rate of 19.8 ml/min and calibration of DSC was done using indium (MP: 156.6 °C, ∆Hf = 28.45 J/g) and n-dodecane (MP: − 9.65 °C, ∆Hf = 216.73 J g−1, Capric acid melting with MP of 31.6 °C controlled the calorimeter’s calibration. The oil samples were weighed around 10–11 mg into aluminum pans and hermetically sealed. An empty hermetically sealed aluminum pan was used as reference. The pan with the sample was located into the calorimeter at 25 °C and the following time–temperature program was applied;
-
i.
Heating from 25 to 60 °C at 5 °C/min to melt all crystals and nuclei,
-
ii.
Cooling at 5 °C/min to − 40 °C and keeping for 3 min at − 40 °C,
-
iii.
Heating at 5 °C/min from − 40 to 60 °C
Determination of Peroxide Value (PV)
One gram of insect oil was weighted and mixed with 10 ml of chloroform. 15 ml of acetic acid and 1 ml of potassium iodide were added to chloroform-oil mixture respectively, then the mixture was agitated for a minute and solution was kept at room temperature in a dark place for 5 min as closed. Then, 75 ml of distilled water and 1 ml of starch were added to solution. The mixture was titrated with 0.002 N of sodium thiosulfate (Na2S2O3) solution because expected peroxide value was less than 12.5. PV was calculated as; [23],
V2 = consumption of Na2S2O3 solution in main test, in mL, V1 = consumption of Na2S2O3 solution in blank test, in mL, F = titer of the thiosulfate solution, m = weighed portion of substance in grams.
Determination of the Total Phenolic Content (TPC)
Folin-Ciocalteu (F–C) reagents were used to determine the TPC of insect oils. The method described by Al-Rimawi et al. [24] was used as reference. Insect powders were dissolved in ethanol:water:acetic acid mixture (50:42:8) at a ratio of 1:10 (g/ml) and kept in mixture for 60 min to assure complete extraction. Then, the mixture was syringed and filtered through a 0.45 μm micro filter. 40 μl of sample was mixed with 1.8 ml of F–C reagent (0.1 M); was kept at room temperature in dark for 5 min. Then, 1.2 ml of sodium bicarbonate (NaHCO3) (7.5% w/v) was added to mixture, vortexed again; later, the mixture was kept at room temperature in dark for 1 h. Afterwards, absorbance values were measured at 760 nm using a UV/VIS spectrophotometer (Optizen Pop Nano Bio, Mecasys Co. LTD, Korea). Blank was prepared by mixing 40 μl of ethanol:water:acetic acid solution (50:42:8), 1.8 ml F–C reagent (0.1 M) and 1.2 ml of NaHCO3.
Determination of the Antioxidant Activity (AA) with DPPH Radical Scavenging Activity
To determine the AA of insect oils, samples were extracted as in method of Brand-Williams et al. [25] with modifications. 0.1 ml of insect oil was weighted and dissolved in ethanol:water:acetic acid (50:42:8) mixture a ratio of 1:10 (ml/ml) and vortexed for 30 s. The mixture was kept in dark for 60 min. After elapsed time, the mixture was syringed and filtered through 0.45 μm micro filter. 3.9 ml of 0.0634 mM (25 ppm) DPPH in methanol (95%) was added to each extract. After 1 h, the mixtures were vortexed and absorbance values were measured at 517 nm using a UV/VIS Spectrophotometer (Optizen Pop Nano Bio, Mecasys Co. LTD, Korea).
Statistical Analysis
The statistical analysis was done for all experiments by using MINITAB (Version 16.2.0.0, Minitab Inc., Coventry, UK). The effect of different oil extraction conditions, methods and the type of insects were investigated by ANOVA (analysis of variance). Tukey’s multiple comparison test with at confidence interval of 95% was used if significance difference was detected. All experiments were repeated at least twice.
Results and Discussion
Oil Content
It was expected that HHP would increase the yield because of its ability on the charge groups’ deprotonation and disruption of hydrophobic bonds and salt bridges; thus, more solvent could penetrate into cells [26, 27]. However, the results showed that oil content decreased with HHP-E (p < 0.05) (Table 1). There was significant difference between HHP-E and CE samples and difference was significant at different temperature values (p < 0.05). It was hypothesized that HHP might have disrupted the structures of triglycerides [5]. Oil content of mealworm and cricket were found in the ranges of 22.9–24.22% and 16.17–18.05% respectively (Table 1. However, oil content of mealworm was reported in the range of 27.2 to 38.3%; and that of cricket was in the range of 16.4 to 19.1% [5, 8]. The reason of this difference was attributed to the duration of extraction in our study. Normally CE could take 30 to 60 min and it could be applied twice [20, 28]. However, in our study to make it comparable with HHP-E it was kept at 15 min.
Fatty Acid Composition
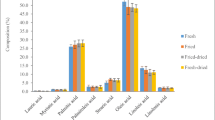
Table 2 shows the fatty acid composition and Tables 3 and 4 shows the total Saturated Fatty Acid (SFA), Monounsaturated Fatty Acid (MUFA) and Polyunsaturated Fatty Acid (PUFA) composition of insect species and total fatty acid composition of oils extracted from different insect species.
In mealworm, there was significant difference in tridecanoic, palmitoleic and stearic acids with HHP-E over CE (p < 0.05). The amount of palmitic acid decreased with pressure; however, the situation was different in tridecanoic acid, because it existed only at 30 °C for both pressurized and non-pressurized samples. Statistical results showed that, HHP-E significantly increased the amount of arachidonic and eicosapentaenoic acids, whereas significant decrease was obtained in palmitic acid (p < 0.05). On the other hand, amount of oleic acid significantly increased by the increase in temperature from 30 to 40 °C; margaric, heptadenoic and strearic acids were negatively affected from temperature rise.
In cricket, the amount of stearic acid decreased significantly with HHP-E (p < 0.05). While HHP-E at 30 °C decreased the amount of linoleic acid significantly, it increased the amount of eicosatrienoic acid. Interestingly, arachidonic acid existed in both CE and HHP-E at only 30 °C, while eicosapentaenoic acid existed in both methods at only 40 °C. Arachidonic acid in both CE and HHP-E at only 30 °C could have been converted into eicosanoids that are small lipids, so it was possible to say that the increasing temperature might have caused the formation of eicosanoids by arachidonic acid conversion [29].
In summary, fatty acid composition of insects was significantly affected from both HHP treatment and temperature variation (p < 0.05). However, desired extraction condition for different fatty acids changed with insect type and application parameters. Although amounts of myristic, palmitoleic and linolenic acids in mealworm and cricket oils were relatively high, the most abundant fatty acids found in both were found to be palmitic, stearic, oleic and linoleic acids.
Overall, it was found that the highest percentage of UFA is obtained at 40 °C for both CE and HHP-E for mealworm, and the highest percentage of UFAs is obtained at 40 °C with the application of HHP for cricket. The total amount of fatty acids decreased significantly at 30 °C with the application of pressure for both insect species while the other combinations of parameters did not cause a significant difference (p > 0.05) (Tables 3 and 4).
UFAs like linoleic acid that is liquid at room temperature can undergo reversible conformational change under the effect of extreme pressure conditions around 700 MPa; however, change in UFAs that are solid at room temperature are irreversible [19]. In this study, pressure conditions were lower compared to 700 MPa and thus effect of pressure was not found significant (p > 0.05). The phase transformations and conformational changes may have occurred under extreme pressure conditions, so it could be indicated that combination of high pressure and temperature with longer processing time caused an increase in content of UFAs [19].
Peroxide Value (PV)
According to Codex Alimentarius [30], the upper limit of PV for fats and oils is 10 meqO2/kg oil. In this study, PV were found in the range of 1.61 and 2.21 meqO2/kg oil for both insect species; that is, they were far from the upper limit and safe to consume; besides, both types of insect oils has high oxidative stability (Table 1). The key factor that stabilizes both primary and secondary oxidation is the existence of antioxidants that insect oils included. Antioxidants scavenge free radicals such as lipid alkyl hydroxyl or lipid peroxyl radicals; also, they quench singlet oxygen. Antioxidants can donate hydrogen atoms to free radicals and thus, free radicals convert into more stable non-radical products. All in all, the existence of antioxidants limits the oxidation in fats and oils, so it is expected that they would have lower secondary oxidation values [31].
Statistical analysis showed that for mealworm the lowest oxidation was observed at 30 °C for both CE and HHP-E. The results showed that increasing temperature caused an increase in PV; that is, the increasing temperature caused oxidation in mealworm oil. In cricket, there was also significant difference for all different extraction conditions (p < 0.05). The lowest oxidation value was obtained for CE samples treated at 30 °C. As in mealworm oil, PV was higher in pressure treated samples when compared to the conventionally extracted oils at same temperature; that is, HHP had a negative effect on oxidation status of cricket oils. The effect of pressure under 300 MPa had slight effect on lipid oxidation, but the oxidation increased above 300 MPa in pork fat [32]. In our study, mealworm and cricket contained high amount of UFAs which were known to be very susceptible to oxidation and high-pressure values of 500 MPa caused increased the oxidation. However still the PV values were below the standards.
Crystallization (CP) and Melting Points (MP)
Significant difference was found in the CP of mealworm oil under different extraction conditions (p < 0.05) (Table 1). HHP caused a decrease in the CP of mealworm oil while there was no significant difference in the MP for all extraction conditions (p > 0.05). It can be explained with the amount of SFAs and UFAs that oil included. The higher UFAs may have caused lower CP, so the lowest CP was obtained at 40 °C with pressure application with the existence of highest percentage of UFAs among others.
In cricket, there was significant difference for both CP and MP when looking at the combined effect of pressure and temperature (p < 0.05) (Table 1). Oil extracted with CE comes to the forefront in terms of lower CP when compared to HHP assisted extracted oils. Besides, it was found that the highest MP was achieved at 30 °C without pressure and the lowest was obtained at 40 °C with pressure.
The reason could be the hydrophobic interactions between lipids being quite sensitive to pressure and lipids that are liquid at room temperature form crystals under pressure effect by increasing the MP of triglycerides [33]. Although the MP of mealworm oil decreased with pressure, for cricket oil increase on melting and crystallization was observed. The hydrophobic interactions between lipid groups of crickets might have been more sensitive than mealworm. Also, the amount of SFAs of both insect oils decreased at 40 °C, and this might have caused a decrease in MP of both mealworm and cricket oils.
Total Phenolic Content (TPC)
It was found that there was significant difference in mealworm oil in terms of TPC (Table 5). Temperature was not found to be significant (p > 0.05); however, the pressure was (p < 0.05). In 30 °C, TPC increased significantly with pressure; however, it decreased at 40 °C (p < 0.05). It may be concluded that pressure had negative effect on the phenolic content with increased temperature.
In cricket, there was also significant difference in pressure and temperature separately (p < 0.05) (Table 5). The pressure caused an increase in TPC. Moreover, there was significant difference in the combined effect of pressure and temperature except for HHP-E of cricket oil at 30 °C. The phenolic compounds may have not be affected with heat and pressure treatment even they are sensitive to heat and pressure [34]. The temperature did not cause a significant difference in mealworm oil (p > 0.05), while it caused a significant difference in cricket oil (p < 0.05). Mealworm oil fit to the situation that Patras et al. [34] explained, but phenolic compounds that cricket included might have oxidized easily with increase in temperature [35]. It could be concluded that phenolic compounds that mealworm oil included were more heat resistant compared to cricket oil. HHP-E might have resulted in cellular walls’ disruption and hydrophobic bonds in the membrane,thus, the distribution and aggregation of phenolic compounds might have changed and interaction between solvent and phenolic compounds increased [27]. Besides, this increase might be related with increased extractability of some antioxidant compounds [36].
Antioxidant Activity (AA) with DPPH Assay
Statistical analyses showed that pressure and temperature caused significant difference on AA in mealworm and cricket oil (p < 0.05) (Table 5). No correlation was found between the TPC values and AA results indicating that the phenolics found in the oils either do not necessarily react with DPPH or do not show antioxidant activity at all. HHP-E might have released antioxidant compounds into the extracellular environment by disrupting the cell walls and different antioxidants showed different AA and the effect of pressure on them differed from one to another [37]. This could be the reason of observed increase in AA with HHP-E in our study. Moreover, the effect of temperature on AA of both species was significant (p < 0.05). The increased temperature increased the rate of extraction and that provided higher recovery of antioxidant compounds [38]. Higher temperatures might have caused degradation to heat sensitive antioxidant compounds [39].
Conclusion
The results of the study indicated that mealworm and cricket oil are potential sources of essential fatty acids and antioxidants. Also, oil extraction with HHP can be an alternative method against CE when the optimum parameters are chosen. However, for industrial applications, it may be required to do a feasibility study between two methods. Extensive research is required to explore the properties of insect oils with different parameters.
References
Tao, J., Davidov-Pardo, G., Burns-Whitmore, B., Cullen, E.M., Li, Y.O.: Effects of edible insect ingredients on the physicochemical and sensory properties of extruded rice products. J. Insects Food Feed 3(4), 263–278 (2017). https://doi.org/10.3920/JIFF2017.0030
Vartiainen, O., Elorinne, A.-L., Niva, M., Vaisanen, P.: Finnish consumers’ intentions to consume insect-based foods. J. Insects Food Feed (2020). https://doi.org/10.3920/JIFF2019.0042
Mariod, A.A., Saeed Mirghani, M.E., & Hussein, I. Principles of oil extraction, processing, and oil composition. In: Unconventional Oilseeds and Oil Sources, pp. 347–350. (2017). https://doi.org/10.1016/b978-0-12-809435-8.00053-6.
Alves, A.V., Sanjinez-Argandoña, E.J., Linzmeier, A.M., Cardoso, C.A.L., Macedo, M.L.R.: Food value of mealworm grown on acrocomia aculeata pulp flour. PLoS ONE (2016). https://doi.org/10.1371/journal.pone.0151275
Bovera, F., Piccolo, G., Gasco, L., Marono, S., Loponte, R., Vassalotti, G., et al.: Yellow mealworm larvae (Tenebrio molitor, L.) as a possible alternative to soybean meal in broiler diets. Br. Poultry Sci. 56(5), 569–575 (2015). https://doi.org/10.1080/00071668.2015.1080815
Makkar, H.P.S., Tran, G., Heuzé, V., Ankers, P.: State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 197, 1–33 (2014). https://doi.org/10.1016/j.anifeedsci.2014.07.008
Ravzanaadii, N., Kim, S.-H., Choi, W.-H., Hong, S.-J., Kim, N.-J.: Nutritional value of mealworm, Tenebrio molitor as food source. Int. J. Ind. Entomol. 25(1), 93–98 (2012). https://doi.org/10.7852/ijie.2012.25.1.093
von Hackewitz, L.: The house cricket Acheta domesticus, a potential source of protein for human consumption. Swedish University of Agricultural Sciences, Dept. of Molecular Sciences, Uppsala, 17 (2018). https://stud.epsilon.slu.se/13728/11/von-hackewitz_l_180906.pdf
Hutchins, R.F.N., Martin, M.M.: The lipids of the common house cricket, Acheta domesticus L. I. Lipid classes and fatty acid distribution. Lipids 3(3), 247–249 (1968). https://doi.org/10.1007/BF02531195
van Huis, A.: Edible crickets, but which species? J. Insects Food Feed (2020). https://doi.org/10.3920/JIFF2020.x001
Elamin, W.M., Endan, J.B., Yosuf, Y.A., Shamsudin, R., Ahmedov, A.: High pressure processing technology and equipment evolution: a review. J. Eng. Sci. Technol. Rev. 8(5), 75–83 (2015)
Liepa, M., Zagorska, J., Galoburda, R.: High-pressure processing as novel technology in dairy industry: a review. Res. Rural Dev. 1, 76–83 (2016)
Orlien, V.: High pressure treatment and the effects on meat proteins. Med. Res. Arch. 5(8), 1–10 (2017)
Yaldagard, M., Mortazavi, S.A., Tabatabaie, F.: The principles of ultra high pressure technology and its application in food processing/preservation: a review of microbiological and quality aspects. Afr. J. Biotechnol. 7, 2739–2767 (2008). https://doi.org/10.5897/AJB07.923
Balasubramaniam, V.M., Martínez-Monteagudo, S.I., Gupta, R.: Principles and application of high pressure-based technologies in the food industry. Ann. Rev. Food Sci. Technol. 6(1), 435–462 (2015). https://doi.org/10.1146/annurev-food-022814-015539
Chawla, R., Patil, G.R., Singh, A.K.: High hydrostatic pressure technology in dairy processing: a review. J. Food Sci. Technol. 48, 260–268 (2011). https://doi.org/10.1007/s13197-010-0180-4
Ginsau, M.A.: High pressure processing: a novel food preservation technique. IOSR J. Environ. Sci. Ver. I 9(5), 2319–2399 (2015). https://doi.org/10.9790/2402-0951109113
Parekh, S.L., Aparnathi, K.D., Sreeja, V.: High pressure processing: a potential technology for processing and preservation of dairy foods. Int. J. Curr. Microbiol. Appl. Sci. 6(12), 3526–3535 (2017)
Povedano, I., Guignon, B., Montoro, Ó.R., Sanz, P.D., Taravillo, M., Baonza, V.G.: Effects of high pressure on unsaturated fatty acids. High Press. Res. 34(4), 428–433 (2014). https://doi.org/10.1080/08957959.2014.970187
Bußler, S., Rumpold, B.A., Jander, E., Rawel, H.M., Schlüter, O.K.: Recovery and techno-functionality of flours and proteins from two edible insect species: meal worm (Tenebrio molitor) and black soldier fly (Hermetia illucens) larvae. Heliyon (2016). https://doi.org/10.1016/j.heliyon.2016.e00218
Jeon, Y.H., Son, Y.J., Kim, S.H., Yun, E.Y., Kang, H.J., Hwang, I.K.: Physicochemical properties and oxidative stabilities of mealworm (Tenebrio molitor) oils under different roasting conditions. Food Sci. Biotechnol. 25(1), 105–110 (2016). https://doi.org/10.1007/s10068-016-0015-9
Tomaszewska-Gras, J.: Rapid quantitative determination of butter adulteration with palm oil using the DSC technique. Food Control 60, 629–635 (2016). https://doi.org/10.1016/j.foodcont.2015.09.001
Scheme, R.: IFRA analytical method: determination of the peroxide value. Int. Fragran. Assoc. Rep. 17, 1–5 (2011)
Al-Rimawi, F., Rishmawi, S., Ariqat, S.H., Khalid, M.F., Warad, I., Salah, Z.: Anticancer activity, antioxidant activity, and phenolic and flavonoids content of wild Tragopogon porrifolius plant extracts. Evid. Based Complement. Altern. Med. (2016). https://doi.org/10.1155/2016/9612490
Brand-Williams, W., Cuvelier, M.E., Berset, C.: Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 28, 25–30 (1995). https://doi.org/10.1016/S0023-6438(95)80008-5
Ahmed, J.: High pressure processing of fruits and vegetables. Stewart Postharv. Rev. (2006). https://doi.org/10.2212/spr.2006.1.8
Prasad, N.K., Yang, B., Zhao, M., Wang, B.S., Chen, F., Jiang, Y.: Effects of high-pressure treatment on the extraction yield, phenolic content and antioxidant activity of litchi (Litchi chinensis Sonn.) fruit pericarp. Int. J. Food Sci. Technol. 44(5), 960–966 (2009). https://doi.org/10.1111/j.1365-2621.2008.01768.x
L’Hocine, L., Boye, J.I., Arcand, Y.: Composition and functional properties of soy protein isolates prepared using alternative defatting and extraction procedures. J. Food Sci. (2006). https://doi.org/10.1111/j.1365-2621.2006.tb15609.x
Norris, D.O., & Carr, J.A.: Synthesis, metabolism, and actions of bioregulators. In: Vertebrate endocrinology (pp. 41–91). (2013). https://doi.org/10.1016/b978-0-12-394815-1.00003-3
Codex Alimentarius: Standard for edible fats and oils not covered by individual standards. CodexAlimentarius, 2–7. (2017).
Choe, E., Min, D.B.: Mechanisms and factors for edible oil oxidation. Comprehens. Rev. Food Sci. Food Safe. 5, 169–186 (2006). https://doi.org/10.1111/j.1541-4337.2006.00009.x
Medina-Meza, I.G., Barnaba, C., Barbosa-Cánovas, G.V.: Effects of high pressure processing on lipid oxidation: a review. Innov. Food Sci. Emerg. Technol. 22, 1–10 (2014). https://doi.org/10.1016/j.ifset.2013.10.012
Cheftel, J.C., Culioli, J.: Effects of high pressure on meat: a review. Meat Sci. 46, 211–236 (1997). https://doi.org/10.1016/S0309-1740(97)00017-X
Patras, A., Brunton, N., Da Pieve, S., Butler, F., Downey, G.: Effect of thermal and high pressure processing on antioxidant activity and instrumental colour of tomato and carrot purées. Innov. Food Sci. Emerg. Technol. 10(1), 16–22 (2009). https://doi.org/10.1016/j.ifset.2008.09.008
Réblová, Z.: Effect of temperature on the antioxidant activity of phenolic acids. Czech J. Food Sci. 30(2), 171–177 (2012)
Cao, X., Zhang, Y., Zhang, F., Wang, Y., Yi, J., Liao, X.: Effects of high hydrostatic pressure on enzymes, phenolic compounds, anthocyanins, polymeric color and color of strawberry pulps. J. Sci. Food Agric. 91(5), 877–885 (2011). https://doi.org/10.1002/jsfa.4260
Briones-Labarca, V., Venegas-Cubillos, G., Ortiz-Portilla, S., Chacana-Ojeda, M., Maureira, H.: Effects of high hydrostatic pressure (HHP) on bioaccessibility, as well as antioxidant activity, mineral and starch contents in Granny Smith apple. Food Chem. 128(2), 520–529 (2011). https://doi.org/10.1016/j.foodchem.2011.03.074
García-Márquez, E., Román-Guerrero, A., Cruz-Sosa, F., Pérez-Alonso, C., Jiménez-Alvarado, R., Vernon-Carter, E.J.: Effect of solvent-temperature extraction conditions on the initial antioxidant activity and total phenolic content of muitle extracts and their decay upon storage at different pH. Rev. Mexi. Ingen. Qum. 11(1), 1–10 (2012)
Liyana-Pathirana, C.M., Shahidi, F.: Antioxidant activity of commercial soft and hard wheat (Triticum aestivum L.) as affected by gastric pH conditions. J. Agric. Food Chem. 53(7), 2433–2440 (2005). https://doi.org/10.1021/jf049320i
Funding
This study was funded by Middle East Technical University Research Fund Project [Grant Number YLT-314–2018-3581].
Author information
Authors and Affiliations
Contributions
All the authors contributed equally and intellectually to this research, met authorship requirements and read and approved the final version of the article.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ugur, A.E., Bolat, B., Oztop, M.H. et al. Effects of High Hydrostatic Pressure (HHP) Processing and Temperature on Physicochemical Characterization of Insect Oils Extracted from Acheta domesticus (House Cricket) and Tenebrio molitor (Yellow Mealworm). Waste Biomass Valor 12, 4277–4286 (2021). https://doi.org/10.1007/s12649-020-01302-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-020-01302-z