Abstract
This research work was performed on olive orchards (Olea europaea L., cv. Chemlali) of different origin areas of olive trees exposed to atmospheric contamination and those growing in a relatively clean area, during two successive years (2015/2016 and 2016/2017). Volatile profile of olive oil samples was carried out by headspace solid-phase micro-extraction coupled with gas chromatography/mass spectrometry (HS-SPME-GC/MS). A total of 93 volatile compounds, belonging to several classes of compounds, such as hydrocarbons, aldehydes, ketones, alcohols, terpenoids, and esters, were identified. Indeed, the analysis of phenols was performed by high-performance liquid chromatography with absorbance and fluorometric detection. Discriminant analysis confirmed that the pattern of volatile compounds includes enough information to predict sample variety amongst discrete monovarietal olive oil. The results indicate that climatic factors strongly effect volatile formation and phenolic composition and are supposed to be suitable markers of the geographic origin.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Volatile compounds responsible for green attributes of virgin olive oils have been proved to be produced through the enzymatic oxidation of linolenic and linoleic acids (Cherfaoui et al. 2018). In addition to their health benefits, extra virgin olive oil (EVOO) is highly appreciated by consumers due to their intense color and aromatic characteristics (Ben Brahim et al. 2018). The chemistry and flavor of a specific EVOO, which define its quality and uniqueness, besides being strongly dependent on the nature of the cultivar used for its production, are also affected by several other factors like climatic and edaphic conditions, agricultural practices, extraction methods, processing techniques, and storage conditions (Peres et al. 2017). The genetic effect related to the cultivar is one of the most important aspects of volatile composition of olive oil (Ben Brahim et al. 2018).
However, climatic and agronomic conditions of olive growing may affect the volatile composition of olive oils obtained by the same cultivar. Within this framework, the relationships between the water availability over fruit ripening and the volatile composition has been studied (Peres et al. 2017). Results revealed that, in the climatic conditions of central Italy, the rainfall impact is pre-eminent with respect to temperature and that some compounds, such as hexanal and isobutyl acetate were negatively correlated to rainfall.
In conclusion, we can consider that several agronomic and climatic parameters can influence the volatile composition of olive oils. For this reason, volatile compounds could be thought out as markers, with sensory impact, that can be used to differentiate them. On the other hand, the oxidative degradation indices assess these primary and secondary products. They are in use as surrogate measures for oil quality and oxidative stability (Frankel 2010; Cherfaoui et al. 2018), especially creating unpleasant flavor and reducing the shelf-life of edible oils. Some of volatile aldehyde compounds, particularly E,E-2,4-decadienal, E,E-2,4-heptadienal, are correlated with common lipid degradation parameters, thus regarded as deterioration “markers” (Molina-Garcia et al. 2017).
Others, as acrolein, are considered to have high relevance, by dint of their capacity to induce toxicological effects (Domínguez et al. 2014). Moreover, unsaturated aldehydes, such as alkenals and alkandienals, show more severe toxicity than alkanals (Domínguez et al. 2014). Thus, understanding the formation mechanisms of these volatiles will help in perceiving the involved reactions occurring due to pollution, showing a useful tool for the evaluation of the existing aroma compounds, the quantitative determination of contaminants, and oil degradation study (Sghaier et al. 2016). The volatile profile is in part responsible for oil flavor, and the sensory attributes have an impact on the appreciation of the oil by consumers (Sghaier et al. 2016). Phenolic and volatile compounds are also responsible for the virgin olive oil (VOO) flavor and, hence, the resulting sensory assessment (Drira et al. 2020). Besides the volatile compounds considered in plant organs of the intact olive fruit as secondary products, other volatile compounds are formed very quickly during disruption of the cell structure due to enzymatic reactions in the presence of oxygen. They are the main compounds responsible for the “green” and fruity desirable aroma attributes of VOO (Zhu et al. 2016). The main precursors for volatile formation are fatty acids (linoleic and a-linolenic), mainly by means of the lipoxygenase cascade (LOX), and amino acids (Aparicio and Harwood 2013). On the contrary, the low-quality VOO have complex profiles composed of a large number of volatiles responsible for off flavors like rancid, mustiness, fusty, and muddy sediment. The volatile compounds responsible for the VOO aroma contain components with diverse chemical natures and molecular masses, and they are present at very low concentrations. But the influence of each volatile on the sensory quality depends on its concentration and odor threshold.
Successive studies, obtained using HS-SPME–GS/MS, confirmed the relationships between the volatile composition of virgin olive oil and their origin area. In this study, the volatile and phenolic composition of oils from olive plants exposed to atmospheric chemical pollution in an area near a fertilizer factory has been investigated. The results were compared with those of olive trees growing in a clean area, not directly exposed to chemical pollution.
Materials and Methods
Chemicals and Reagents
All chemicals were of analytical grade hydroxytyrosol, tyrosol, oleuropein, gallic acid, catechin, vanillic acid, p-coumaric acid, 3.4-dihydroxy phenylacetic acid, chlorogenic acid, and epicatechin and were obtained from Sigma (St. Louis MO, USA). The polyphenolic compound 2,5-dihydroxybenzaldehyde was used as internal standard at concentration 34.4 mg/l. HPLC-grade methanol, acetic acid, diethyl ether, and ethanol were obtained from Merck (Darmstadt, Germany). Hydrochloric acid was obtained from Merck. Ultrapure water from Milli-Q system (Millipore, Bedford, MA, USA) with resistivity of 18 MΏ cm was used in all cases. All solution was filtered through 0.45-mm membranes (Millipore) and degassed prior to use.
Samples
The analyses were run on six virgin olive oils from two different geographical areas. Olive oils were obtained from unwashed and washed olive fruits (Olea europaea L.cv. Chemlali) manually picked in olive groves located in a land plot at 0.7 km from the Industrial Society of Phosphoric Acid and Fertilizers (Société Industrielle d'Acide Phosphorique et d'Engrais, SIAPE) close to Sfax city, and they are referred as “polluted oils” (PO). On the other hand, the control plot (with control oils, CO) was located in Menzel Chaker region, 45 km north of the “SIAPE,” in an inland rural area, without any industries. Sampling was performed during two successive crops (2015/2016 and 2016/2017).
For each sample, about 2 kg of handpicked fresh olives were used for olive oil production. Each sample from polluted oils was divided into two portions. One of them was washed and rinsed with deionized water and is referred as “washed polluted olive oils” (WPOO), and the other one is referred as “unwashed polluted olive oils” (UWPOO). Cold extractions were realized in laboratory by oleodoseur system (composed of crusher, vertical malaxator, and centrifuge). Then, the produced oils were filled in dark bottles and conserved for analysis at 4 °C.
Preparation of the Samples: Headspace-SPME Extraction (HS-SPME)
The HS-SPME extractions were performed using a carboxen/divinylbenzene/polydimethylsiloxane fiber (CAR/DVB/PDMS, 1 cm, 50-/30-μm film thickness (df)) supplied from Supelco, Bellefonte, PA, USA. Prior to use, the fiber was conditioned following the manufacturer’s recommendations. Fiber blanks were run periodically to ensure the absence of contaminants and/or carryover. The samples, with 4.0-mL volume each, were introduced in a 22.0-mL vial, sealed with a Teflon Lined rubber septum/magnetic screw cap. The vial was equilibrated for 10 min at 50 °C and then extracted for 50 min at the same temperature. The thermal desorption of the analytes was carried out by exposing the fiber to the GC injection port at 260 °C for 3 min in a splitless mode.
GC/MS Conditions
The analyses were performed on a GC/MS system consisting of a Bruker GC 456 with a Bruker mass-selective detector Scion TQ. An automatic sampler injector was used: CTC Analysis auto-sampler CombiPAL. Data were acquired with MSWS 8.2 Bruker and analyzed with Bruker MS Data Review 8.0. The chromatographic separation was achieved on a ZB-WAX PLUS capillary column (60 m × 0.32 mm i.d., 1.0 μm df) supplied by Phenomenex, Torrance, CA, USA. The oven temperature program began at 40 °C hold for 5 min, raised at 4 °C min−1 up to 240 °C, and hold for 5 min. Helium was used as carrier gas constant pressure of 35.0 psi at the electronic flow control (EFC 21) and 23.0 psi at the EFC 24. The MS transfer line and source temperatures were set at 240 °C and 220 °C, respectively. The spectra were matched by NIST MS Search Program, version 2.0 g. To determine characteristic mass fragments, electron ionization (EI) at 70 eV mass spectra of the analytes was recorded at full scan, from 40 to 450 Da. Linear retention indices (LRIs) were calculated using a commercial hydrocarbon mixture (C8–C20) and compared with the LRIs described in the literature.
High-Performance Liquid Chromatography with Absorbance and Fluorimetric Detection
Extraction of the Phenolic Fraction
Sample extraction was made according to Gargouri et al. (Gargouri et al. 2013). Four grams of the oil sample was added to 2 mL of n-hexane and 4 mL of a methanol/water (60:40, v/v) solution in a 20-mL centrifuge tube. After vigorous mixing, they were centrifuged for 3 min at 1490 g. The hydroalcoholic phase was collected, and the organic phase was re-extracted twice with 4 mL of methanol/water (60:40, v/v) solution each time. Finally, the hydroalcoholic fractions were combined, washed with 4 mL of n-hexane to remove the residual oil, and then concentrated and dried by evaporative centrifuge in a vacuum at 35 °C.
Chromatographic Analysis of Phenols
The analyses were carried out in a Waters liquid chromatography (Agilent Infinity) equipped with two pumps (Model 510), an automated gradient controller (Model 680), an injector (Rheodyne Model 7125 with a 20-ml loop), a programmable fluorescence detector (Model 470), and a tunable absorbance detector (Model 486). Baseline Workstation 810 software (Waters) and a personal computer were employed for data storage and evaluation. The analytical column was a Waters (Mildford, MA, USA) Nova-Pak C18 3, 9 × 150 mm particle diameter. A Nova-Pak C18 precolumn was employed to protect the analytical column and kept at ambient temperature (30 °C) with an injection volume of 10 µL. The mobile phase comprised of 2% acetic acid in water (solvent A)/methanol (solvent B) at a flow rate of 0.5 mL/min, with the steps as follows: 0 min, 100% A; 15 min, 85% A; 25 min, 50% A; and 34 min, 30% A. A wavelength of 280 nm was used for absorbance detector. The fluorescence detector was operated at an excitation wavelength set at 280 nm and emissions at 320 and 350 nm (Selvaggini et al. 2006).To check the peak purity of each compound, a Beckman diode array detector (Model 168) controlled by the Beckman System Gold software was used. Peaks were considered pure when there was a match factor ≥ 99.5. Injected samples were interspersed with standards to ensure accurate quantitation. Chromatographic peaks were identified by comparing retention times of samples with those of standard compounds. Quantitation was carried out by internal standardization. The hydroxytyrosol, tyrosol, and oleuropein standards were prepared in 10% methanol + 2% CH3COOH, the remaining standards in 50% MeOH. The determination of the signal of interest was done by comparing retention times between the samples and the injected standards at 280 nm (Rodrıguez-Delgado et al. 2001).
Statistical Analysis
For all the experiments, six samples were analyzed, and all the assays were carried out in triplicate. The significant differences between the values of all parameters were identified at p < 0.05 following the ANOVA: Student–Newman–Keuls test by means of SPSS Statistics 17.0 for Windows (SPSS Inc. 2008). The aim of applying non-standardized principal component analysis (PCA) was to demonstrate the effect of all investigated parameters (n = 93) on the olive oil samples using the varimax rotation together with Kaiser normalization. The PCA type was Pearson (n), the biplot type was correlation biplot, and the coefficient was automatic. A uniform hierarchical cluster analysis (HCA) methodology was applied on data. Each cluster was determined by the following parameters: similarity, Euclidean distance matrix, and the Ward’s method, generating a dendogram for olive oil samples. All the chemometric analyses were carried out using XLSTAT software for Windows (v.2013.2.03, Addinsoft, NY, USA).
Results and Discussion
Identification of Volatile Compounds in Oil Samples
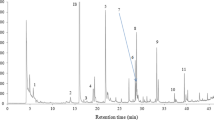
Concentrations of volatile compounds, whether major or minor, are crucial to virgin olive oil quality. Consequently, the identification of the compounds causing the flavor or off-flavor is therefore the key for quality control. Virgin olive oil has a delicate and unique flavor (Cherfaoui et al. 2018). The volatile compounds identified in oil and linear retention index values on the multidimensional chromatographic system for these compounds are presented in Table 1. The identification was made by comparing the obtained spectra with MS NIST version 2.0 (Johnson 2014). A total of 93 volatile compounds were isolated and identified, of the following chemical families: 8 alkanes, 11 alkenes, 8 alkylbenzenes, 9 alkanals, 7 alkenals, 2 alkandienals, 22 alcohols, 4 esters, 8 ketones, 12 terpenes, and 2 other compounds. Most of these components represented groups of characteristic volatiles produced through the lipoxygenase pathway. In the six oleaster oils, the isolated and identified compounds are mainly alcohols such as 1-hexanol at 32.13 min (LRI 1391) ([M + H]+, m/z 102) (Fig. 2d), 2-hexen-1-ol (isomer 1) at 34.16 min (LRI 1443) ([M + H]+, m/z 100) (Fig. 2f), and 3-hexen-1-ol (isomer 2) (Fig. 2e) at 33.50 min (LRI 1426) ([M + H]+, m/z 100). Among the aldehyde compounds identified, we can mention hexanal (Fig. 2b) at 21.96 min (LRI 1105) ([M + H]+, m/z 100) and the other linear aldehydes such as pentanal, heptanal, octanal, and nonanal. In addition, the monounsaturated aldehydes were also identified in our oil samples, as 3-hexenal at 24.76 min (LRI 1173) and (([M + H]+, m/z 98) and 2-hexenal (isomer 1) at 27.15 min (LRI 1231) ([M + H]+, m/z 98) and 2-hexenal (isomer 2) at 27.97 min (LRI 1250) ([M + H]+, m/z 98) (Fig. 2c). In addition, the volatile compounds of the virgin olive oil do not contribute to its whole aroma with the same importance. Chemical factors such as volatility and the hydrophobic character, size, shape, conformational structure, type, and position of functional groups seem to be more related to the odor intensity of a volatile compound than its concentration (Cherfaoui et al. 2018). Such compounds do not originate from the LOX pathway, and they are mostly the result of other, mostly undesirable processes (Purcaro et al. 2014). Ethyl acetate, at 13.10 min (LRI 903) ([M + H]+, m/z 88), which are responsible for winey-vinegar defect and, together with 3-methylbutanol, clearly indicated that olives underwent fermentation (ben Hammouda et al., 2017). Non-LOX C4 and C5 branched compounds are known to derive from the conversion of certain amino acids, while linear acids, esters, and ketones originate from fatty acid metabolism (Cherfaoui et al. 2018). All of the mentioned processes are commonly linked to more or less degraded raw olive fruit material, due to physical damage, inadequate sanitary conditions, or unsuitable storage of fruit before processing, and they are often found in VOOs with sensory defects (Ben Mansour et al. 2015; Polari et al. 2018). The possibility that particular non-LOX volatile compounds were formed and/or increased in concentration as a result of various oxidative processes, as shown previously by other authors (ben Hammouda et al. 2017), should not be neglected.
Determination and Comparison of the Composition of the Volatile Compounds in Oil Samples
Figure 1 shows the typical GC/MS chromatogram data for the volatile’s compounds extracted from olive oils by HS-SPME. The mean values (%) of the GC analyses of triplicate extractions and standard deviations are reported in Table 2. A total of 93 volatile compounds were isolated and tentatively identified: 27 hydrocarbons families, 8 alkanes, 11 alkenes, and 8 alkylbenzenes; 18 total aldehydes with 9 alkanals, 7 alkenals, and 2 alkandienals; 22 alcohols; 4 esters; 9 ketones; 12 terpenes, and 2 other compounds. Comparing the six oleasters collected at the same stage of ripeness, we can observe that there were some differences in the constituents of the volatile fraction. Only in control olive oils (CO), 2-pentanol; 1-butanol, 3-methyl; 1-nonanol; 1-decanol; 8-heptadecene; and 2,6-dimethyl-1,3,5,7-octatetraene were present, whereas 1-pentanol; 2-buten-1-ol; 1,5-hexadien-3-ol; 1-octanol; 1-penten-3-one; o-xylene; β-phellandrene; β-Myrcene; and decane were present only in polluted olive oils (PO) (Table 2). The most important mechanisms responsible for these volatile compounds are the thermal degradation of lipid (mainly oxidation), Maillard reaction, interaction between Maillard reaction products with lipid-oxidized products, and vitamin degradation (Van Ba et al. 2012). Among the volatile compounds, aldehydes might play an important role in the flavor because of the low odor threshold values, and their sensory characteristics are mainly associated with a fatty aroma (Song et al. 2011; ben Hammouda et al. 2017).
Alkanes, Alkenes, and Alkylbenzenes
The hydrocarbons of olive oils have been studied by different authors as possible markers to distinguish virgin olive oil from different olive varieties or different geographical origins (Molina-Garcia et al. 2017). The hydrocarbons octane; hexane; undecane; 1,7-nonadiene, 4,8-dimetyl; 1-octene, 6-methyl-; 1.3-pentadiene; 1.4-pentadiene; and 3-ethyl-1.5-octadiene have been detected in the aroma fractions of the tested oils. Mono-ring aromatic hydrocarbons (MAHs), such as ethylbenzene, styrene, and 1,2,4-trimetylbenzene, were observed in tested EVOOs at low levels. The presence of very small traces of benzene derivatives could be explained by environmental contaminants present in the fruit. On the other hand, styrene could be formed by decarboxylation of trans-cinnamic acid which is produced by L-phenylalanine deamination. Thereafter, ethylbenzene could be formed by reduction of styrene (Sabatini et al. 2009).
Aldehydes
As observed in Table 2, taking into consideration the effect of the growing area conditions, it is important to note that control oil samples (CO) had the highest level of aldehydes comparing to samples grown under the vicinity of phosphoric acid factory (PO). These compounds increased distinctly for the WPOO from 30.696% (2015) to 31.269% (2016), whereas they clearly increased for the UNPOO from 11.423% (2015) to 15.691% (2016). Furthermore, alkanals, alkenals, and alkandienals are the family of volatiles presenting a greater and more marked rise when oils are subjected to pollution (Table 2). They were of most concern from health point of view (Molina-Garcia et al. 2017). Among alkanals, with 5 to 10 carbon atoms present in the volatile fraction of oil samples were butanal, pentanal, hexanal, heptanal, octanal, and nonanal, which have been generated from lipid oxidation. This result is consistent with those found by Martins et al. (2020), which have shown that lipids generally have the greatest influence on the production of aroma components. In fact, hexanal (Fig. 2b) and 2-hexenal (isomer 2) (Fig. 2c) are the major volatile compounds detected in the oil blends by the SPME method. Thus, the hexanal and 2-hexenal (isomer 2) levels for polluted olive oils in the year of 2016 reached 2.55% and 25.369%, respectively, for WPOO and 2.28% and 26.838%, respectively, for UWPOO. These compounds are reported as the products of lipid oxidation of linoleic acid and linolenic acid (Zhu et al. 2016). The predominance of hexanal over the other compound in the volatile profile can be attributed to the multiplicity of its synthesis pathways. These results are in accordance with those described in the literature, which have reported that hexanal is the major aldehyde in oil samples and can be generated from oleic acid, linoleic and arachidonic acids, and through the degradation of other unsaturated aldehydes, such as 2,4-decadienal (Nieto et al. 2011; ben Hammouda et al. 2017). Among the other alkanals and alkenals which were detected in the oil samples, but at lower amount, 3-hexenal, 2-hexenal (isomer1) and nonanal can be mentioned; their levels for polluted olive oils in the year of 2016 reached 0.4%, 0.291%, and 0.243%, respectively, for WPOO and 0.33%, 0.447%, and 0.174%, respectively, for UWPOO. On the other hand, alkandienals were significantly present. In fact, 2,4-hexadienal (isomer) is formed in a greater amount for WPOO and UWPOO from 0.082 to 0.111% in 2016. These results are in agreement with those mentioned in the literature (Veneziani et al. 2018), which have proven that 2,4-hexadienal is one of the important unsaturated aldehydes, especially for the fat aroma and play some part in species characteristic flavor. In general, unsaturated aldehydes, such as alkenals and alkandienals, show much more severe toxicity than alkanals (Molina-Garcia et al. 2017).
Alcohols
Alcohols with six carbon atoms are associated with sweetness and contribute favorably to the aroma of extra-virgin olive oil (Reboredo-Rodríguez et al. 2012). These were among the other volatile compound affected by pollution. In fact, the highest compounds formed for oil samples WPOO and UWPOO were 2-hexen-1-ol (isomer1) (Fig. 2f) 1.149% and 0.412%, respectively (Table 2). This compound was found to contribute to a mild, fruit, and balsamic odor (Zhu et al. 2016). Indeed, as observed in Table 2, an important formation of 1-octen-3-ol, heptan-1-ol, and octan-1-ol was obtained. Both compounds were two cleavage products from methyl oleate hydroperoxides (Molina-Garcia et al. 2017).
Esters and Ketones
Nine ketones were detected in the oil samples, and they underwent a decrease in UWPOO. The content of 3-pentanone (Fig. 2a) is by far the major C5 ketone compound in all studied oils. Two unsaturated ketones, 1-penten-3-one and 6-methyl-5-hepten-2-one, were detected by GC/MS. To 1-penten-3-one, a mushroom aroma is described (Martins et al. 2020), whereas 6-methyl-5-hepten-2-one affected sweet, fruity, and green odors (Reboredo-Rodríguez et al. 2012). Esters were formed in small quantities in polluted oils, being totally absent in control olive oils (Table 2). These results are in agreement with a recent study (Bubola et al. 2014), which has demonstrated that an increase in C5 ketones have been observed in olive oil stored in the dark, and these compounds were proposed as markers of virgin olive oil quality freshness. Esters, compounds associated with fruity sensory notes (Zhu et al. 2016) as acetic acid, methyl ester, and acetic acid, hexyl ester, were present in aroma of control olive oils, but there are minor components compared with aldehydes or alcohols (Table 2). The low level of esters in samples indicates a lower content of alcohol acetyl transferase (AAT) in these olive oils (Peres et al. 2017).
Terpenes
Several terpene hydrocarbons (mono- and sesquiterpenes) were often detected. Most of them are minor compounds of the volatile fraction not derived from fatty acid transformations; however, even if they occur in EVOO below their olfactory threshold, they may provide useful quality markers (Ben Brahim et al. 2018). Interestingly, the anti-microbial activity of EVOO sesquiterpene hydrocarbons against a range of different microorganisms was evidenced (Cecchi and Alfei 2013). Among sesquiterpenes, molecular formulas C15H24, 3-carene, p-xylene, and o-xylene were present in the largest amounts with respect to the other sesquiterpenes (a-farnesene, α-acorenol, trans-β-ocimene, and β-ocimene) in almost all of the oils analyzed. According to previous reports, terpenoid hydrocarbons show large differences that depend on both the olive variety and geographical origin (Pouliarekou et al. 2011; Ben Mansour et al. 2015). Actually farnesene, which vary among samples in the present study, was often reported to be the most representative terpenic compound in EVOOs from different geographical region (Ben Brahim et al. 2018).
Phenolic Compounds Content
Phenolic compounds are a group of polar components, which contain one or more aromatic hydroxylated rings, including functional derivatives and are part of non-glycerin components in virgin olive oil (Seker 2010). The amount of phenolic compounds in EVOO is an important factor when evaluating its quality, given that the natural phenols improve its resistance to oxidation and, to certain extent, are responsible for its sharp bitter taste (Bubola et al. 2014). The quantity of phenols is influenced by the cultivar, the climatic conditions during growth, and the degree of ripening (Gargouri et al. 2013; Ammar et al. 2014). In all of the investigated EVOO samples, 10 phenols were identified and quantified (Table 3) when using a wavelength of 280 nm for detection. Moreover, the molecular ions of each compound were used to confirm the identification of the analytes. The use of a fluorescence detector in series with absorbance detector allowed increasing selectivity and sensitivity for the determination of catechin, vanillic acid, chlorogenic acid, epicatechin, and 3.4-dihydroxy phenylacetic acid in oil samples. Differences in bioactive and antioxidant compounds between oils from the polluted and control olive oils samples (PO and CO, respectively) were recorded.
Moreover, differences were observed comparing the analytical data of oils of the same field in the two consecutive years. Generally, the most abundant was tyrosol, followed by hydroxytyrosol. A higher concentration was observed in UWPOO and WPOO for 2016, while the WPOO for 2015 was distinguished by higher amount of p-coumaric Ac. The effects of the environment and pedo-climatic conditions on these compounds were clearly observed based on the diverse behaviors of each growing area (Rekik et al. 2019).
Method Validation
To assess the validity of the method, validation tests were run. All test parameters were carefully chosen to cover the range of samples and concentrations involved (Table 4). The linearity of standard curves was expressed in terms of the determination coefficient from plots of the integrated peak area versus concentration of the same standard (mg/L). These equations were obtained over a wide concentration range in accordance with the levels of these compounds found in the olive samples. Details are given in Table 4. The system was linear in all cases (R2 > 0.99). LOD and LOQ were studied to check the sensitivity of the methods under the working conditions proposed. These limits, referring to the concentrations in olive oil needed if they were to be detected and quantified, were of the order of milligrams. The method has excellent sensitivity (Table 4).
Chemometric Analysis
In the preliminary analysis of the olive oil data, PCA was performed to investigate any potentially, existing clustering of samples on the basis of geographical origin and the year of harvest. PCA was applied to the dataset of volatile compounds of VOO from the different samples studied (Fig. 3). The first principal compound (PC) accounted for 44.47% of variance, while the second PC contributed 17.35%, resulting 65% of total variance. As Fig. 3a and b shows, using this approach, the olive oils were divided in three groups. The formed clusters indicated a large variation in the profiles of olive oil volatiles between the two harvest years, possibly caused by different weather conditions during the growing season. The majority of the investigated aldehydes and ketones that derive from the LOX pathway, including the major ones, such as 2-hexenal (isomer 1 and 2) (v67 and v71) and 3-hexenal (v52), as well as 1-penten-3-one (v33), 3-pentanone (v29) (Fig. 2a), 5-hepten-3-one, 5-methyl- (v49), and unsaturated hydrocarbons, were characteristic for monovarietal EVOO, washed and unwashed olive oils samples for the year 2016, and they could have contributed to generating positive green and fruity notes (Ben Brahim et al. 2018).
Chemometric analysis. Principal component analysis (PCA) obtained from the volatile compounds (listed in Tables 1 and 2) on PC1 and PC2 of all olive oil samples analyzed, considering 93 variables presented in the loading plot (a) and 6 observations shown in the score plot (b). Hierarchical cluster analysis [HCA]: dendrogram showing the clustering of the all analyses performed on oils from washed and unwashed polluted olives under two seasons 2015–2016 and 2016–2017 in the score plot (c)
The application of cluster analysis to the volatiles divided all the samples into three groups: UWPOO 2016, WPOO 2016, CO 2015, and CO 2016 comprised the first group, the second group consisted of UWPOO 2015, while WPOO 2015 belonged to the thirrd group. The dendrogram of Fig. 3c shows that washing olive samples has no relevant influence on the production of volatiles since samples of the same variety and the same year of harvest 2016 have a great similarity. UWPOO 2015 and WPOO 2015 presented an extreme dissimilarity in their composition not only between each other, but also in comparison with the other samples. In fact, these samples can be distinguished from the others by the high levels of alkenes, alkylbenzenes, esters, and terpenes, whose contents are by far the highest in WPOO 2015 samples. Nonetheless, in the same group, there is a high similarity between polluted oils in the year 2016 and CO 2015. It can be explained by the levels of alkenals in both. The geographic origin of oils plays a fundamental role in defining the volatile compound profile of virgin olive oils. Early studies conducted by Ben Mansour et al. (Ben Mansour et al. 2015) show the ability of volatiles, sampled with the static headspace, in discriminating different oils coming from different Tunisian regions.
Conclusion
The application of SPME to the analysis of virgin olive oil headspace allowed the detection of significant differences in the proportion of volatile constituents from oils of different geographical origins. The results indicate that besides the genetic factor, environmental conditions influence the volatile profiles. However, the study of a larger number of samples from various years of production would lend support to the results obtained by this first screening. The semi-quantitative determination of the volatile compounds can provide very useful information on EVOO quality and its control. A method for analysis of 10 phenolic compounds in oil samples by HPLC using fluorescence and absorbance detectors in series is presented. Even that the number of samples analyzed were limited, the influence of the pollution on volatile profiles of Chemlali virgin olive oils should be considered preliminary.
References
Ammar S, Zribi A, Mansour AB et al (2014) Effect of processing systems on the quality and stability of Chemlali olive oils. J Oleo Sci 63:311–323
Aparicio R, Harwood J (2013) Handbook of olive oil. Anal Prop, 2nd edn. Springer, New York
Ben Brahim S, Amanpour A, Chtourou F et al (2018) Gas chromatography–mass spectrometry–olfactometry to control the aroma fingerprint of extra virgin olive oil from three Tunisian cultivars at three harvest times. J Agric Food Chem 66:2851–2861
ben Hammouda I, Freitas F, Ammar S et al (2017) Comparison and characterization of volatile compounds as markers of oils stability during frying by HS–SPME-GC/MS and chemometric analysis. J Chromatogr B 1068:322–334
Ben Mansour A, Flamini G, Ben Selma Z et al (2015) Comparative study on volatile compounds, fatty acids, squalene and quality parameters from whole fruit, pulp and seed oils of two Tunisian olive cultivars using chemometrics. Eur J Lipid Sci Technol 117:976–987
Bubola KB, Koprivnjak O, Sladonja B, Belobrajić I (2014) Influence of storage temperature on quality parameters, phenols and volatile compounds of Croatian virgin olive oils. Grasas Aceites 65:e034-e41
Cecchi T, Alfei B (2013) Volatile profiles of Italian monovarietal extra virgin olive oils via HS-SPME–GC–MS: newly identified compounds, flavors molecular markers, and terpenic profile. Food Chem 141:2025–2035
Cherfaoui M, Cecchi T, Keciri S, Boudriche L (2018) Volatile compounds of Algerian extra-virgin olive oils: effects of cultivar and ripening stage. Int J Food Prop 21:36–49
Domínguez R, Gómez M, Fonseca S, Lorenzo JM (2014) Effect of different cooking methods on lipid oxidation and formation of volatile compounds in foal meat. Meat Sci 97:223–230
Drira M, Kelebek H, Guclu G et al (2020) Targeted analysis for detection the adulteration in extra virgin olive oil’s using LC-DAD/ESI–MS/MS and combined with chemometrics tools. Eur Food Res Technol. https://doi.org/10.1007/s00217-020-03522-y
Frankel EN (2010) Chemistry of extra virgin olive oil: adulteration, oxidative stability, and antioxidants. J Agric Food Chem 58:5991–6006
Gargouri B, Ammar S, Zribi A et al (2013) Effect of growing region on quality characteristics and phenolic compounds of chemlali extra-virgin olive oils. Acta Physiol Plant 35:2801–2812
Johnson SG (2014) NIST Standard Reference Database 1A v17. In: NIST. https://www.nist.gov/srd/nist-standard-reference-database-1a-v17. Accessed 9 Apr 2019
Martins N, Jiménez-Morillo NT, Freitas F et al (2020) Revisiting 3D van Krevelen diagrams as a tool for the visualization of volatile profile of varietal olive oils from Alentejo region, Portugal. Talanta 207:120276
Molina-Garcia L, Santos CSP, Cunha SC et al (2017) Comparative fingerprint changes of toxic volatiles in low PUFA vegetable oils under deep-frying. J Am Oil Chem Soc 94:271–284
Nieto G, Bañón S, Garrido MD (2011) Effect of supplementing ewes’ diet with thyme (Thymus zygis ssp. gracilis) leaves on the lipid oxidation of cooked lamb meat. Food Chem 125:1147–1152
Peres F, Martins LL, Ferreira-Dias S (2017) Influence of enzymes and technology on virgin olive oil composition. Crit Rev Food Sci Nutr 57:3104–3126
Polari JJ, Garcí-Aguirre D, Olmo-García L et al (2018) Impact of industrial hammer mill rotor speed on extraction efficiency and quality of extra virgin olive oil. Food Chem 242:362–368
Pouliarekou E, Badeka A, Tasioula-Margari M et al (2011) Characterization and classification of Western Greek olive oils according to cultivar and geographical origin based on volatile compounds. J Chromatogr A 1218:7534–7542
Purcaro G, Cordero C, Liberto E et al (2014) Toward a definition of blueprint of virgin olive oil by comprehensive two-dimensional gas chromatography. J Chromatogr A 1334:101–111
Reboredo-Rodríguez P, González-Barreiro C, Cancho-Grande B, Simal-Gándara J (2012) Dynamic headspace/GC–MS to control the aroma fingerprint of extra-virgin olive oil from the same and different olive varieties. Food Control 25:684–695
Rekik O, Ben Mansour A, Jabeur H et al (2019) Effect of pollution on the quality of olive oils from trees grown near a phosphoric acid factory. Eur J Lipid Sci Technol 121:1800490
Rodrıguez-Delgado MA, Malovana S, Perez JP et al (2001) Separation of phenolic compounds by high-performance liquid chromatography with absorbance and fluorimetric detection. J Chromatogr A 912:249–257
Sabatini N, Perri E, Marsilio V (2009) An investigation on molecular partition of aroma compounds in fruit matrix and brine medium of fermented table olives. Innov Food Sci Emerg Technol 10:621–626
Seker M (2010) Effects of geographical origin and cultivar on phytosterol and tocopherol components in olive (Olea europaea L.) cultivars cultivated in Turkey. In: XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010): Olive Trends Symposium-From the 924. pp 393–400
Selvaggini R, Servili M, Urbani S et al (2006) Evaluation of phenolic compounds in virgin olive oil by direct injection in high-performance liquid chromatography with fluorometric detection. J Agric Food Chem 54:2832–2838
Sghaier L, Vial J, Sassiat P et al (2016) An overview of recent developments in volatile compounds analysis from edible oils: Technique-oriented perspectives. Eur J Lipid Sci Technol 118:1853–1879
Song S, Zhang X, Hayat K et al (2011) Formation of the beef flavour precursors and their correlation with chemical parameters during the controlled thermal oxidation of tallow. Food Chem 124:203–209
Van Ba H, Hwang I, Jeong D, Touseef A (2012) Principle of meat aroma flavors and future prospect. In: Latest research into quality control. IntechOpen
Veneziani G, Esposto S, Taticchi A et al (2018) Characterization of phenolic and volatile composition of extra virgin olive oil extracted from six Italian cultivars using a cooling treatment of olive paste. LWT 87:523–528
Zhu H, Wang SC, Shoemaker CF (2016) Volatile constituents in sensory defective virgin olive oils. Flavour Fragr J 31:22–30
Acknowledgements
The authors thank the Ministry of Higher Education Scientific Research (Laboratory LR14ES08), Tunisia, and ARIMNET 2 MedOOmics “Mediterranean Extra Virgin Olive Oil Omics: profiling and fingerprinting”—“Arimnet2/0001/2015” for the support of this research work. This work was supported by Portuguese National Funds through FCT – Foundation for Science and Technology under the Associate Laboratory for Green Chemistry – LAQV [UID/QUI/50006/2020] and the project [UIDB/05183/2020]. This work has received also funding from the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No. 778045 and was also anchored by the RESOLUTION LAB, an infrastructure at NOVA School of Science and Technology. The authors would like to extend their thanks to Mrs Leila Mahfoudhi, teacher of English in the Sfax Faculty of Science, for proofreading and polishing the language of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to Participate
Not applicable.
Conflict of Interest
Ons Rekik declares that he has no conflict of interest. Amir ben Mansour declares that he has no conflict of interest. M.D.R. Gomes Da Silva declares that he has no conflict of interest. Mohamed BOUAZIZ declares that he has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rekik, O., Mansour, A.b., Da Silva, M.D.R.G. et al. Identification of Trace Volatile and Phenolic Compounds in Olive Oils with Trees Growing in Different Area Conditions: Using SPME/GC–MS . Food Anal. Methods 14, 2494–2510 (2021). https://doi.org/10.1007/s12161-021-02061-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-021-02061-w