Abstract
Artificial neural networking (ANN) based models are being increasingly and effectively used in the prediction and estimation of response variables during various food processing applications. We demonstrated the feasibility of a feed forward back propagation ANN model to estimate ascorbic acid content in fresh amla juice that was ohmic heating assisted vacuum evaporated (OHVC) with/without ultrasonication (US) from color attributes (L*, a*, b* and ΔE) during 4 weeks of ambient storage. There was a positive effect of synergistic processing of OHVC and US on the quality of stored amla juice with the fresh untreated sample witnessing ascorbic acid and total phenolic degradation of up to 60% and 14% higher than the OHVC + US treated samples respectively. At the end of the storage period, the color change was lowest in OHVC samples stored in glass containers (ΔE = 5.00) in comparison to untreated samples stored in PET containers (ΔE = 9.61). The results further suggested that coefficient of determination (R2) for the fitted ANN model was approximately 0.93 with a mean square error of 0.15, which was 20% higher and 66% lower than that obtained using multiple linear regression model, respectively. Furthermore, a number of training algorithms were tested out of which Levenberg–Marquardt algorithm showed the highest efficiency in model prediction. Overall, the quality in OHVC and US processed juice was retained better in comparison to control and the fitted ANN model effectively predicted ascorbic acid content from input color parameters. The results provided can be helpful in the non-destructive evaluation of ascorbic acid content in stored amla juice using AI mediated modelling.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The increased health consciousness among consumers worldwide have increased the market demand for fresh fruit juices providing significant nutritional benefits. Amla or the Indian gooseberry (Emblica officinalis) is an important source of bioactive compounds including ascorbic acid, tannins, phenols, flavonoids and antioxidants [1]. The fruit has a high reverence in the Indian subcontinent as a constituent of indigenous medicinal formulations [2]. The bioactive compounds in amla exhibit hypolipidemic and hypoglycemic properties and form a major constituent of hepatoprotective formulations [3]. Because of its high perishability, amla fruits are only available for a short duration of time. In addition, poor taste, astringency, high acidity are some factors that limit its acceptability as a table fruit [4]. As such, fresh fruit is usually processed into value-added products to maximize the returns. One of the most commonly available value-added amla product is the processed juice. During thermal processing of fruit juice, reduction in ascorbic acid and browning are two main challenges faced by the juice industry [5].
Novel food processing techniques have evolved rapidly over the recent past to process high quality and safe food products [6]. High intensity, probe type ultrasonication is one such non-thermal technique that has been employed in the fruits and vegetable juice industry to modify physiochemical properties, rheological behaviour, enzymatic activity, microbial spoilage and antioxidant properties [7, 8]. The process works on the mechanical effects of cavitation, wherein cavitation bubbles are formed and exploded due to the ultrasound induced pressure changes [9]. On the other hand, ohmic heating is an alternate thermal processing technique in which the food product is held between two current carrying electrodes and behaves like a resistance. As such, rapid volumetric heat is generated which has been extensively used to promote microbial safety, inactivation of spoilage causing enzymes and modifications of sensory properties [10].
Ascorbic acid is one of the most important bioactive compound present in amla juice which is quantified by several methods including wet chemistry, UPLC, and HPLC [11]. Overall, these analytical methods are often cumbersome and require complex sample preparation methods [12] and are not always environmentally friendly [13]. Consequently, there is a growing interest, especially in large scale production and quality assessment, to develop a non-destructive analysis technique that could predict ascorbic acid content from input physical parameters.
Artificial neural networks (ANNs) are a type of machine learning algorithm inspired from the structure and function of the human brain [14]. They are designed to recognize patterns and make predictions or decisions based on input data. The learning process of an ANN involves adjusting the weights of the synapses based on the input data and the desired output. This is done through a process called “backpropagation,” which involves adjusting the weights to minimize the difference between the actual output and the desired output [15]. ANN models are efficient tools that handle several parameters and predict the behaviour of multivariable, non-linear and linear regression problems which are common in food processing applications [16]. Several researchers have applied ANN models and expressed a better generalization capability, higher tolerance to noise and missing data [17]. Multilayer Perceptron (MLP) with feed forward back propagation is a kind of supervised learning technique which uses back propagation for training the network. The main purpose of the process is to readjust weights and biases on the basis of training data so that the error of prediction is minimized [14]. Such networks are widely used in the food processing applications for behavior recognition, prediction, regression and curve fitting purposes [18]. The food processing applications of ANNs that have been explored mainly include modelling and optimization of bioactive compound extraction from plant matrices using novel techniques [19, 20], food drying [15, 21, 22], extrusion [23], fermentation [24, 25], shelf-life estimation [26] among other processes for a wide range of fresh and processed food products. However, to our knowledge, there is no systematic study attempted to establish a relationship between color attributes (L*, a*, b* and ΔE) and ascorbic acid content in fruit juices using feed forward back propagation artificial neural network model. As such the present study was aimed to develop a prediction model based on ANN to estimate ascorbic acid content in fresh, optimized Ohmically heated with/without ultrasound treatment during 4 weeks of ambient storage in amber glass and opaque polyethylene terepthalate (PET) bottles from color attributes.
Materials and methods
Sample procurement and juice preparation
Mature and ripened amla fruits (Emblica officinalis L. var. Neelum) were kindly supplied by fruit farms of Punjab Agricultural University, Ludhiana, India. The fruits were frozen at − 20 ± 1 °C for 24 h followed by thawing at 20 °C for another 10–12 h after sanitization with 100 ppm sodium hypochlorite solution. The freeze–thaw cycle softens the tissue and eases manual deseeding process [5]. Juice was extracted in a household juicer and filtered using double-layered muslin cloth. All chemicals used in the experiments were of analytical grade and were procured from Molychem chemicals (Mumbai, India).
Ohmic heating assisted vacuum evaporation and ultrasonication treatment systems
Ohmic heating treatments for amla juice were carried out in a custom designed treatment chamber (Fig. 1) consisting of concentric SS-316 electrodes and a vacuum headspace of 100% of the treatment volume. The input ac electric current was varied through a 30 A variable single-phase transformer. The current was cut-off using a proportional–integral–derivative temperature controller (UTC-221P, Multispan), A digital ammeter and voltmeter assembly (AV-22, Multispan) assembled inside an insulated SS-304 enclosure was also provided for a better control over variables. In addition to this, the voltage and current readings were also cross calibrated with the help of a digital multimeter (RISH Multi 12 s, Rishabh Industries, IN). The juice (3 L) was treated at an optimized set of conditions for voltage gradient (12.5 V/cm), temperature (65 °C) and process time (44 min) using a Box–Behnken design in response surface methodology (unpublished results). The optimized ultrasonication pre-treatments, on the other hand were conducted based on the results of our previous study [5] at an optimized set of conditions viz, ultrasound intensity (70%); process time (8 min) and pulse duration (5 s on, 5 s off). The amla juice samples were stored in triplicates after optimized ultrasonication pretreatment followed by optimized ohmic heating treatments (OHVC + US), ohmic heating alone (OHVC) while as fresh untreated juice was kept as control.
Storage behaviour and keeping quality
The keeping quality of processed amla juice samples (Fresh, optimized OHVC and optimized OHVC + US) was evaluated under ambient conditions in the month of December (10–23 °C, 40 ± 10% RH). The samples were stored in amber glass and opaque PET bottles of 100 mL capacity. The readings for quality attributes were taken at a regular interval of 5 days for color attributes and ascorbic acid to generate data for kinetic modelling and after 7 days for total phenolic content.
Total phenolic content
Total phenols in the treated amla juice were determined using the Folin–Ciocalteu method as described by Waterhouse [27] with some modifications. Briefly, 0.5 mL of treated/fresh amla juice was mixed with 9.5 mL of 80% methanol and refluxed at 80 °C for 10 min. The mix was then centrifuged at 10,000×g and filtered using a Whatman filter paper to make final volume 10 mL with the addition of 80% methanol. Half millilitre of 20-fold diluted amla juice was then mixed with equal volumes of 1:1 diluted Folin–Ciocalteu reagent. The mix was allowed to react for 5 min after which 1 mL of 35% Na2CO3 (w/v) was added. The contents were placed in dark for one hour and the absorbance was measured at 765 nm using a UV–Vis spectrophotometer. Standard gallic acid dissolved in 80% methanol in different concentrations was used to develop a standard calibration curve. Total phenolic content was expressed as milligrams of gallic acid equivalent per millilitre of amla juice (mg GAE/mL).
Ascorbic acid
Ascorbic acid content (mg/100 mL) of amla juice was determined using titration with standardized 2,6-Dichlorophenol-Indophenol dye (DID) solution as discussed in our previous study [5]. Briefly, 0.25 mL of amla juice was added to 10 mL of metaphosphoric acid solution (15 g metaphosphoric acid + 40 mL acetic acid in 450 mL double distilled water). Only 5 mL from this solution were further titrated with the dye (52 mg DID + 42 mg NaHCO3) until a pink color appeared.
Color attributes
The color attributes of the treated juice were measured using Color Reader CR-10 (Konica Minolta Sensing Inc, Japan) in terms of the CIE LAB parameters viz. L* (lightness/darkness), a* (red/green) and b* (blue/yellow). The samples were analysed in a 250/100 mL glass beakers and the sensing bulb was so placed that no light could pass through except for the illumination generated by the equipment. The color change was calculated as per the following equation.
where \({L}_{o}^{*}\), \({a}_{o}^{*}\) and \({b}_{o}^{*}\) are the tristimulus values for fresh juice respectively.
Kinetic modelling of ascorbic acid using artificial neural networks
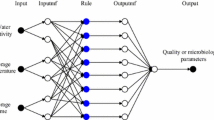
The prediction modelling for ascorbic acid was performed using four input color parameters viz. ‘L*’, ‘a*’, ‘b*’ and color change (ΔE). A multilayer feed forward backpropagation (MLFFBP) network was used for the prediction using the ‘nntool’ of MATLAB v.R2016a (MathWorks Inc, Massachusetts, US). The effectiveness of the neural networks was checked by using networks with different number of layers and neurons and the combination that provided best correlation coefficient between the input and output layer was selected. The transfer function for the hidden layers was set as ‘tansig’ (TANSIGMOID) while as PURELIN transfer function was used for the output layers as suggested by Tarafdar et al. [21] and Dorofki et al. [28]. Such functions are believed to increase the process efficiency while significantly decreasing the processing time. The model topology and the associated transfer functions for hidden and output layers are shown in Fig. 2. The training parameters were set as to use mean square error as a performance indicator with an error goal of < 1e−07. Training of the model was performed using the Levenberg–Marquardt (TRAINLM) algorithm using 30% of the input data. The performance characteristics of TRAINLM algorithm were further compared with different algorithms providing better adaptive learning rates and efficient backpropagation. These algorithms included Gradient descent with momentum and Adaptive learning rate (TRAINGDX); Resilient Backpropagation (TRAINRP); BFGS Quasi Newton (TRAINBFG); Conjugate Gradient with Beale Powell Restarts (TRAINCBG); Gradient Descent (TRAINGD); One Step Secant (TRAINOSS); Random Weight Bias Rule (TRAINR) and Scaled Conjugate Gradient (TRAINSCG). The algorithms were tested at different configurations of hidden layer neurons and based on the coefficient of determination, correlation coefficients and mean square errors, the most feasible algorithm was selected. The adaption behaviour of the fitted neural network was performed based on gradient descent function with momentum and bias learning functions (LEARNGDM). Models having a different number of layers, neurons were evaluated and the one with highest correlation coefficient (R), minimum mean square error (MSE) and lowest complexity of model architecture was selected. The evaluation parameters to analyze the accuracy of neural network were calculated as per the following equations [21]:
where MSE is the mean square error, N is total number of observations, exp and pred are the experimental and predicted values respectively.
Statistical analysis
The statistical analysis for analyzing variance of the completely randomized designs at a least significant difference of p < 0.05 was carried out using the Statistical Package for the Social Sciences (SPSS) software (v.20) (SPSS, Chicago, IL, USA). The design of experiments was done using Design Expert v 13.0 (StatEase Inc). The regression models and graphical presentations were obtained using GraphPad (Prism) and the data are presented as means of at least three replications.
Results and discussion
Color attributes
Color is one of the most important parameters that determines the acceptance of amla juice after storage. In the present study, the lowest and highest magnitude of color change was witnessed after processing with OHVC and OHVC + US treatments (Fig. 3a). Immediately after the treatments, a high color change (9.95) value was observed for a combination of optimized OHVC and ultrasonication optimized OHVC treatments. These results indicate that ohmic heating treatments under optimized process conditions caused a significant change in the ΔE values in comparison to fresh samples. However, the color change values for OHVC and OHVC + US treatments improved as the storage proceeded irrespective of the storage material (Fig. 3a) and was comparable to fresh untreated juice at 20th day of ambient storage. The data further corroborates this finding that there was a non-significant effect of treatment as well as packaging material on the color change values of amla juice on 15th and 20th day. It can further be understood from the multivariate analysis of variance between storage period and treatment type, that the order of changes in ΔE values followed OHVC + US glass > OHVC + US PET ≥ OHVC PET > OHVC Glass > Fresh glass = Fresh PET. There was a non-significant effect of packaging material on the color change values in fresh untreated and optimized OHVC processed amla juice samples. However, amla juice treated with optimized conditions of OHVC and ultrasonication, stored in PET bottles witnessed a relatively higher retention (p < 0.05) of color attributes as compared to amber glass. Ayhan et al. [29] studied the change in color attributes of pulsed electric field processed orange juice stored for 112 days in glass, PET, HDPE and LDPE containers. The authors observed that glass and PET showed a non-significant difference in the changes of color attributes. However, HDPE and LDPE fared poorly and experienced a much higher degradation in color. These findings suggest that amber glass and PET have good gas and light barrier properties and are chemically inert. Detrimental effects on color change during storage of amla juice is primarily linked to enzymatic browning and photolytic effects and temperature being the most critical factor that influences enzymatic activity.
Comparisons of L*, a* and b* values for all treatments over the 30-day storage period are presented in Fig. 3b–d respectively. It can be observed that OHVC treatments significantly affected the lightness of amla juice. However, as the storage proceeded, an improvement of L* value was observed. At the end of 4 weeks, there was a significant difference among L* values of all stored samples. On the other hand, there was a definitive increase of ‘a*’ values during the ambient storage of amla juice, irrespective of the treatment applied (Fig. 3c). It was observed that the minimum ‘a*’ value was recorded by fresh untreated amla juice (− 7.45) which degraded to 0.3 after 4 weeks of ambient storage. This resulted in a loss of light green color that is a characteristic feature of fresh amla juice. The corresponding ‘a*’ value for OHVC and OHVC + US treatments was however significantly lower than the fresh untreated samples (p < 0.05). The difference between packaging materials at the end of storage was also non-significant. There was a significant increase in ‘b*’ values of stored amla juice which corresponded to an increase in browning. Among the samples, fresh untreated samples had a significantly (p < 0.05) higher ‘b*’ value after 30 days in ambient storage as compared to OHVC and OHVC + US treatments. The maximum ‘b*’ value was observed for fresh untreated samples in glass bottles (17.55), while as the minimum value was recorded for amla juice after immediate treatment with OHVC (9.85). The fresh amla juice has ‘b*’ value of 14.8. The results are in accordance with the literature, wherein ohmic heating is believed to cause a significant change in color attributes of treated food products. There can be a number of reasons for the trends observed. However, it is pertinent to mention that at low frequencies, electrode corrosion in SS-316 electrodes occur via electro-dissolution resulting in the generation of metallic ions. Since food systems are rich in bioactive compounds in the form of ligands, various coordination complexes could be produced due to their interactions with the migrated ions [30]. At the time of treatment, these metal complexes could have altered the color of processed juice with their characteristic colors. However, metal ligands with low crystal field stabilization energy and of non-chelating nature tend to be unstable and their dissociation might have resulted in the improvement of color change with storage [31].
Ascorbic acid
Effects of packaging materials, treatment type and storage period on the concentration of ascorbic acid of amla juice during storage under ambient conditions are shown in Fig. 3e. The highest ascorbic acid concentration was recorded in OHVC + US treatment (613 mg/100 mL) which was mainly due to the release of bound ascorbic acid during ultrasonication. At the end of storage, the lowest ascorbic acid content was observed in control samples stored in glass containers (113.8 mg/100 mL), which was around 60% lower than OHVC + US treated samples stored in PET bottles. The results indicate a positive effect of synergistic processing of ohmic heating and ultrasonication on the quality of stored amla juice. It can be observed that there is a non-significant difference among packaging material and treatment type between OHVC and OHVC + US treatments at the end of 4-week storage period (p < 0.05). Comparison of overall means using Tukeys’-b test for the treatments suggest that PET containers were better for fresh untreated and OHVC + US while as there was no significant difference among the containers for OHVC treatment. Among the storage periods, ascorbic acid witnessed a significant time dependent decline. The retention of ascorbic acid after 1 month storage period was observed to be better in the case of OHVC and OHVC + US treatments as compared to control samples. This could have possibly been due to the inactivation of quality degrading enzymes and pasteurization effects of OHVC that provided a better stability to the compound. Ascorbic acid is an important quality characteristic and has been used as an indicator of quality and shelf life in many food products including pineapple juice [32]. Vitamin C and flavor are degraded in containers that have a permeability to atmospheric oxygen [33]. In addition, the degradation is ameliorated in the presence of light. Some other factors that accelerate the ascorbic acid degradation include availability of oxygen in the headspace and storage temperature [34]. The results suggest that both PET and glass containers showed a significant retention of ascorbic acid due to the gas and light barrier properties.
Total phenols
Figure 4 presents the total phenolic values of amla juice processed under different conditions, packaging material over a 30-day ambient storage period. Initially, the total phenolic content varied from 16.09 mg/mL in OHVC + US treatments to 19.21 mg/mL in fresh untreated samples. The percentage degradation of TPs during storage was lowest for OHVC + US treated amla juice in glass containers (7.45%), OHVC + US treated amla juice in PET containers (13.03%), OHVC treated amla juice in glass containers (13.92%), OHVC treated amla juice in PET containers (20.2%), fresh untreated amla juice in glass containers (19.9%) and fresh untreated amla juice stored in glass containers (21.4%) respectively. The overall trends in the changes of TP of amla juice during ambient storage are presented in Fig. 4. As discussed in the preceding sections, OHVC and US treatments caused a significant decrease in the total phenolic compounds and resulted in their degradation or conversion into secondary metabolites. However, the differences in phenolic content over storage was not as significant as ascorbic acid. The juice samples observed a marked decline after 1st week whereafter the phenolic content stabilized with time. Among the treatments, it was observed that the overall means for OHVC and OHVC + US treatments did not differ significantly. The effect of packaging material was also observed to be non-significant. There are a number of research works that have studied the stability of phenolic compounds during storage. For example, Piljac-Zegarac et al. [35] observed that phenolic compounds in different juice samples are stable and that the content first increases and then decreases with storage. The phenolic compounds like flavan-3-ol monomers and dimers are considered to be primary substrates for enzymatic oxidation induced by polyphenol oxidase enzyme. As a result, total phenolics degrade with storage converting into secondary compounds [36, 37]. During the storage of litchi pericarp at room temperature, Deng et al. [38] observed a phenolic degradation upto the extent of 48%. Similar results were also observed in strawberries, sour cherries and raspberries [39]. Furthermore, from Fig. 4, it can be observed that TP content decreased most significantly during the 1st week of storage in fresh untreated samples. This could have been due to the activity of polyphenol oxidase enzyme that exhibits highest activity after juice extraction. Similar results were also observed by Deng et al. [38] where the authors observed the most rapid phenolic degradation under room temperature during the first 12 h of storage under ambient conditions.
Predictive modelling using artificial neural network
The basis of any neural network program is an algorithm, which is an indicator of the generalization ability of the selected model, precision of fitting data, mapping ability and overall efficiency [15]. A neural network model was fitted to the input data (‘L*’, ‘a*’, ‘b*’ and ΔE; n = 84) to find a correlation with the output variable (ascorbic acid, mg/mL) in stored amla juice. Levenberg–Marquardt (TRAINLM) training algorithm was used to train the network based on the suggestions of Sun et al. [15] as it shows better correlation and minimum mean square error as compared to other training algorithms. In the present work, the ANN model showed a good correlation coefficient and satisfactory prediction of data at 10 hidden layer neurons and two layers. This helped in the reduction of complexity of the network at a significantly lower processing time. The final model matrix containing the topology, weights and bias for each network layer is provided in Table 1. The correlation plot of the fitted model having one hidden layer of 10 neurons and trained at 70% input data is provided in Fig. 5. It can be observed that the correlation coefficient for training, validation and testing data is 0.97, 0.96 and 0.93 following the correlation coefficient order Training > Validation > Testing. The overall correlation coefficient was 0.96, suggesting a good fit of the input data. The calculated values of R2 for the fitted model was around 0.93 with a mean square error of 0.15. There are different algorithms that have been applied by researchers and each have their individual advantages and disadvantages. However, in the application of prediction of responses in food processing operations like microwave assisted drying, freeze drying etc., it has been observed that TRAINLM function has the best fit accuracy. In addition, the mathematical transfer function, viz. ‘tansig’ for hidden layers and ‘purelin’ showed best adequacy of fit in the applied ANN models [11]. The results are in accordance with the literature, wherein Sun et al. [15] and Tarafdar et al. [21] reported the best fit ANN feed forward back propagation model having low number of hidden layer neurons (10–15).
Comparison of different ANN training algorithms
The performance characteristics of Levenberg–Marquardt (TRAINLM) algorithm were compared with different algorithms providing better adaptive learning rates and efficient backpropagation. These algorithms included Gradient descent with momentum and Adaptive learning rate (TRAINGDX); Resilient Backpropagation (TRAINRP); BFGS Quasi Newton (TRAINBFG); Conjugate Gradient with Beale Powell Restarts (TRAINCBG); Gradient Descent (TRAINGD); One Step Secant (TRAINOSS); Random Weight Bias Rule (TRAINR) and Scaled Conjugate Gradient (TRAINSCG). The results highlighted that TRAINLM algorithm for the correlation between input color attributes and ascorbic acid (mg/mL) showed better results in terms of a higher R2 and low MSE as compared to other algorithms that were studied. In addition, TRAINLM achieved the termination criterion earlier, thereby reducing processing time. The comparison of algorithm performance parameters is provided in Table 2 and the relative differences have been shown in Fig. 6. It can be observed that the TRAINLM correlation coefficient for training (0.97), validation (0.96) and testing (0.93) are also higher than other algorithms. Among other algorithms that were studied it was observed that the coefficient of determination (R2) for TRAINLM (0.93) was highest, followed by TRAINOSS (0.86), TRAINBFG (0.86), TRAINGDX (0.85), TRAINSCG (0.835), TRAINRP (0.83), TRAINCBG (0.827), TRAINR (0.729) and TRAINGD (0.716) respectively. Almost similar trends were observed for MSE values which were lowest for TRAINLM (0.15) followed by TRAINOSS (0.246), TRAINBFG (0.25), TRAINGDX (0.27), TRAINRP (0.31), TRAINCBG (0.329), TRAINR (0.424) and TRAINGD (0.509) respectively. The results therefore suggest that TRAINLM can be efficiently used in food processing applications to achieve a better correlation function between process variables and responses due to its faster convergence. Similar observations have been made in previous literature by Tarafdar et al. [21], Jafari et al. [40]; Raj and Dash [20] and others.
ANN vs. multiple linear regression
Multiple Linear Regression (MLR) of input parameters (‘L*’, ‘a*’, ‘b*’ and ΔE) was chosen for the comparison of prediction performance of selected ANN model. The evaluation of regression models was based on the R2 and MSE to that of ANN for prediction of ascorbic acid content in amla juice (Fig. 6). The linear regression equation that was observed to best fit the input data is provided below:
The R2 value for multiple linear correlation was observed to be 0.74 for the same dataset as used for ANN while as a MSE value of 0.45. It was observed that ANN model gave best fit for the prediction data with an R2 value of 0.93 and MSE value of 0.15. The corresponding R2 value for linear regression model was almost 20% lower than the ANN model, while as the MSE was 66% higher. The experimental vs predicted values for ANN and MLR are plotted in Fig. 7. The results suggested that the developed model using ANN was able to perform better than MLR model for the prediction of ascorbic acid content of amla juice during ambient storage. Since there are no ANN models available in literature for the correlation of color attributes and ascorbic acid content, no comparisons were possible. In other applications like microwave assisted drying and freeze drying, a higher R2 value was recorded for ANN models by Sun et al. [15] and Tarafdar et al. [21] for carrots and white button mushrooms respectively. Although drying processes are complex in nature and depend on a number of variables, there is a definitive trend of decrease or increase as the drying proceeds with the processing variables. Furthermore, the adequacy of fit using ANN modelling approaches can be improved with the use of a significantly larger dataset. It can thus be inferred that it is more suitable to fit complex multi-variable data patterns with the help of ANN models, rather than using simple regression trends that are rendered inadequate.
Conclusions
In the present study, storage behaviour of ohmic heating with/without ultrasound pretreatments during 4 weeks of ambient storage in PET and amber glass bottles was reported. The ohmic heating treatments were observed to cause a significant color change in the juice right after treatment, which however improved during storage. At the end of the storage period, the processed samples witnessed a better color retention (color change value of 5.00 in OHVC samples stored in glass containers) in comparison to untreated samples (color change value of 9.61 in PET containers). Ascorbic acid and total phenols were also observed to decrease with storage, however, polyphenolic content in amla juice was more stable than ascorbic acid content. There was not an overall significant difference between the PET and glass storage bottles and therefore the findings merit a long-term study to further establish differences between the two. A feed forward back propagation ANN model was fitted to the color attributes (L*, a*, b* and ΔE) vs. ascorbic acid and a significant correlation with a coefficient of determination of 0.93 and a low mean square error. The model fared significantly well in non-destructively analysing ascorbic acid from physical parameters of color. The R2 and MSE values of ANN model were significantly better than those obtained using model developed by multiple linear regression technique. Among the training algorithms studied, Levenberg–Marquardt algorithm returned better model efficiency and suitability with the input dataset. However, further research efforts encompassing a higher amount of input data and using image processing algorithms rather than L*, a* and b* values would further increase the effectiveness and reduce the time consumed in non-destructive and rapid assessment of ascorbic acid from treated juice.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.
References
B.P. Gaire, L. Subedi, Phytochemistry, pharmacology and medicinal properties of Phyllanthus emblica Linn. Chin. J. Integr. Med. (2014). https://doi.org/10.1007/s11655-014-1984-2
K. Rose, C. Wan, A. Thomas, N.P. Seeram, H. Ma, Phenolic compounds isolated and identified from amla (Phyllanthus emblica) juice powder and their antioxidant and neuroprotective activities. Nat. Prod. Commun. (2018). https://doi.org/10.1177/1934578X1801301019
M.S. Baliga, A.R. Shivashankara, K.R. Thilakchand, M.P. Baliga-Rao, P.L. Palatty, T. George, S. Rao, Hepatoprotective effects of the Indian gooseberry (Emblica officinalis Gaertn): a revisit, in Dietary Interventions in Liver Disease (Academic Press, 2019), pp. 193–201
R. Tewari, V. Kumar, H.K. Sharma, Thermal and nonthermal processing of an underutilized fruit Emblica officinalis (Amla): a sustainable approach. Sustain. Food Technol. (2023). https://doi.org/10.1039/D3FB00058C
R. Aslam, M.S. Alam, A. Ali, Y. Tao, S. Manickam, A chemometric approach to evaluate the effects of probe-type ultrasonication on the enzyme inactivation and quality attributes of fresh amla juice. Ultrason. Sonochem. 92, 106268 (2023)
R. Pandiselvam, S. Mitharwal, P. Rani, M.A. Shanker, A. Kumar, R. Aslam et al., The influence of non-thermal technologies on color pigments of food materials: an updated review. Curr. Res. Food Sci. 6, 100529 (2023)
B.M. Lepaus, B.S. Valiati, B.G. Machado, M.M. Domingos, M.N. Silva, L. Faria-Silva et al., Impact of ultrasound processing on the nutritional components of fruit and vegetable juices. Trends Food Sci. Technol. 138, 752–765 (2023)
F. Salehi, Physico-chemical properties of fruit and vegetable juices as affected by ultrasound: a review. Int. J. Food Prop. 23(1), 1748–1765 (2020)
R. Aslam, M.S. Alam, J. Kaur, A.S. Panayampadan, O.I. Dar, A. Kothakota, R. Pandiselvam, Understanding the effects of ultrasound processng on texture and rheological properties of food. J. Texture Stud. 53(6), 775–799 (2022)
N.K. Doan, D.Q. Lai, T.K.P. Le, Ohmic heating: its current and future application in juice processing. Food Rev. Intl. 39(9), 6908–6933 (2023)
I. Klimczak, A. Gliszczyńska-Świgło, Comparison of UPLC and HPLC methods for determination of vitamin C. Food Chem. 175, 100–105 (2015)
D. Valinger, M. Kušen, A. Jurinjak Tušek, M. Panić, T. Jurina, M. Benković et al., Development of near infrared spectroscopy models for quantitative prediction of the content of bioactive compounds in olive leaves. Chem. Biochem. Eng. Q. 32(4), 535–543 (2018)
A. Khoddami, M.A. Wilkes, T.H. Roberts, Techniques for analysis of plant phenolic compounds. Molecules 18(2), 2328–2375 (2013)
G.V.S. Raj, K.K. Dash, Comprehensive study on applications of artificial neural network in food process modeling. Crit. Rev. Food Sci. Nutr. 62(10), 2756–2783 (2022)
Q. Sun, M. Zhang, A.S. Mujumdar, P. Yang, Combined LF-NMR and artificial intelligence for continuous real-time monitoring of carrot in microwave vacuum drying. Food Bioprocess Technol. 12, 551–562 (2019)
S. Abdullah, R.C. Pradhan, D. Pradhan, S. Mishra, Modeling and optimization of pectinase-assisted low-temperature extraction of cashew apple juice using artificial neural network coupled with genetic algorithm. Food Chem. 339, 127862 (2021)
M. Fazaeli, Z. Emam-Djomeh, M. Omid, A. Kalbasi-Ashtari, Prediction of the physicochemical properties of spray-dried black mulberry (Morus nigra) juice using artificial neural networks. Food Bioprocess Technol. 6, 585–590 (2013)
J. Nayak, K. Vakula, P. Dinesh, B. Naik, D. Pelusi, Intelligent food processing: journey from artificial neural network to deep learning. Comput. Sci. Rev. 38, 100297 (2020)
Z. Dolatabadi, A.H. Elhami Rad, V. Farzaneh, S.H. Akhlaghi Feizabad, S.H. Estiri, H. Bakhshabadi, Modeling of the lycopene extraction from tomato pulps. Food Chem. 190, 968–973 (2016)
G.B. Raj, K.K. Dash, Ultrasound-assisted extraction of phytocompounds from dragon fruit peel: optimization, kinetics and thermodynamic studies. Ultrason. Sonochem. 68, 105180 (2020)
A. Tarafdar, N.C. Shahi, A. Singh, Freeze-drying behaviour prediction of button mushrooms using artificial neural network and comparison with semi-empirical models. Neural Comput. Appl. 31, 7257–7268 (2019)
N. Karakaplan, E. Goz, E. Tosun, M. Yuceer, Kinetic and artificial neural network modeling techniques to predict the drying kinetics of Mentha spicata L. J. Food Process. Preserv. 43(10), e14142 (2019)
S. Neelam, B.K. Kumbhar, M. Kulshreshtha, Modeling of extrusion process using response surface methodology and artificial neural networks. J. Eng. Sci. Technol. 1(1), 31–40 (2006)
Z. Zheng, X. Guo, K. Zhu, W. Peng, H. Zhou, The optimization of the fermentation process of wheat germ for flavonoids and two benzoquinones using EKF-ANN and NSGA-II. RSC Adv. 6(59), 53821–53829 (2016)
E. Betiku, A.E. Taiwo, Modeling and optimization of bioethanol production from breadfruit starch hydrolyzate vis-a-vis response surface methodology and artificial neural network. Renew. Energy 74, 87–94 (2015)
G.K. Goyal, S. Goyal, Cascade artificial neural network models for predicting shelf life of processed cheese. J. Adv. Inf. Technol. 4(2), 80–83 (2013)
A.L. Waterhouse, Determination of total phenolics. Curr. Protocol Food Anal. Chem. 6(1), I1-1 (2002)
M. Dorofki, A.H. Elshafie, O. Jaafar, O.A. Karim, S. Mastura, Comparison of Artificial Neural Network transfer functions abilities to simulate extreme runoff data, in International Conference on environment, Energy and Biotechnology, vol. 33 (IACSIT Press, Singapore, 2012), pp. 39–44
Z. Ayhan, H.W. Yeom, Q.H. Zhang, D.B. Min, Flavor, color, and vitamin C retention of pulsed electric field processed orange juice in different packaging materials. J. Agric. Food Chem. 49(2), 669–674 (2001)
C.P. Samaranayake, S.K. Sastry, In-situ pH measurement of selected liquid foods under high pressure. Innov. Food Sci. Emerg. Technol. 17, 22–26 (2013)
E. Habeych, V. van Kogelenberg, L. Sagalowicz, M. Michel, N. Galaffu, Strategies to limit colour changes when fortifying food products with iron. Food Res. Int. 88, 122–128 (2016)
A. Akyildiz, T.S. Mertoglu, E. Agcam, Kinetic study for ascorbic acid degradation, hydroxymethylfurfural and furfural formations in orange juice. J. Food Compos. Anal. 102, 103996 (2021)
S. Basak, L. Shaik, S. Chakraborty, Effect of ultraviolet and pulsed light treatments on ascorbic acid content in fruit juices—a review of the degradation mechanism. Food Chem. Adv. 2, 100333 (2023)
V. Gérard, E. Ay, B. Graff, F. Morlet-Savary, C. Galopin, W. Mutilangi, J. Lalevée, Ascorbic acid derivatives as potential substitutes for ascorbic acid to reduce color degradation of drinks containing ascorbic acid and anthocyanins from natural extracts. J. Agric. Food Chem. 67(43), 12061–12071 (2019)
J. Piljac-Žegarac, L. Valek, S. Martinez, A. Belščak, Fluctuations in the phenolic content and antioxidant capacity of dark fruit juices in refrigerated storage. Food Chem. 113(2), 394–400 (2009)
Y. Sun, L. Zhong, L. Cao, W. Lin, X. Ye, Sonication inhibited browning but decreased polyphenols contents and antioxidant activity of fresh apple (Malus pumila Mill, cv. Red Fuji) juice. J. Food Sci. Technol. 52, 8336–8342 (2015)
Z. Fang, Y. Zhang, Y. Lü, G. Ma, J. Chen, D. Liu, X. Ye, Phenolic compounds and antioxidant capacities of bayberry juices. Food Chem. 113(4), 884–888 (2009)
M. Deng, Y. Deng, L. Dong, Y. Ma, L. Liu, F. Huang et al., Effect of storage conditions on phenolic profiles and antioxidant activity of litchi pericarp. Molecules 23(9), 2276 (2018)
D. Huang, B. Ou, R.L. Prior, The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 53(6), 1841–1856 (2005)
S.M. Jafari, M. Ganje, D. Dehnad, V. Ghanbari, Mathematical, fuzzy logic and artificial neural network modeling techniques to predict drying kinetics of onion. J. Food Process. Preserv. 40(2), 329–339 (2016)
Funding
The first author was financially assisted through a student fellowship from the Indian Council of Agricultural Research, Senior Research Fellowship programme. The second author has received financial support for the work by the ICAR-All India Coordinated Research Project on Post Harvest Engineering and Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests to declare relevant to this article’s content.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aslam, R., Alam, M.S. Storage behaviour of ohmic heated and ultrasonicated amla juice: AI mediated correlation between ascorbic acid content and color attributes. Food Measure 18, 4972–4983 (2024). https://doi.org/10.1007/s11694-024-02549-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-024-02549-z