Abstract
Two hundred and twenty-three olive samples of different olive cultivars (Koroneiki, Asprolia, Lianolia, Ntopia, Thiaki, Mavrolia, and Others) grown in the Ionian islands (Kefalonia, Kerkyra, Leukada, and Zakynthos) were subjected to headspace solid phase microextraction coupled to gas chromatography/mass spectrometry analysis. The aim of the study was to characterize the aroma pattern of these olive oil cultivars, and track whether specific volatile compounds could be used for olive oil cultivar authentication using chemometrics. Multivariate analysis of variance implemented on the semi-quantitative data of volatile compounds (alcohols, aldehydes, benzene derivatives, esters, hydrocarbons, ketones, and terpenoids), showed that olive cultivar had a significant impact on the volatile composition of olive oil samples. Factor analysis and linear discriminant analysis indicated those specific volatile compounds that could be related to olive oil cultivar and established statistical models for the olive oil cultivar authentication from Ionian islands, thus indicating a characteristic aroma fingerprint of these olive oils.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Νowadays the increased demand for authentic products of special characteristics, nutritional properties, and potential health benefits by both the consumers and food authorities, has led to the development of consecutive research at an international level to achieve this purpose. Among the food products of nutritional interest with an important global production [1], olive oil comprises a basic food source in the Mediterranean food culture [2]. It is a liquid source of lipid molecules, obtained by pressing the olives (Olea europaea L.) and extracting the respective oil. Some typical bio-molecules include oleic acid with smaller amounts of linoleic and palmitic acids, phenols, tocopherols, sterols, phospholipids, waxes, squalene, and other hydrocarbons [3].
Olive oil has a complex composition that varies according to different factors such as olive cultivar, altitude, harvesting year, extraction processing techniques, etc. [3, 4]. The unique characteristics of each olive cultivar in relation to the climatic conditions, agronomic practices, geographical production area, harvesting practices, and processing technology, are closely related to the olive oil quality and composition [3, 4]. The unique quality characteristics of a genuine olive oil may allow its labeling with a special product status indicative if its origin (PDO-Protected Designation of Origin or PGI-Protected Geographical Indication) as indicated by the European Commission [5]. Therefore, authenticity of olive oil is an important topic for the food sector and different regulation bodies. The term authentication is a multi-side topic, since it concerns the characterization, geographical origin determination, cultivar differentiation, and adulteration control [6,7,8,9,10]. In this context, the determination of olive oil authenticity is achieved after implementation of different instrumental and conventional techniques to collect data regarding its quality indices, chemical markers, sensory and compositional characteristics during the production procedure, storage, and distribution [11,12,13]. Analyses were performed in combination with multivariate analysis, supervised and unsupervised chemometrics, such as multivariate analysis of variance (MANOVA), linear discriminant analysis (LDA), principal component analysis (PCA) or factor analysis (FA) [14,15,16,17].
Among the different chemical indices of olive oil, the determination of volatile compounds has a special impact, as it has been highly correlated with its organoleptic properties. Volatile compounds are responsible for both positive and negative olfactory characteristics [16,17,18], thus contributing to the further understanding of olive oil quality [19, 20].
It has been reported that during the production process of olive oil and its chemical oxidation, most of the C5 and C6 volatile compounds, which in turn are responsible for the typical fruity and green aroma notes of olive oil, are produced by the lipoxygenase (LOX) enzyme pathway [10, 18, 21]. The LOX pathway involves enzymes such as lipoxygenase and hydroperoxide lyase that oxidize and cleave polyunsaturated fatty acids to aldehydes, respectively. These in turn, are reduced to alcohols (by the action of alcohol dehydrogenase) and esterified to produce esters (by the action of alcohol acyltransferase).
Based on the above, the aim of the present study was to characterize the aroma profile of a large number of olive oil samples (two hundred and twenty-three) of 7 different olive cultivars grown in 4 Ionian islands, some studied for the first time. In addition, the potential correlation of specific volatile compounds with the cultivar authentication of these olive oils was investigated, in combination with supervised and unsupervised chemometrics. To the best of our knowledge, limited studies are available in the recent literature reporting volatile compounds analysis data of Greek olive oil samples derived from different olive cultivars grown in the Ionian islands [8, 15, 17, 22]. The potential to implement these data in cultivar authentication control constitutes a major novelty of the present study. The study also contributes to the understanding of the flavor complexity of these different olive oil cultivars and may comprise a solid basis for the potential use of the volatile compounds’ data in future studies (e.g. for the purity control -undisputed botanical origin- of olive fruits, especially for the less studied olive cultivars grown in the Ionian islands).
Materials and methods
Olive oil samples
Two hundred and twenty-three virgin olive oil (VOO) samples (N = 223) harvested in 2017–2018 (November 2017 to January 2018) of different olive cultivars grown in four Ionian islands in Greece [Kefalonia (38° 15′ 54″ N 20° 33′ 09″ E), Kerkyra (39° 35′ 28.60″ N 19° 51′ 50.54″ E), Leukada (38° 43′ N 20° 39′ E) and Zakynthos (37° 48′ N 20° 45′ E)] were used in the study (Supplementary Fig. 1). Olive oil samples belonged to Koroneiki (N = 47), Lianolia (N = 37), Asprolia (N = 36), Ntopia (N = 64), Thiaki (N = 13), Mavrolia (N = 8), and 18 additional samples which belonged to Myrtada (N = 2), Italian (N = 1), Ladolia (N = 1), Hontrolia (N = 1), Throumpa (N = 1), and Vassilikada (N = 1) from Kerkyra island; Korfolia (N = 5), Hontrolia (N = 1), Kerkyraiki (N = 1), and Plexoudenia (N = 1) from Kefalonia island; Plexydolia (N = 2) and Tragolia (N = 1) from Leukada island. These 18 samples were grouped as “Others’’ to evaluate further the chemometric models to be established for the authentication of olive oil samples according to cultivar. The samples of collected olives had the following characteristics: (i) the fruits had the same degree of maturity (the time of harvesting of the olive fruit was defined as the time when the fruit begun to change color), and (ii) collection of samples was done by covering as much as possible all the olive growing areas of the Ionian islands. Soon after receiving the raw material (c.a. 3 kg/sample), the following steps were followed: Selection of olives and leaves involved using only healthy olives without any imperfections, followed by crushing of olives and removal of olive core, grinding in a blender, addition of an equal amount of water, and mixing the olive oil for 45 min at a temperature below 27 °C, followed by centrifugation for 4 min at 3500 revolutions per minute (rpm), and then receiving the olive oil, archiving, and placing samples in dark vials under chilled temperature [17].
Chemicals and reagents
4-Methyl-2-pentanone [(CH3)2CHCH2COCH3, MW = 100.16] used as internal standard was purchased from Fluka (Germany). The standard mixture of alkanes C8-C20 (40 mg/L each in n-hexane) was purchased from Sigma-Aldrich (Germany).
Determination of volatile compounds
Preparation of olive oil samples for headspace-solid phase microextraction (HS-SPME)
Four grams (4 g) of olive oil were placed in 20 mL screw-cap vials equipped with polytetrafluoroethylene (PTFE)/silicone septa and afterwards 100 μL of the internal standard (4-methyl-2-pentanone of initial concentration of 500 μg/L) were added. The vials were vortexed and maintained in a water bath at 45 °C under stirring at 600 rpm during the extraction procedure with the fiber. The HS-SPME extraction procedure followed in the study was optimized according to the following conditions: 15 min equilibration time, 15 min sampling/exposure time of the fiber, weight of sample 4 g, vial volume 20 mL, and constant extraction temperature of the water bath at 45 °C [17]. The fiber used for the extraction of volatile compounds in the headspace of olive oil samples was a divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) fiber (50/30 μm) having 2 cm length (Supelco, Bellefonte, PA, USA). Before the analysis of samples the fiber was cleaned daily using the’’clean” program method [17].
Instrumentation and analytical conditions
A gas chromatograph (GC) unit (Agilent 7890A) coupled to a mass spectrometry (MS) detector (Agilent 5975) was used for the analysis of the volatile compounds of olive oil samples. A DB-5MS [cross-linked (5%-Phenyl)-methylpolysiloxane)] capillary column (J & W Scientific, Agilent Technologies, Santa Clara, CA, USA), with dimensions of 60 m × 320 μm i.d.,1 μm film thickness was used, with helium as the carrier gas (purity 99.999%), at a flow rate of 1.5 mL/min. The temperature for the injector and MS-transfer line were maintained constant at 260 °C and 270 °C, respectively. The oven temperature was held at 40 °C for 4 min and was further increased to 160 °C at a rate of 4 °C/min and maintained for 2 min, increasing to 250 °C at a rate of 8 °C/min and maintained for 2 min. The electron impact mass spectra were recorded in the mass range of 29–500 amu (atomic mass units). The ionization energy was 70 eV. A split ratio of 2:1 was used. To handle contamination problems, that could cause memory effects, blank runs were carried out before and after the analysis of consecutive olive oil samples [17].
Identification of volatile compounds and expression of results
The identification of volatile compounds of olive oil samples was done using the Wiley 7 NIST (National Institute of Standards and Technology) mass spectral library (NIST 2005) (J. Wiley & Sons Ltd., West Sussex, England), the determination of the linear retention time indices (LRIs), and reference compounds (for marker volatile compounds indicated by LDA and factor analysis) [for dodecane, (E)-3,7-dimethyl-1,3,6-octatriene heptanal, 1-propanol, and (Z)-3-hexen-1-ol]. The identified volatile compounds in replicated samples that had > 80% probability according to the NIST mass spectral library were considered for the statistical evaluation. For the determination of the linear retention index values (Kováts formula), the mixture of n-alkanes (C8–C20) was used following the IUPAC methodology as compiled by McNaught and Wilkinson [23]. Results were expressed as semi-quantitative data (μg/L) with reference to the internal standard 4-methyl-2-pentanone. The yield of the internal standard was > 95% [17].
Statistical analysis
The semi-quantitative data (μg/L) of volatile compounds were subjected to chemometrics to investigate the impact of olive cultivars on the volatile composition of olive oil samples. Comparison of the average values was done using MANOVA to determine which volatile compounds showed significant differences (p < 0.05) in their composition among olive oil samples of different cultivar (Koroneiki, Lianolia, Asprolia, Ntopia, Thiaki, Mavrolia, and Others). MANOVA creates a new dependent variable based on the linear combination of all the dependent variables in the model (i.e., volatile compounds), which maximizes as far as possible the differences in the average values between the level groups of the independent variable (i.e., olive oil cultivar). The Wilks' Lambda and Pillai’s Trace test statistics were implemented to study the main effects and interaction of the independent variables at the multidimensional level [24]. The effectiveness of the used sample size in the experiment was estimated by the observed power. Power is the probability of rejecting the hypothesis that the means are equal when they are in fact not equal. The power during MANOVA depends on the sample size, the magnitudes of the variances, the alpha level, and the actual differences among the population means in a given group of objects. In that sense, high power is much desirable. The high power means that there is a high probability of rejecting the null hypothesis when the null hypothesis is false. This is a critical measure of precision in hypothesis testing during the application of multivariate statistics [25].
FA, as a dimension reduction technique (unsupervised statistical technique), describes the variability (variance) that exists between an initial number of measured (obvious) and associated variables, and a smaller number of non-obvious variables, called factors. The purpose of factor analysis is to summarize the relationships between the initial and the factor variables in a comprehensive and accurate way by providing percentages of variance (% variance) associated with those factors. During FA, the Keiser-Meyer-Olkin index (KMO) assesses the sample adequacy (it should be > 0.50), while Bartlett’s Test of Sphericity (p-value should be < 0.05) assesses whether the correlations between the variables allow the actual implementation of factor analysis. The extraction method was PCA with Varimax rotation and Keiser Normalization [17, 26]. LDA is a supervised statistical technique that aims to find a linear combination of the statistically significant objects of interest (i.e., volatile compounds indicated during MANOVA) that separate two or more groups of objects (i.e., olive oil cultivars). The suitability of the prediction ability of the LDA models was evaluated by the cross-validation method during which each case is classified by the functions derived from all cases other than that particular case [24]. Regarding the LDA analysis, the cultivar of olive oil samples was considered as the factor variable (grouping variable), while the semi-quantitative data of the volatile compounds as the independent variables. Statistical analysis was done using the Statistical Package for the Social Sciences (SPSS) version 26.0 statistics software (SPSS, IBM Inc. [24]).
Results and discussion
Volatile compounds of olive oil of different olive cultivars
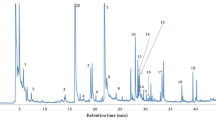
Table 1 lists the semi-quantitative data (μg/L) of the volatile compounds that were identified among olive oil samples of different olive cultivars from Ionian islands. In total, 24 volatile compounds were tentatively identified belonging to alcohols, aldehydes, benzene derivatives, esters, hydrocarbons, ketones, and terpenoids. A typical gas chromatogram of olive oil sample (no. 53) of the Korfolia olive cultivar from Kefalonia is shown in Fig. 1. It should be noted that the volatile compounds that were identified in the present study are in line with previous studies concerning the cultivar or geographical origin authentication of olive oil from different countries [8, 12, 15,16,17, 27].
A typical gas-chromatogram of olive oil (sample no.53) of the ‘‘Korfolia’’ olive cultivar from Kefalonia. 1: Ethanol; 2: Pentanal; 3: Toluene; 4: Hexanal; 5: (E)-2-Hexenal; 6: 2,2,4,6,6-Pentamethylheptane; 7: Decane; 8: (Z)-3-Hexen-1-ol, acetate; 9: Nonanal; 10: Dodecane; 11: 1,3-Bis(1,1-dimethylethyl)benzene; IS: Internal standard
Differences in the volatile composition of olive oil samples of different olive cultivars were observed (Table 1). In addition, substantial differences were observed in the sum of volatile compounds classes according to olive oil cultivars. The most dominant volatile compounds were aldehydes, followed by hydrocarbons, and alcohols. The respective order for the alcohols composition (μg/L) was Mavrolia > Koroneiki > Ntopia > Lianolia > Asprolia > Thiaki > Others. Regarding the aldehydes composition (μg/L), the respective order was Lianolia > Ntopia > Others > Mavrolia > Thiaki > Asprolia > Koroneiki. In the case of benzene derivatives, the respective composition (μg/L) followed the order: Others > Lianolia > Mavrolia > Thiaki > Ntopia > Koroneiki > Asprolia. Similarly, esters had the following order in their composition (μg/L): Thiaki > Others > Koroneiki > Asprolia. As far as the hydrocarbons are concerned, their composition (μg/L) had the following order: Koroneiki > Thiaki > Others > Ntopia > Lianolia > Mavrolia > Asprolia. For ketones, the respective order in their composition (μg/L) was: Ntopia > Koroneiki > Lianolia. Finally, terpenoids showed the following order in their composition (μg/L): Lianolia > Mavrolia.
The basic volatiles that contribute to the aroma of olive oil are hexanal, the aroma of which is associated with green apple and cut grass, trans-2-hexenal or (E)-2-hexenal, whose aroma is associated with that of bitter almonds, and other green, fruity, sharp, bitter and astringent aroma notes, and the 1-hexanol whose aroma is related to that of tomato and other fruity, soft, aromatic, alcoholic or even harsh aromas [4, 22].
Among aldehydes, pentanal recorded the highest amount (μg/L) in olive oil samples of the Koroneiki cultivar. Hexanal recorded the higher amount (μg/L) in olive oil samples of the Thiaki cultivar, whereas (E)-2-hexenal, the most abundant volatile compound among the analyzed olive oil samples, recorded the highest amount (μg/L) in olive oil samples of the Lianolia cultivar. Of the remaining aldehydes, nonanal recorded the highest amount (μg/L) in olive oil samples of the Koroneiki cultivar, while heptanal and octanal were identified only in olive oil samples of Ntopia, Thiaki, and Lianolia cultivars. It should be stressed that the role of aldehydes in olive oil cultivar differentiation (along with other C6 compounds), such as hexanal and (E)-2 hexenal, has been reported to serve as characteristic volatile compounds of different olive oil cultivars [19, 22, 28, 29], including Koroneiki among others, in different parts of the world [8, 19].
Concerning the alcohols, ethanol was identified in all olive oil, while the oil samples of the Mavrolia cultivar had the highest amounts (Table 1). Ethanol may provide a fermented-like, ripe fruit, and pungent aroma in olive oil, while in combination with other alcohols such as 2-methyl propanol, cis-2-penten-1-ol, cis-3-hexenol and octanol may impart a sweet and fruity odor, resulting in positive effects to the aroma and quality of olive oil [3]. In previous studies dealing with the determination of volatile compounds of olive oil from Morocco (Picholine marocaine cultivar) [9], Tunisia (Jemri, Toffehi, and Fakhari cultivars) [28], Brazil (Arbequina, Arbosana, Picual, Koroneiki, Grapollo, Coratina and Frantoio cultivars) [27], Greece (Koroneiki cultivar) [22], and Italy (Leccino cultivar) [29], ethanol was not reported to contribute to the aroma of olive oil. However, the only report on the contribution of ethanol in the volatile profile of olive oil was for the Italian olive cultivar “Alperujo” [16], Therefore, we postulate that ethanol may be linked as a characteristic volatile compound of olive oil associated with its cultivar, grown in a specific region [17].
1-Hexanol was identified only in olive oil samples of the Ntopia cultivar. In a previous study 1-hexanol was associated with the olive oil cultivar [4]. Concerning the other alcohols, (Z)-3-hexen-1-ol, and (E)-2-hexen-1-ol recorded the highest amounts (μg/L) in olive oil samples of Koroneiki and Lianolia cultivars (Table 1). These compounds have been associated with a “green” and “grassy” odor as well as an astringent-bitter taste of olive oil [3]. Finally, 1-propanol was identified only in olive oil samples of Asprolia and Thiaki cultivars, in much lower concentration (Table 1). 1-Propanol was previously reported to contribute to the aroma of olive oil of the Leccino olive cultivar from Italy [29].
Hydrocarbons may also be derived from the LOX pathway [10]. The most abundant hydrocarbons were 2,2,4,6,6-pentamethylheptane and decane. Decane recorded the highest amount (μg/L) in olive oil samples of Lianolia cultivar, whereas 2,2,4,6,6-pentamethylheptane presented the highest amount (μg/L) in olive oil samples of Lianolia and Koroneiki cultivars. The contribution of dodecane in the aroma of all olive oil samples, is also worth mentioning. The highest amount (μg/L) of dodecane was recorded in olive oil samples of Ntopia and Koroneiki cultivars (Table 1). Another critical point to discuss is that these hydrocarbons were not reported to contribute to the aroma of olive oil of the Koroneiki, Thiaki, Asprolia, and Lianolia olive cultivars from Ionian islands [8, 22]. Depending on the carbon chain, hydrocarbons may give an aromatic, sweet, apple-like, and oily odor in olive oil [3].
Among ketones, only 6-methyl-5-hepten-2-one was identified in olive oil samples of Ntopia, Koroneiki, and Lianolia cultivars. The highest amount (μg/L) of this compound was determined in olive oil samples of the Ntopia cultivar. This compound contributes to the pungent, green, and fruity odor of olive oil [30]. The contribution of this ketone in olive oil aroma is in agreement with the results reported by Pouliarekou et al. [8] and Theodosi et al. [22].
Acetic acid hexyl ester and (Z)-3-hexen-1-ol, acetate were the only esterified compounds that were identified in olive oil samples. Acetic acid hexyl ester was identified only in olive oil samples of Asprolia cultivar, whereas (Z)-3-hexen-1-ol, acetate recorded the highest amount (μg/L) in olive oil samples of Thiaki cultivar. Acetic acid hexyl ester was previously reported to contribute to the aroma of olive oil from olive cultivars grown in the Ionian islands [8, 22]. These compounds also derive from the LOX pathway and are responsible for the fruity, sweet and pleasant aromatic notes of olive oil [3, 15].
Regarding the benzene derivatives, toluene was identified in highest amounts (μg/L) in olive oil samples of other cultivars, followed by Koroneiki and Thiaki cultivars. On the contrary, 1,3-bis(1,1-dimethylethyl)-benzene was identified in all olive oil samples, recording the highest amount (μg/L) in olive oil samples of Mavrolia cultivar. 1,3-Bis(1,1-dimethylethyl)benzene was not previously reported to contribute to the aroma of olive oil of Koroneiki, Thiaki, Ntopia, Asprolia, and Lianolia olive cultivars from Ionian islands [8, 22], nor Picholine marocaine olive cultivar from Morocco [9], and Alperujo olive cultivar from Florence (Italy) [16]. Toluene and 1,3-bis(1,1-dimethylethyl)benzene may lead to a bitter and spicy olive oil flavor [3].
Finally, terpenoids such as dl-limonene and trans-β-ocimene may vary in their respective concentration according to olive cultivar and geographical origin [17, 22, 30]. In the present study, dl-limonene was identified only in Koroneiki and Mavrolia cultivars, whereas trans-β-ocimene was identified only in olive oil samples of the Lianolia cultivar.
Supplementary to the above, it should also be considered the effect of territory (climate and soil), including agronomic and technological aspects, on the volatile composition of the studied olive oil cultivars [19]. Studying the relevant literature, it is well documented that geographical and botanical origin of olive oil has, in most cases, a strong positive correlation considering the aforementioned [8, 17, 19, 20, 29].
Cultivar authentication of olive oil from Ionian islands
Multivariate analysis of variance
The qualitative criteria of the multivariate hypothesis Pillai’s Trace = 3.079 (F = 12.022, df = 120, p = 0.000), and Wilks' Lambda = 0.005 (F = 13.843, df = 120, p = 0.000) both having an observed power of 1.000, showed that there was a significant impact of olive cultivar on the volatile composition of olive oil samples. The multivariate effect of the olive cultivar on the volatile compounds of olive oil samples is showed by the F-value tests. Among the volatile compounds identified in olive oil samples, twenty-three showed significant differences (p < 0.05) in their composition according to the olive cultivar (Table 1).
Factor analysis
Factor analysis showed that the 23 statistically significant volatile compounds adequately describe the variability in the multidimensional space. The Keiser-Meyer-Olkin (KMO) index was 0.652 while Bartlett’s Test of Sphericity index had the values X2 = 1427.604, df = 253, p = 0.000, indicating the existence of correlations between the variables (volatile compounds) allowing the application of factor analysis. The main volatile compounds that showed the highest correlation (factors) are provided in bold numbers in Table 2.
Based on the first 9 principal components (PCs), the variance explained was 69.835%, a value considered as satisfactory (Fig. 2). The volatile compounds for which the correlation value in the rotated component matrix of the multidimensional space was the largest were: dodecane (PC1, 12.179% of the total variance), (E)-3,7 dimethyl-1,3,6-octatriene (PC2, 10.952% of the total variance), heptanal (PC3, 10.284% of the total variance), pentanal (PC4, 8.469% of the total variance), 1-propanol (PC5, 6.520% of the total variance), ethanol (PC6, 5.553% of the total variance), acetic acid hexyl ester (PC7, 5.552% of the total variance), 3-methylbutanal (PC8, 5.267% of the total variance), and toluene (PC9, 5.058% of the total variance).
Linear discriminant analysis
The results of LDA showed that five discriminant functions were formed: Wilks' Lambda = 0.005 (X2 = 992.346, df = 115, p = 0.000) for the first; Wilks' Lambda = 0.031 (X2 = 658.659, df = 88, p = 0.000) for the second; Wilks' Lambda = 0.119 (X2 = 403.122, df = 63, p = 0.000) for the third; Wilks' Lambda = 0.292 (X2 = 233.125, df = 40, p = 0.000) for the fourth; and Wilks' Lambda = 0.603 (X2 = 95.840, df = 19, p = 0.000) for the fifth. The first discriminant function accounted for 44.4% of the total variance and had the highest eigenvalue (4.818) and canonical correlation (0.910). The second discriminant function had a significantly lower eigenvalue (2.852) and canonical correlation (0.860), while accounted for 26.3% of the total variance. The third discriminant function had a lower eigenvalue (1.452) and canonical correlation (0.770) accounting for 13.4% of the total variance. Similarly, the fourth discriminant function had a lower eigenvalue (1.064) and canonical correlation (0.718) accounting for 9.8% of the total variance. Finally, the fifth discriminant function had the lowest eigenvalue (0.658) and canonical correlation (0.630) accounting for 6.1% of the total variance All discriminant functions accounted for 100% of the total variance.
During LDA, the eigenvalue of the discriminant function is an essential parameter, since it provides information on how well the function differentiates the initial groups (olive oil cultivar). In parallel, the group centroid values comprise another essential parameter in LDA. The group centroid values are considered for the estimation of the classification ability of the LDA model and refer to the unstandardized canonical discriminant functions, evaluated at group means. The centroid values have two numbers which represent the coordinates (the abscissa is the first discriminant function, and the ordinate is the second discriminant function) [17].
The group centroid values were: (− 2.056, 0.481), (4.247, 1.149), (− 0.658, − 0.845), (− 0.079, − 1.409), (− 2.429, 5.112) and (− 0.025, − 1.376) for olive oil samples of Koroneiki, Lianolia, Asprolia, Ntopia, Thiaki, and Mavrolia olive cultivars, respectively (Fig. 3).
The best results (based on the cross-validation method) were obtained for the olive oil samples of the Lianolia cultivar, where of the 37 initial samples, 34 were correctly allocated in Lianolia cultivar (correct prediction rate of 91.9%), while 3 samples were allocated in Ntopia cultivar. Similarly, for the Koroneiki cultivar of the 47 initial samples, 42 were correctly allocated in Koroneiki cultivar (correct prediction rate of 89.4%), while 2 samples were allocated in Asprolia and 3 samples in Ntopia cultivars. Furthermore, for the olive oil samples of the Ntopia cultivar of the 64 initial samples, 57 were correctly allocated in Ntopia cultivar (correct prediction rate of 89.1%), while 3 samples were allocated in Koroneiki, 1 sample in Lianolia, 2 samples in Asprolia, and 1 sample in Mavrolia cultivars. For the olive oil samples of the Asprolia cultivar of the 36 initial samples, 31 were correctly allocated in Asprolia cultivar (correct prediction rate of 86.1%), and 5 samples were allocated in Ntopia cultivar. Regarding the olive oil samples of the Thiaki cultivar, of the 13 initial samples, 9 were correctly allocated in Thiaki cultivar (correct prediction rate of 69.2%), and 4 samples were allocated in Koroneiki cultivar. Finally, for the olive oil samples of the Mavrolia cultivar the results were not satisfactory, since of the 8 initial samples 4 were correctly allocated in Mavrolia (correct prediction rate of 50%), 3 samples in Ntopia and 1 sample in Asprolia cultivars (Table 3).
The overall correct classification rate was 89.3% for the original and 86.3% for the cross-validation method (Table 3). Between these values, the latter is considered very satisfactory given that samples of olive oil of 6 different olive cultivars were studied together in the linear discrimination model.
The volatile compounds that mostly contributed to the discrimination of olive oil samples from Ionian islands according to olive cultivar, were those pooled with the highest absolute correlation value (in bold) within the discriminant functions (Table 4). These were (E)-3,7 dimethyl-1,3,6-octatriene for discriminant function 1, 1-propanol for discriminant function 2, (Z)-3-hexen-1-ol for discriminant function 3, acetic acid hexyl ester for discriminant function 4, and 3-methyl butanal for discriminant function 5. By setting a lower demand of the correlation value to be ≥ 0.45, these volatile compounds were: trans-β-ocimene for discriminant function 1, 1-propanol and (Z)-3-hexen-1-ol acetate for discriminant function 2, (Z)-3-hexen-1-ol for discriminant function 3, acetic acid hexyl ester for discriminant function 4, and 3-methylbutanal for discriminant function 5. Therefore, these volatile compounds are most strongly related to the cultivars of olive oil from Ionian islands.
Complementary evaluation of the results of multivariate statistics using additional olive oil samples of less known olive cultivars
To further evaluate the results of multivariate statistics, the semi-quantitative data of 18 additional olive oil samples belonging to other olive cultivars grown in the four Ionian islands were subjected to analysis simultaneously with the former 205 olive oil samples. Full data for the statistical analysis carried out in this section are given in supplementary material (Supplementary Tables 1–8) to avoid repetition and an extended study. Briefly, multivariate statistical analysis showed again that olive cultivar had a significant impact on the volatile composition of olive oil samples of different cultivar (Supplementary Table 1). Even though the number of olive oil samples was increased (223), the significant volatile compounds remained constant and explained 68.64% of the total variance during factor analysis (Supplementary Table 4) (Fig. 4). The discrimination results after implementation of linear discriminant analysis were 84.8% for the original and 79.8% for the cross-validation method, respectively (Supplementary Table 7) (Fig. 5). As it can be observed, these classification results were slightly lower compared to the previous results, given the lower prediction rates that were obtained for olive oil samples of the Koroneiki (78.7%), Lianolia (86.5%), and Asprolia (80.6%) cultivars. The classification rate of olive oil samples of other cultivars was rather poor (55.6%) as 8 samples were allocated partially to the group of Koroneiki, Lianolia, Ntopia, and Thiaki cultivars (Supplementary Table 7). However, the overall classification results may be considered satisfactory given that olive oil samples of rare olive cultivars (for which no data is available for their volatile profile or authenticity) were studied together with common olive cultivars grown in the Ionian islands. It is also worth mentioning that the correlation values of volatile compounds identified in olive oil samples of Koroneiki, Lianolia, Asprolia, Ntopia, Thiaki, Mavrolia, and other olive cultivars from Ionian islands between groups (cultivar) within each discriminant function was also differentiated (Supplementary Table 8) when the number of olive oil samples increased, thus providing, information on the impact of olive oil samples of the ‘‘Others’’ olive cultivars, in the discrimination model.
Differentiation of olive oil of Koroneiki, Lianolia, Asprolia, Ntopia, Thiaki, Mavrolia, and other olive cultivars from Ionian islands based on 23 volatile compounds in combination with LDA (two-dimensional display-2D). 1: Koroneiki. 2: Lianolia. 3: Asprolia. 4: Ntopia. 5: Thiaki. 6: Mavrolia. 7: Others
The classification results of multivariate statistics for olive oil samples of 6 or 7 olive cultivars obtained in the present study support and further strengthen those of similar studies in the literature concerning the cultivar authentication of olive oil using volatile compounds analysis and chemometrics [4, 7, 15, 31].
Conclusions
Olive oil of different olive cultivars grown in the Ionian islands had a characteristic aroma owed to different proportions of alcohols, aldehydes, benzene derivatives, esters, hydrocarbons, ketones, and terpenoids. Among the studied olive cultivars, some of them studied for the first time, olive oil of the Lianolia olive cultivar had the richer aroma. The implementation of chemometric analysis on the semi-quantitative data of volatile compounds resulted in a satisfactory differentiation of olive oil samples according to olive cultivar. To the best of our knowledge, this is the first report in the literature that presents semi-quantitative volatile compounds’ data for 223 olive oil samples from different olive cultivars grown in the Ionian islands, establishing at the same time chemometric models for the olive oil cultivar authentication. The understanding and the characterization of the volatile fingerprint of olive oils is still a challenge, in terms of promoting research data in areas of regional specialization, and by creating new competitive zones at an international level for the commercialization of authentic olive oils or unique blends of olive oils of different olive cultivars. However, the use of reference compounds to collect quantitative data related to the whole volatile fingerprint of olive oil, in a future work, will further validate the results of the present study.
Data Availability
The manuscript includes all the relevant data.
References
United States Department of Agriculture (USDA) (2020) National Agricultural Statistics Service Pacific Regional Office, P.O. Box 1258 Sacramento, CA 95812.
D. Rongai, N. Sabatini, L. Del Coco, E. Perri, P. Del Re, N. Simone, D. Marchegiani, F.P. Fanizzi, 1H NMR and multivariate analysis for geographic characterization of commercial extra virgin olive oil: a possible correlation with climate data. Foods 6(11), 96 (2017). https://doi.org/10.3390/foods6110096
N. Caporaso, Virgin olive oils: environmental conditions, agronomical factors and processing technology affecting the chemistry of flavor profile. J. Food Chem. Nanotechnol. 2(1), 21–31 (2016). https://doi.org/10.17756/jfcn.2016-007
C.M. Kalua, R.J. Mailer, J. Ayton, M.S. Allen, D.R. Bedgood, A.G. Bishop, P.D. Prenzler, Discrimination of olive oils and fruits into cultivars and maturity stages based on phenolic and volatile compounds. J. Agric. Food Chem. 54(21), 8390 (2006). https://doi.org/10.1021/jf068011k
European Commission 182/2009 of 6 March 2009 amending Regulation (EC) no. 1019/2002 on marketing standards for olive oil. Off. J. Eur. Union. 2009, L63, 6–8.
P. Zunin, R. Boggia, P. Salvadeo, F. Evangelisti, Geographical traceability of West Liguria extra virgin olive oils by the analysis of volatile terpenoid hydrocarbons. J. Chrom. A 1089(1–2), 243–249 (2005). https://doi.org/10.1016/J.CHROMA.2005.07.005
C. Pizarro, S. Rodríguez-Tecedor, N. Pérez-del-Notario, J.M. González-Sáiz, Recognition of volatile compounds as markers in geographical discrimination of Spanish extra virgin olive oils by chemometric analysis of non-specific chromatography volatile profiles. J. Chrom. A 1218(3), 518–523 (2011). https://doi.org/10.1016/J.CHROMA.2010.11.045
E. Pouliarekou, A. Badeka, M. Tasioula-Margari, S. Kontakos, F. Longobardi, M.G. Kontominas, Characterization and classification of Western Greek olive oils according to cultivar and geographical origin based on volatile compounds. J. Chrom. A 1218(42), 7534–7542 (2011). https://doi.org/10.1016/J.CHROMA.2011.07.081
A. Βajoub, A. Sánchez-Ortiz, E.A. Ajal, N. Ouazzani, A. Fernández-Gutiérrez, G. Beltrán, A. Carrasco-Pancorbo, First comprehensive characterization of volatile profile of north Moroccan olive oils: a geographic discriminant approach. Food Res. Int. 76, 410–417 (2015). https://doi.org/10.1016/J.FOODRES.2015.05.043
X. Zhou, Q. Zhang, X. Chen, X. Li, C. Han, In-situ assessment of olive oil adulteration with soybean oil based on thermogravimetric-gas chromatography/mass spectrometry combined with chemometrics. Food Cont. 130, 108251 (2021). https://doi.org/10.1016/j.foodcont.2021.108251
S. Vichi, L. Pizzale, L.S. Conte, S. Buxaderas, E. López-Tamames, Solid-phase microextraction in the analysis of virgin olive oil volatile fraction: Modifications induced by oxidation and suitable markers of oxidative status. J. Agric. Food Chem. 51(22), 6564–6571 (2003). https://doi.org/10.1021/jf030268k
B. Berlioz, C. Cordella, J.-F. Cavalli, L. Lizzani-Cuvelier, A.-M. Loiseau, X. Fernandez, Comparison of the amounts of volatile compounds in French protected designation of origin virgin olive oils. J. Agric. Food Chem. 54(26), 10092–10101 (2006). https://doi.org/10.1021/JF061796+
L. Conte, A. Bendini, E. Valli, P. Lucci, S. Moret, A. Maquet, F. Lacoste, P. Brereton, D.L. García-González, W. Moreda, T.G. Toschi, Olive oil quality and authenticity: a review of current EU legislation, standards, relevant methods of analyses, their drawbacks and recommendations for the future. Trends Food Sci. Technol. 105, 483–493 (2020). https://doi.org/10.1016/j.tifs.2019.02.025
F. Longobardi, A. Ventrella, C. Napoli, E. Humpfer, B. Schütz, H. Schäfer, M.G. Kontominas, A. Sacco, Classification of olive oils according to geographical origin by using 1H NMR fingerprinting combined with multivariate analysis. Food Chem. 130, 177–183 (2012). https://doi.org/10.1016/j.foodchem.2011.06.045
I. Kosma, A. Badeka, K. Vatavali, S. Kontakos, M. Kontominas, Differentiation of Greek extra virgin olive oils according to cultivar based on volatile compound analysis and fatty acid composition. Eur. J. Lipid Sci. Technol. 118, 849–861 (2016). https://doi.org/10.1002/ejlt.201500293
L. Cecchi, M. Migliorini, E. Giambanelli, A. Cane, N. Mulinacci, B. Zanoni, Volatile profile of two-phase olive pomace (Alperujo) by HS-SPME GC-MS as a key to defining volatile markers of sensory defects caused by biological phenomena in virgin olive oil. J. Agric. Food Chem. 69, 5155–5166 (2021). https://doi.org/10.1021/acs.jafc.1c01157
E. Eriotou, I.K. Karabagias, S. Maina, D. Koulougliotis, N. Kopsahelis, Geographical origin discrimination of “Ntopia” olive oil cultivar from Ionian islands using volatile compounds analysis and computational statistics. Eur. Food Res. Technol. 247, 3083–3098 (2021). https://doi.org/10.1007/s00217-021-03863-2
S. Esposto, A. Taticchi, M. Servili, S. Urbani, B. Sordini, G. Veneziani, L. Daidone, R. Selvaggini, Overall quality evolution of extra virgin olive oil exposed to light for 10 months in different containers. Food Chem. 351, 129297 (2021). https://doi.org/10.1016/j.foodchem.2021.129297
F. Angerosa, M. Servili, R. Selvaggini, A. Taticchi, S. Esposto, G. Montedoro, Volatile compounds in virgin olive oil: occurrence and their relationship with the quality. J. Chrom. A 1054(1–2), 17–31 (2004). https://doi.org/10.1016/J.CHROMA.2004.07.093
C.M. Kalua, M.S. Allen, D.R. Bedgood, A.G. Bishop, P.D. Prenzler, K. Robards, Olive oil volatile compounds, flavour development and quality: a critical review. Food Chem. 100(1), 273–286 (2007). https://doi.org/10.1016/j.foodchem.2005.09.059
F. Angerosa, Influence of volatile compounds on virgin olive oil quality evaluated by analytical approaches and sensor panels. Eur. J. Lipid Sci. Technol. 104(9–10), 639–660 (2002). https://doi.org/10.1002/1438-9312(200210)104:9/10%3c639::AID-EJLT639%3e3.0.CO;2-U
S. Theodosi, I.S. Kosma, A.V. Badeka, Quality characteristics of Koroneiki olive oil from Zakynthos island (Greece) and differentiation depending on the altitude level. Eur. Food Res. Technol. 247, 1235–1248 (2021). https://doi.org/10.1007/s00217-021-03705-1
IUPAC. Compendium of Chemical Terminology, 2nd ed. (the "Gold Book"). Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford (1997). Online version (2019-) created by S. J. Chalk. ISBN 0–9678550–9–8. https://doi.org/10.1351/goldbook.
A. Field, Discovering statistics using SPSS, 3rd edn. (Sage Publications Ltd, London, 2009)
C.J. Huberty, S. Olejnik, Applied MANOVA and discriminant analysis, 2nd edn. (John Wiley, New Jersey, 2006)
I.T. Jolliffe, Principal component analysis (Springer-Verlag, New York, 2002)
J.R.O. da Costa, S.M. Dal Bosco, R.C.D.S. Ramos, I.C.K. Machado, J. Garavaglia, S.S. Villasclaras, Determination of volatile compounds responsible for sensory characteristics from Brazilian extra virgin olive oil using HS-SPME/GC-MS direct method. J. Food Sci. 85(11), 3764–3775 (2020). https://doi.org/10.1111/1750-3841.15467
S. Benbrahim, A. Amanpour, F. Chtourou, H. Kelebek, S. Selli, M. Bouaziz, Gas chromatography-mass spectrometry-olfactometry to control the aroma fingerprint of extra virgin olive oil from three Tunisian cultivars at three harvest times. J. Agric. Food Chem. 66(11), 2851–2861 (2018). https://doi.org/10.1021/acs.jafc.7b06090
A.-M. Dourou, S. Brizzolara, F. Famiani, P. Tonuttia, Changes in volatile organic composition of olive oil extracted from cv. ‘Leccino’ fruit subjected to ethylene treatments at different ripening stages. J. Sci. Food Agric. 101, 3981–3986 (2021). https://doi.org/10.1002/jsfa.11024
S. Kesen, H. Kelebek, S. Selli, Characterization of the volatile, phenolic and antioxidant properties of monovarietal olive oil obtained from cv. Halhali. J. Amer. Oil Chem’. Soc. 90, 1685–1696 (2013). https://doi.org/10.1007/s11746-013-2327-8
B. Quintanilla-Casas, J. Bustamante, F. Guardiola, D.L. García-González, S. Barbieri, A. Bendini, T.G. Toschi, S. Vichi, A. Tres, Virgin olive oil volatile fingerprint and chemometrics: towards and instrumental screening tool to grade the sensory quality. LWT-Food Sci. Technol. 121, 108936 (2020). https://doi.org/10.1016/j.lwt.2019.108936
Acknowledgements
The authors are grateful to Assoc. Prof. Anastasia Badeka for the access she provided to the GC/MS unit of the Laboratory of Food Chemistry at the Department of Chemistry of the University of Ioannina and to Mr. Vassilios Karabagias for the complementary support in the data processing.
Funding
Open access funding provided by HEAL-Link Greece. We acknowledge support of this work by the project “Probing the Bioactive and Health Protective Compounds of Ionian islands’ Olive Oil” (MIS 5005497) which is implemented under the Action “Targeted Actions to Promote Research and Technology in Areas of Regional Specialization and New Competitive Areas in International Level”, funded by the Operational Programme “Ionian islands 2014–2020” and co-financed by Greece and the European Union (European Regional Development Fund).
Author information
Authors and Affiliations
Contributions
Nikolaos Kopsahelis: Conceptualization, Formal analysis and investigation, Methodology, Resources, Supervision, Writing—review and editing; Ioannis K. Karabagias: Formal analysis and investigation, Methodology, Writing—original draft preparation, Writing—review and editing; Harris Papapostolou: Formal analysis and investigation, Methodology, Writing—original draft preparation; Effimia Eriotou: Conceptualization, Funding acquisition, Methodology, Resources, Supervision, Writing—original draft preparation.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human and animal rights
Not applicable.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kopsahelis, N., Karabagias, I.K., Papapostolou, H. et al. Cultivar authentication of olive oil from Ionian islands using volatile compounds and chemometric analyses. Food Measure 18, 4402–4416 (2024). https://doi.org/10.1007/s11694-024-02502-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-024-02502-0